Abstract
Phosphatidylinositol-3-kinase (PI3K), and its downstream effector Akt, or protein kinase Bα (PKBα), play a major regulatory role in control of apoptosis, proliferation, and angiogenesis. PI3K and Akt are amplified or overexpressed in a number of malignancies, including sarcomas, ovarian cancer, multiple myeloma, and melanoma. This pathway regulates production of the potent angiogenic factor vascular endothelial growth factor (VEGF), and protects tumor cells against both chemotherapy and reactive oxygen–induced apoptosis through phosphorylation of substrates such as apoptotic peptidase–activating factor-1 (APAF-1), forkhead proteins, and caspase 9. Given its diverse actions, compounds that suppress the PI3K/Akt pathway have potential pharmacologic utility as angiogenesis inhibitors and antineoplastic agents. Using the SVR angiogenesis assay, a screen of natural products, we isolated the alkaloid solenopsin, and found that it is a potent angiogenesis inhibitor. We also found that solenopsin inhibits the PI3K signaling pathway in cells upstream of PI3K, which may underlie its affects on angiogenesis. Consistent with inhibition of the activation of PI3K, solenopsin prevented the phosphorylation of Akt and the phosphorylation of its substrate forkhead box 01a (FOXO1a), a member of the forkhead family of transcription factors. Interestingly, solenopsin also inhibited Akt-1 activity in an ATP-competitive manner in vitro without affecting 27 of 28 other protein kinases tested.
Introduction
The serine/threonine kinase c-Akt-1, or protein kinase Bα (PKB), is the cellular homolog of a transforming oncogene initially isolated from a lymphoma. Akt is a downstream target of phosphatidylinositol-3-kinase (PI3K), a family of at least 4 different enzymes, with the prototypical PI3K heterodimer consisting of a p85 (regulatory) and a p110 (catalytic) subunit. The PI3K/Akt pathway is involved in the regulation of diverse cellular functions including proliferation, cytoskeletal organization, survival, and malignant transformation.1–4 Upon binding of PI3K products to its pleckstrin homology domain, Akt is translocated to the plasma membrane where it is activated by upstream phosphorylated kinases, including PI3K-dependent kinases 1 and 2 (PDK1 and PDK2) and mammalian target of rapamycin complex 2 (mTORC2). The PI3K/Akt pathway is stimulated by numerous receptor tyrosine kinases and oncogenes, including receptors for insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), ras, Her2/neu, and polyoma middle T oncogenes.5–10 Because Akt plays a central role in regulating apoptosis, angiogenesis, and metabolism of cells, Akt is an attractive pharmacologic target for the treatment of cancer and inflammation.11,12 Small-molecular-weight inhibitors of PI3K include LY 294002 and the fungal metabolite wortmannin,13 as well as ether phospholipids, including perifosine, which has entered clinical trials.14,15
Using the SVR angiogenesis assay,16–18 we found that solenopsin A,19,20 the primary alkaloid from the fire ant Solenopsis invicta, has antiangiogenic activity. We also discovered that solenopsin disrupted angiogenesis in vivo in embryonic zebrafish. In order to determine the mode of action, we examined the ability of solenopsin to inhibit a battery of cellular kinases and found that solenopsin A inhibited Akt relatively selectively in an ATP-competitive manner without affecting its upstream activator PDK1 or PI3K. However, in cells, solenopsin prevented the activation of PI3K, the phosphorylation of Akt-1 at both Thr308 and Ser473, and the phosphorylation and subsequent subcellular localization of forkhead box O1a (FOXO1a), a physiologic substrate of Akt.21 In contrast, solenopsin did not affect the activity of purified PI3K or PDK1, which is the kinase that phosphorylates Akt at Thr308. Taken together, our results imply that solenopsin exerts its effects on Akt activity in cells by inhibiting a step in the signaling pathway that lies upstream of PI3K.
Materials and methods
Synthesis of solenopsin and solenopsin derivatives
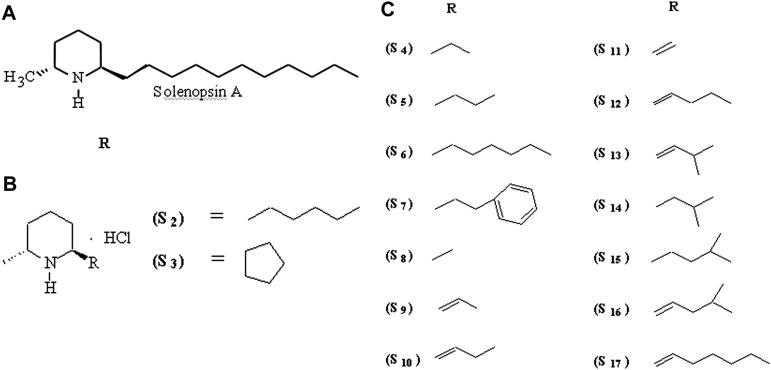
Solenopsin analogs were synthesized beginning with 4-chloropyridine and alkyl magnesium halides, resulting in a 2-alkyl-4-chloro tetrahydropyridine, which was further modified by lithiation and reduction with a hydrogen and palladium/carbon catalyst.22 Derivatives S1, S2, and S3 were prepared this way from commercially available alkyl Grignard reagents (Sigma-Aldrich, St Louis, MO). Other reagents were synthesized according to the method of Beak and Lee,23 in which N-Boc–substituted piperidine is lithiated and methylated at the 6 position with dimethylsulfate, followed by addition of a formyl group at the 2′ position. The 2′ position was modified by a Wittig reaction, generating 2′alkenyl–substituted 6-methylpiperidines, which were subsequently hydrogenated to generate derivatives S5 to S19. Derivatives were converted to hydrochloride salts through passage of hydrogen chloride through solutions of the solenopsin derivatives.
Other reagents were synthesized by lithiating N-Boc–substituted piperidine and methylating it at the 6 position with dimethylsulfate, followed by addition of a formyl group at the 2′ position.23,24 The 2′ position was modified by a Wittig reaction, generating 2′alkenyl substituted 6-methylpiperidines, which were subsequently hydrogenated to generate derivatives S5 to S19. Derivatives were converted to hydrochloride salts through passage of hydrogen chloride through solutions of the solenopsin derivatives.
Structure activity relationship in SVR angiogenesis assay
SVR cells are murine endothelial cells immortalized by infection with an ecotropic retrovirus encoding SV40 large T antigen, followed by transformation with oncogenic H-ras, and have been used extensively as a screen for angiogenesis inhibitors.16–18,25 SVR cells were plated at a concentration of 10 000 cells/well, as previously described,18 and treated with solenopsin or solenopsin analogs (Figure 1) at concentrations varying from 0 to 6 μg/mL for 48 hours, at which point cells were counted with a Coulter Counter (Coulter, Hialeah, FL).
Figure 1.
Structure of solenopsin and solenopsin analogs.
Kinase inhibition assays
Solenopsin and its analogs were tested in vitro for inhibitory activity against the following enzymes as previously described26: MKK1, ERK2, JNK1, p38α MAPK, p38β MAPK, p38γ MAPK, p38δ MAPK, RSK1, MAPKAP-K2, MSK1, PRAK, PKA, PKCα, PDK1, AKT1, SGK, S6K1, GSK3β, ROCK-II, AMPK, CHK1, CK2, PHK, LCK, CSK, CDK2/cyclin A, CK1, DYRK1a, and PP2a as previously performed for honokiol.18 Solenopsin and its analogs were tested against purified recombinant PI3K p110alpha/p85alpha complex measuring the conversion of phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3).27
Western blot analysis
Cells were grown on 10-cm dishes and allowed to reach approximately 80% confluence before protein isolation. Sample aliquots normalized for protein quantities were size fractionated by SDS-PAGE, and the proteins were transferred to a PVDF membrane. The blots were incubated in blocking solution; PBS with 5% (wt/vol) powdered nonfat milk for 1 hour at room temperature (RT). The blots were then incubated overnight in sheep polyclonal anti-Akt (Upstate Biotechnology, Charlottesville, VA) or rabbit polyclonal antiphosphorylated Akt (Ser 473; Cell Signaling Technology, Danvers, MA). Rabbit polyclonal antibodies against insulin receptor substrate 1 (IRS1) were from Upstate Biotechnology, and mouse monoclonal antiphosphotyrosine (P-Tyr-100) was from Cell Signaling Technology. Protein A–agarose beads and secondary antibodies conjugated to horseradish peroxidase (HRP) were from Santa Cruz Biotechnology (Santa Cruz, CA). Dulbecco modified Eagle medium (DMEM) was from Hyclone (Logan, UT), and serum was from Valley Biomedical (Winchester, VA). All chemicals, unless otherwise mentioned, were obtained from Sigma-Aldrich.
FOXO1a and RevGFP export assay
The FOXO1a export assay is described in Kau et al.25 Briefly, 786-O cells were plated onto black, 384-well, clear-bottom plates (Costar, Corning, NY) in 50 μL DMEM/5% fetal clone and infected with Ad-FKHR adenovirus. For compound treatment, solenopsin A was serially diluted starting from 80 μM in a separate 384-well plate before transfer onto infected cells. Cells were incubated with compound for 1 hour before fixation with 3.7% formaldehyde and then stained with M5 anti-FLAG antibody (Sigma-Aldrich), Alexa Fluor 594 goat antimouse antibody (Invitrogen, Carlsbad, CA) and Hoechst 33258 (Sigma-Aldrich).
RevGFP export inhibition is also described in Kau et al.25 U2OS-RevGFP cells were seeded onto clear-bottom, black, 384-well plates in 50 μL complete media. Cells were allowed to attach and grow overnight before treatment with solenopsin A. Solenopsin A dilutions were made as described in the FOXO1a export assay. Cells were incubated with compound for 1 hour before fixation with 3.7% formaldehyde and nuclei staining with Hoechst 33258.
Zebrafish stocks
Zebrafish were grown and maintained at 28.5°C. Matings were routinely carried out at 28.5°C, and the embryos were staged according to established protocols.28 Embryos were staged according to time after fertilization and morphology. Transgenic Fli-EGFP fish (TG(fli1:EGFP)y1)29 were purchased from the Zebrafish Information Network (ZFIN, Eugene, OR).
Drug studies
A number of embryos (20-40) from a transgenic mating pair were collected and incubated at 28.5°C with 2.0 mL of egg water in a 6-well plate. At 6 hours after fertilization, egg water was replaced with fresh egg water (2.0 mL) containing drugs (solenopsins A and S3) at concentrations (3.75, 5, and 6 μg/mL) determined from a preliminary dose curve study. DMSO controls were also included each time an experiment was performed. Phenylthiourea (PTU) was added to a final concentration of 0.003% to prevent pigmentation at 10 to 12 hours after fertilization. Embryos were allowed to develop to the required stages at 28.5°C until ready for confocal microscopy. Fish were placed onto glass coverslips embedded into 35-mm dishes (MatTek Corp, Ashland, MA), anesthetized with tricaine (0.016%), and examined with a Zeiss Axiovert 100M microscope using 10× 10.3 NA and 25× 10.3 NA objectives (Zeiss, Thornwood, NY). Images were generated using a Zeiss LSM 510 laser-scanning microscope and were processed using Zeiss Aim software. These experiments were performed in triplicate. Confocal images were captured at the Cell and Cancer Biology Branch Confocal Microscopy Care facility (National Cancer Institutes, National Institutes of Health).
Studies of the effect of solenopsin on cells
3T3-L1 cells were serum-starved in DMEM + 0.2% BSA for 2 hours and treated with 30 μM solenopsin A for 20 minutes prior to stimulating with 1 μM insulin for 10 minutes. Plates were washed 3 times with ice-cold PBS. Cells were scraped into lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 4 mM Na3VO4, 200 mM NaF, 20 mM Na4P2O7, 10 mM EDTA, 10% glycerol, 1% Triton X-100, and 1:100 protease inhibitor cocktail), and the assay was performed on the cell lysates as mentioned previously.30 Solenopsin (5 nM-100 μM) was tested in NIH3T3 cells for its ability to inhibit PDGF-induced phospho-Akt formation. Phospho-Akt was determined using the LI-COR Odyssey (Lincoln, NE) in-cell Western protocol.31
Results
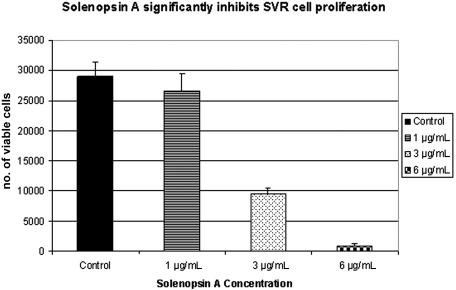
The SVR proliferation assay is a broad screen that examines the ability of compounds to inhibit the growth of ras-transformed endothelial cells by inhibiting either ras−-specific17,25 or endothelial-specific18 signaling. Compounds that inhibit SVR cells are tested routinely on nonendothelial cells to determine whether or not the inhibition is endothelial specific. Solenopsin and a series of related tetrahydropyridines (Figure 1) were tested as angiogenesis inhibitors using the SVR endothelial cell proliferation assay; among all the closely related solenopsin analogs, only solenopsin A significantly impaired SVR proliferation (Figure 2). We thus chose solenopsin for further mechanistic studies.
Figure 2.
Structure-activity relationship of solenopsin and analogs in SVR angiogenesis assay. Solenopsin and its analogs 1 to 17 were tested for their ability to inhibit the proliferation of SVR endothelial cells at 1 μg/mL, 3 μg/mL, and 6 μg/mL. Only solenopsin A exhibited dose-dependent inhibition of SVR proliferation (P < .05). Bars represent average of duplicate experiments, each performed in triplicate. Analog data not shown for brevity.
Inhibition of kinases
Solenopsin was tested against a panel of 28 kinases and 1 protein phosphatase in vitro (Table 1). These experiments revealed that solenopsin at 10 μM inhibited Akt by 50%. Apart from ribosomal protein S6 kinase 1 (RSK1), which was inhibited to a similar extent, no other enzyme in the panel was inhibited significantly. The inhibition of Akt by solenopsin was found to be competitive with respect to ATP, as inhibition of Akt increased with decreasing concentrations of ATP in the assay (Table 2). Solenopsin did not inhibit PDK1 (Table 1), an upstream activator of Akt, nor did it inhibit the purified recombinant PI3K p110alpha/p85alpha complex in a cell-free assay measuring the conversion of PIP2 to PIP3.
Table 1.
Effect of solenopsin on the activities of protein kinases
| Protein kinase | Activity, % control ± SD |
|---|---|
| MKK1 | 110 ± 1 |
| ERK2 | 93 ± 3 |
| JNK1 | 105 ± 7 |
| p38α MAPK | 82 ± 3 |
| p38β2 MAPK | 96 ± 2 |
| p38γ MAPK | 91 ± 2 |
| p38δ MAPK | 100 ± 6 |
| RSK1 | 46 ± 1 |
| MAPKAP-K2 | 93 ± 0 |
| MSK1 | 86 ± 4 |
| PRAK | 121 ± 6 |
| PKA | 137 ± 4 |
| PKCα | 99 ± 9 |
| PDK1 | 136 ± 6 |
| AKT1 | 51 ± 5 |
| SGK | 87 ± 6 |
| S6K1 | 81 ± 13 |
| GSK3β | 108 ± 2 |
| ROCK-II | 104 ± 1 |
| AMPK | 98 ± 7 |
| CHK1 | 84 ± 5 |
| CK2 | 116 ± 1 |
| PHK | 105 ± 0 |
| LCK | 89 ± 0 |
| CSK | 101 ± 3 |
| CDK2/cyclin A | 95 ± 1 |
| CK1 | 109 ± 4 |
| DYRK1a | 86 ± 5 |
| PP2a | 114 ± 6 |
Each protein kinase was assayed in duplicate at 0.1 mM ATP in the presence and absence of 10 μM solenopsin. The results are presented as the percentage of activity remaining in the presence of solenopsin (average of duplicate determinations). Similar results were obtained in another independent experiment. Each protein was expressed, purified, and assayed as described previously.26,32 MKK indicates MAPK kinase; ERK, extracellular signal-related kinase; JNK, c-Jun N-terminal kinase; RSK, p90 ribosomal S6 kinase; MAPKAP-K2, MAPK-activated protein kinase 2; MSK, mitogen- and stress-activated kinase; PRAK, p38-regulated/activated kinase; PKA, cAMP-dependent protein kinase; PKC protein kinase C; PDK1, 3-phosphoinositide–dependent protein kinase 1; AKT1, protein kinase Bα; SGK, serum and glucocorticoid-induced protein kinase; S6K, p70 ribosomal protein S6 kinase; GSK3, glycogen synthase kinase-3; ROCK, Rho-dependent kinase; AMPK, AMP-activated protein kinase; CHK1, checkpoint kinase 1; CK2, casein kinase 2; PHK, phosphorylase kinase; LCK, lymphocyte kinase; CSK; C-terminal Src kinase; CDK2, cyclin-dependent kinase 2-cyclin A complex; DYRK, dual specificity tyrosine phosphorylation regulated kinase; and PP2a, protein phosphatase 2A.
Table 2.
Solenopsin inhibition of PKB/Akt is competitive with ATP
| ATP concentration, μM | Activity, % control ± SD |
|---|---|
| 1 | 25.6 ± 9.6 |
| 5 | 48.4 ± 3.6 |
| 20 | 64.3 ± 5.5 |
| 100 | 75.4 ± 7.3 |
Solenopsin A (10 mM) was tested for its ability to inhibit PKBα at a range of ATP concentrations. The results are presented as the percentage of activity remaining in the presence of solenopsin relative to the activity measured in the absence of solenopsin. Results are expressed as the average of duplicate determinations. Similar results were obtained in several other experiments.
Solenopsin antagonizes Akt function in cell-based assays
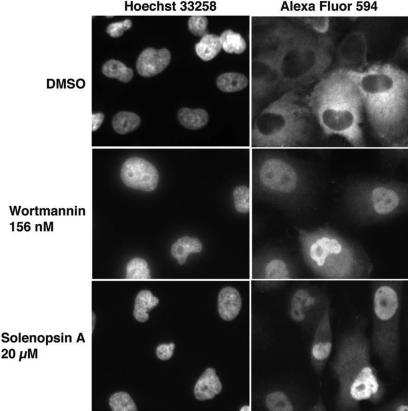
Given that solenopsin inhibited Akt in vitro, we wanted to examine its ability to inhibit Akt in cells. Akt is a serine-threonine kinase that phosphorylates multiple substrates, including members of the forkhead family of transcription factors (FKHD/FOXO). Once these FOXO proteins are phosphorylated, they are transported from the nucleus to the cytoplasm. Recently, Kau et al described a high-throughput assay using FLAG-epitope–tagged FOXO1a in cells null in PTEN to rapidly assess the ability of compounds to inhibit nuclear export of FOXO proteins.25 Consistent with its inhibitory activity on Akt, solenopsin A inhibited the nuclear export of FOXO1a. This inhibition was specific, as export of RevGFP, a protein whose nuclear export does not depend on Akt, was not inhibited by solenopsin A (Figure 3).
Figure 3.
FOXO1a localization after treatment with solenopsin. 786-O PTEN−/− cells were infected with AdFKHR and treated with DMSO, wortmannin, or solenopsin. Treatment with the negative control, DMSO, resulted in FOXO1a in the cytoplasm, while treatment with the PI3K inhibitor wortmannin relocalized FOXO1a to the nucleus. Similarly, solenopsin also relocalized FOXO1a to the nucleus. Wortmannin is used as a positive control.
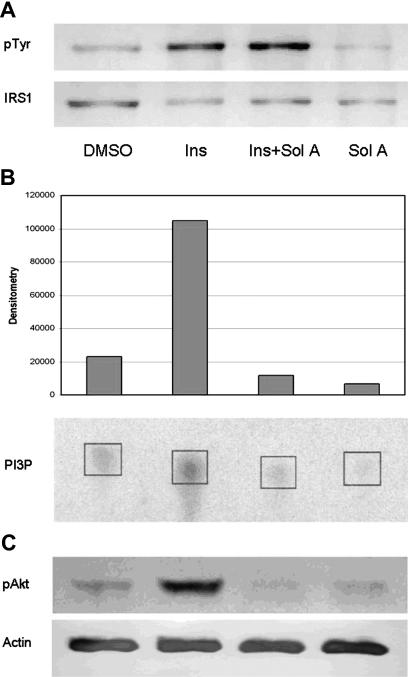
Solenopsin inhibits insulin-mediated PI3K activation
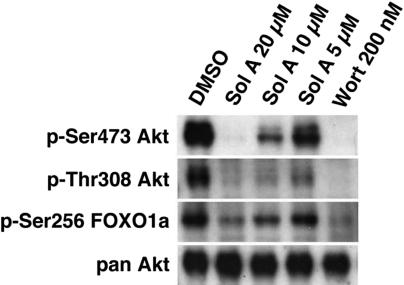
Treatment of cells with solenopsin did not inhibit insulin-stimulated tyrosine phosphorylation of IRS1 (Figure 4A), but blocked insulin-induced, PI3K-dependent generation of 3-phosphoinositides (Figure 4B). Consistent with inhibition in cells occurring at a level between IRS1 and PI3K, solenopsin also inhibited signaling downstream of PI3K, namely the phosphorylation of Akt at Thr308 (Figure 5) and Ser473 (Figures 4C, 5), which are catalyzed by PDK1 and the mTORC2, respectively, and the phosphorylation of FOXO1A at Ser256 (Figure 5). In contrast, solenopsin did not inhibit PDGF-induced phosphorylation of Akt in NIH3T3 cells except at cytotoxic doses (data not shown), suggesting that solenopsin's activity may be specific to insulin signaling or pathway and/or cell dependent.
Figure 4.
Solenopsin inhibits insulin-stimulated PI3K signaling in 3T3-L1 cells. 3T3-L1 fibroblasts were treated with 30 μM solenopsin A for 20 minutes prior to stimulating with 1 μM insulin for 10 minutes. (A) Insulin-stimulated, IRS-1–associated PI3K activity was assessed by measuring the incorporation of labeled phosphate from [γ32P]ATP into phosphatidylinositol. Labeled phosphatidylinositol 3-phosphate was resolved from ATP by thin-layer chromatography, which was visualized using a Storm Phosphorimager. Intensity of the bands was quantified by ImageQuant software (Molecular Dynamics, Sunnyvale, CA). (B) Cell lysates were resolved by SDS-PAGE, Western-blotted with anti–phospho-Akt (Ser473) antibodies, and detected by enhanced chemiluminescence. (C) IRS-1 was immunoprecipitated from cell lysates as in panel A, but the resulting immunoprecipitates were then resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine or anti–IRS-1 antibodies.
Figure 5.
Phospho-Akt and phospho-FOXO1a immunoblot. AdFKHR-infected 786-O cells were treated with DMSO, wortmannin, or solenopsin in decreasing concentrations. Solenopsin, like wortmannin, reduces phospho-Ser473 Akt, phospho-Thr308 Akt, and phospho-Ser256 FOXO1a levels.
Solenopsin A inhibits embryonic angiogenesis in zebrafish
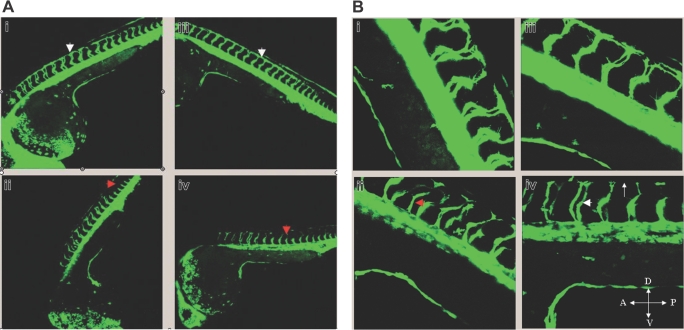
Given that solenopsin A inhibited angiogenesis in vitro, we used a zebrafish model system to determine whether it could suppress angiogenesis in vivo. Zebrafish embryos are transparent, and drugs dissolved in DMSO are readily permeable through the chorion. Solenopsin A and an inactive solenopsin analog (S3) were incubated with embryos from a transgenic (TG(fli1:EGFP)y1)29 zebrafish line that carries a 15-kb promoter of friend leukemia integration-1 transcription factor (fli-1), which drives GFP expression in the endothelium. Treatment with solenopsin, but not the inactive solenopsin analog S3, delayed intersomitic vessel sprouts arising from the dorsal aorta (Figure 6). These effects differ from those reported previously for VEGFR2 inhibitors in zebrafish development33 in which they injected dominant active Akt constructs that rescued VEGFR2's intersomitic vessel defect when tested at 24 hours after fertilization. Here, we tested the effect of solenopsin at 6 hours after fertilization. The vasculogenic vessels such as the dorsal aorta and the posterior cardinal vein formed appropriately in solenopsin-treated embryos, suggesting that solenopsin may delay angiogenic precursors or sprouts from reaching their target. A potential reason for these differences is that VEGFR2 inhibitors may affect the development of tissues that express VEGFR2, while Akt inhibitors such as solenopsin may affect nonvascular tissues, leading to the different phenotype.
Figure 6.
Effect of solenopsin on vascular development in zebrafish. Confocal images of drug-treated embryos shown here are primarily lateral or dorsolateral views of the trunk vasculature of different TG(fli1:EGFP)y1 embryos at 32 hours after fertilization. In both panels (A, 10 ×; B, 25 ×), i and iii represent solenopsin 3–treated embryos while ii and iv represent solenopsin A–treated embryos at 5 and 6 μg/mL, respectively. (A) In i and iii, the primary sprouts from the dorsal aorta and posterior cardinal vein have split at the level of the dorsolateral surface of the neural tube, and branches from adjacent primary sprouts are interconnected to form the paired longitudinal anastomotic vessels (DLAV; white arrowhead). In ii and iv, the primary sprouts (red arrowhead) have delayed considerably in reaching the dorsolateral surface of the neural tube. (B) In high power (25 ×), the absence of DLAV is denoted by an arrow (iv), and the spacing between the sprouts (white arrowhead in iv; red arrowhead in ii) is distinct.
Discussion
Solenopsis invicta, the fire ant, is a major pest in the United States, infesting more than 290 million acres. The ant is capable of multiple stings, and secretes venom that consists of the alkaloid solenopsin and venom proteins.34–36 Repeated stings can cause death to animals and humans through a direct action of the alkaloid or allergic reaction to the protein. The mechanism of action of solenopsin has not previously been determined. Solenopsin and solenopsin analogs were initially synthesized in an effort to inhibit production of solenopsin by a feedback mechanism in fire ants.
We tested solenopsin and solenopsin analogs in the SVR angiogenesis assay, which measures the ability of compounds to inhibit ras-transformed endothelial cells. Of the compounds tested, only the naturally occurring solenopsin A had activity against SVR cells.
The PI3K signaling pathway is known to play a critical role in angiogenesis; therefore, we investigated whether solenopsin affected this pathway. Interestingly, we found that solenopsin did not affect insulin-induced tyrosine phosphorylation of IRS1, but suppressed the activation of PI3K and hence the phosphorylation events that lie downstream of PI3K, such as the insulin-induced phosphorylation of Akt at Thr308 and Ser473 and the phosphorylation of FOXO1A, a physiologic substrate of Akt. However, we also found that solenopsin did not inhibit purified PI3K or PDK1 (the protein kinase which phosphorylates Akt at Thr308) in vitro. Taken together, our results suggest that solenopsin blocks the signaling pathway downstream of IRS1 but upstream of PI3K, perhaps by disrupting the interaction between IRS1 and the p85 regulatory subunit of PI3K or by altering the location of IGFR in lipid rafts.
Interestingly, we also found that solenopsin inhibits Akt in vitro, and that the inhibition was relatively selective, since only 1 other protein kinase (RSK1) of 28 other kinases tested was inhibited. However, the inhibition was competitive with respect to ATP, and the IC50 value determined at 0.1 mM ATP was 5 to 10 μM (Table 2). It is therefore unclear whether Akt would be inhibited significantly in cells at the concentrations used in this study (20-30 μM), since the intracellular concentration of ATP is in the millimolar range. Nevertheless, the relatively selective inhibition of Akt by solenospin in vitro is of interest because relatively few inhibitors of Akt have been developed, and Akt is a prominent pharmacologic target in cancer and inflammatory disorders.
Phospholipid ethers have been demonstrated to have activity against Akt, as well as potential alternative targets.15 Solenopsin shares the long alkyl side chains seen in phospholipid ethers and resembles miltefosine and perifosine by having a positively charged amine group and alkyl chain. Miltefosine has been shown to have antileishmanial activity in humans and to cause insulin resistance in muscle.37 Perifosine is in clinical trials as a PI3K/Akt inhibitor in advanced cancer.38,39
Solenopsin and its derivatives are amenable to large-scale synthesis, and because of its free secondary amine structure solenopsin can be readily conjugated to other molecules for targeted delivery. Recently, advances have been made in the identification of molecules that are specific for tumors and tumor endothelium. Conjugation of solenopsin to these molecules may provide a novel and specific therapy for advanced neoplasms, both in terms of treatment of patients directly and purging of autologous bone marrow for transplantation.40,41
Acknowledgments
We thank Yvona Ward and Ross Lake for their assistance with confocal images.
This work was supported by the ARA grant from Pfizer, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) RO1 AR47901 from the National Institutes of Health (NIH), and a Veterans Administration Merit Award (J.L.A.); the UK Medical Research Council and the Royal Society (P.C.); the Research Career Development Award from the Dermatology Foundation (B.G.); and a T32 NIH National Research Service Award (NRSA) Institutional Training Grant (B.N.P.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Patent application: U.S. Patent Application 20050038071 Kind Code A1 Bowen, J. Phillip et al, February 17, 2005.
References
- 1.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci U S A. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 3.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 4.Watton SJ, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- 5.Klafter R, Arbiser JL. Regulation of angiogenesis and tumorigenesis by signal transduction cascades: lessons from benign and malignant endothelial tumors. J Investig Dermatol Symp Proc. 2000;5:79–82. doi: 10.1046/j.1087-0024.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 6.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summers SA, Lipfert L, Birnbaum MJ. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3- kinase-dependent manner. Biochem Biophys Res Commun. 1998;246:76–81. doi: 10.1006/bbrc.1998.8575. [DOI] [PubMed] [Google Scholar]
- 8.Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 10.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 12.Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappa B and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- 13.Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 14.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 15.Gills JJ, Holbeck S, Hollinshead M, Hewitt SM, Kozikowski AP, Dennis PA. Spectrum of activity and molecular correlates of response to phosphatidylinositol ether lipid analogues, novel lipid-based inhibitors of Akt. Mol Cancer Ther. 2006;5:713–722. doi: 10.1158/1535-7163.MCT-05-0484. [DOI] [PubMed] [Google Scholar]
- 16.Arbiser JL, Moses MA, Fernandez CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbiser JL, Klauber N, Rohan R, et al. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 18.Bai X, Cerimele F, Ushio-Fukai M, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 19.Arbiser JL, Panigrathy D, Klauber N, et al. The antiangiogenic agents TNP-470 and 2-methoxyestradiol inhibit the growth of angiosarcoma in mice. J Am Acad Dermatol. 1999;40:925–929. doi: 10.1016/s0190-9622(99)70080-0. [DOI] [PubMed] [Google Scholar]
- 20.Moriyama Y, Doanhuyn D, Monneret C, Khuonghuu Q. Piperidine alkaloids 4: new synthesis of (+/−) solenopsin-A via heterocyclization-aminomercuration. Tetrahedron Lett. 1977;18:825–828. [Google Scholar]
- 21.Leclercq S, Thirionet I, Broeders F, Daloze D, Vandermeer R, Braekman JC. Absolute-configuration of the solenopsins, venom alkaloids of the fire ants. Tetrahedron. 1994;50:8465–8478. [Google Scholar]
- 22.Comins DL, Brooks CA, Ingalls CL. Reduction of N-acyl-2,3-dihydro-4-pyridones to N-acyl-4-piperidones using zinc/acetic acid. J Org Chem. 2001;66:2181–2182. doi: 10.1021/jo001609l. [DOI] [PubMed] [Google Scholar]
- 23.Beak P, Lee WK. Alpha-lithioamine synthetic equivalents from N-Boc piperidine. Abstr Pap Am Chem Soc. 1990;200:248. ORGN. [Google Scholar]
- 24.Wilkinson TJ, Stehle NW, Beak P. Enantioselective syntheses of 2-alkyl- and 2,6-dialkylpiperidine alkaloids: preparations of the hydrochlorides of (-)-coniine, (-)-solenopsin A, and (-)-dihydropinidine. Org Lett. 2000;2:155–158. doi: 10.1021/ol9912534. [DOI] [PubMed] [Google Scholar]
- 25.Kau TR, Schroeder F, Wojciechowski CL, et al. Phosphoinositol-3 kinase/akt signaling: a chemical genetic screen for inhibitors of FOXO1a nuclear export. Clin Can Res. 2003;9:6138S–6139S. [Google Scholar]
- 26.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier TI, Cook JA, Thomas JE, et al. Cloning, expression, purification, and characterization of the human Class Ia phosphoinositide 3-kinase isoforms. Protein Expr Purif. 2004;35:218–224. doi: 10.1016/j.pep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic-development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 29.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 30.Wang LP, Summers SA. Measuring insulin-stimulated phosphatidyl-inositol 3-kinase activity. Methods Mol Med. 2003;83:127–136. doi: 10.1385/1-59259-377-1:127. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Kovar J, Sissons S, et al. A cell-based immunocytochemical assay for monitoring kinase signaling pathways and drug efficacy. Anal Biochem. 2005;338:136–142. doi: 10.1016/j.ab.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Bain J, McLauchlan H, Elliot M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaMontagne KR, Jr, Moses MA, Wiederschain D, et al. Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. Am J Pathol. 2000;157:1937–1945. doi: 10.1016/s0002-9440(10)64832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 35.deShazo RD, Butcher BT, Banks WA. Reactions to the stings of the imported fire ant. N Engl J Med. 1990;323:462–466. doi: 10.1056/NEJM199008163230707. [DOI] [PubMed] [Google Scholar]
- 36.deShazo RD, Williams DF, Moak ES. Fire ant attacks on residents in health care facilities: a report of two cases. Ann Intern Med. 1999;131:424–429. doi: 10.7326/0003-4819-131-6-199909210-00005. [DOI] [PubMed] [Google Scholar]
- 37.Verma NK, Dey CS. The anti-leishmanial drug miltefosine causes insulin resistance in skeletal muscle cells in vitro. Diabetologia. 2006;49:1656–1660. doi: 10.1007/s00125-006-0260-1. [DOI] [PubMed] [Google Scholar]
- 38.Knowling M, Blackstein M, Tozer R, et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- 39.Ernst DS, Eisenhauer E, Wainman N, et al. Phase II study of perifosine in previously untreated patients with metastatic melanoma. Invest New Drugs. 2005;23:569–575. doi: 10.1007/s10637-005-1157-4. [DOI] [PubMed] [Google Scholar]
- 40.Burg MA, Pasqualini R, Arap W, Ruoslahti E, Stallcup WB. NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res. 1999;59:2869–2874. [PubMed] [Google Scholar]
- 41.Ran S, Gao BN, Duffy S, Watkins L, Rote N, Thorpe PE. Infarction of solid Hodgkin's tumors in mice by antibody-directed targeting of tissue factor to tumor vasculature. Cancer Res. 1998;58:4646–4653. [PubMed] [Google Scholar]