Kendomycin (1), also known as (−)-TAN 2162, is a novel ansamycin isolated from various Streptomyces species.1,2 It is a potent antagonist of the endothelin receptor and a calcitonin receptor agonist that functions as an anti-osteoporotic agent.1 In addition to these modulatory roles on cellular receptors, this bacterial metabolite also displays strong antibiotic activities against a wide range of bacteria, including MRSA strains.2 Furthermore, kendomycin possesses remarkable cytostatic effects on the growth of several human cancer cell lines with a potency higher than or comparable to that of some clinically used drugs.2 Structurally, kendomycin represents a unique ansa system in which a densely substituted tetrahydropyran ring is directly attached to the quinone methide chromophore within a macrocyclic scaffold. This fully carbogenic ansa framework is unprecedented among all of the ansamycins isolated so far.3 Thus, the novel molecular architecture, along with the impressive biological profile, renders kendomycin an important subject of bio-2 and chemical4,5 synthetic studies. Described herein is the first enantioselective total synthesis of kendomycin (1).
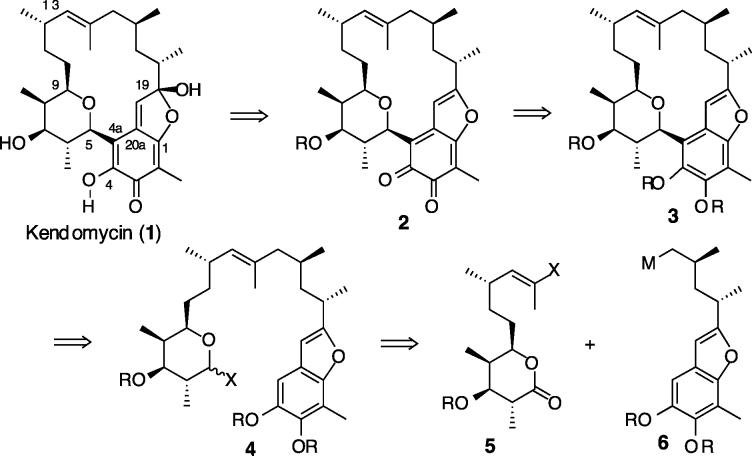
From a synthetic viewpoint, the C13–C14 bond offers an attractive handle for macrocyclization by a ring-closing olefin metathesis (RCM) reaction. However, the trisubstitution and (E)-geometry of this alkene cast uncertainties as to the efficacy of this approach. Moreover, Mulzer and co-workers have noted a hindered rotation about the C4a–C5 bond in their advanced synthetic intermediates,4 which might prove prohibitive for an RCM strategy. Our approach is based on the idea that the very C4a–C5 bond that causes atropisomerism might be formed by a ring closure, thereby avoiding complications arising from the conformational issues (Scheme 1). Hence, the challenge of constructing a macrocycle and the ansa bridge could be simultaneously reduced to a potentially more manageable problem of an aryl C-glycoside synthesis.6 Our synthetic plan projects a late stage fashioning of the ansa core by an oxidative hydration of benzofuran (3 → 2 → 1). The secomacrocycle 4 is envisioned to be assembled by the union of two subunits, 5 and 6, of roughly equal complexity, which in turn can be prepared from readily available building blocks.
Scheme 1.
Structure and Retrosynthetic Analysis of Kendomycin
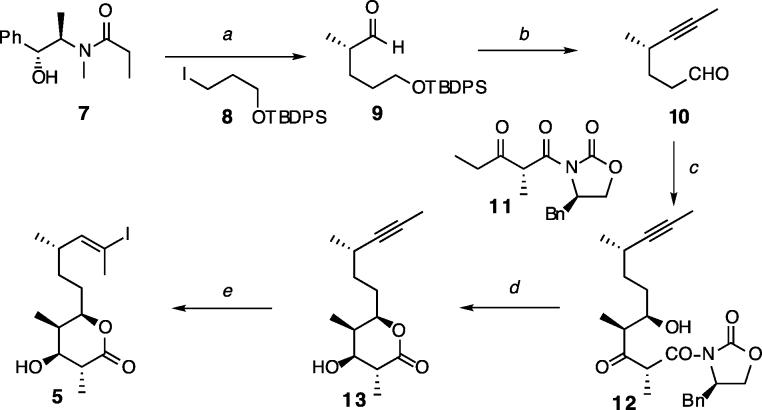
The synthesis of the tetrahydropyranyl subunit 5 employed chiral enolate chemistry to establish the key bonds and stereogenic centers (Scheme 2). The route commenced with the alkylation of Myers' amide 7 with iodide 8, which set the C12 stereocenter.7 After reductive detachment of the chiral auxiliary, the resulting aldehyde 9 was advanced to 10 by alkynylation8 of the aldehyde and oxidation9 of the desilylated alcohol. The THP ring, adorned with contiguous stereogenic centers, was then constructed through a sequence involving stereoselective aldolation, reduction, and lactonization.10 The sequential hydrostannylation–iodination of 13 was carried out with high regioselectivity to afford subunit 5.11
Scheme 2.
Synthesis of the Tetrahydropyran Domaina
a Reagents and conditions: (a) ref 7; (b) (i) CBr4, PPh3, Zn, DCM, 83%, (ii) n-BuLi, MeI, THF, 99%, (iii) TBAF, THF, 99%, (iv) Dess-Martin, DCM, 85%; (c) Sn(OTf)2 TEA, DCM, −78 °C, 82% (dr = 7:1); (d) (i) NaBH(OAc)3, AcOH, 5 °C, 84% (dr = 20:1), (ii) DBU, DCM, 90%; (e) (i) cat. Pd(OAc)2–PCy3, n-Bu3SnH, hexanes–THF, (ii) I2, DCM, 83% (dr = 7–10:1).
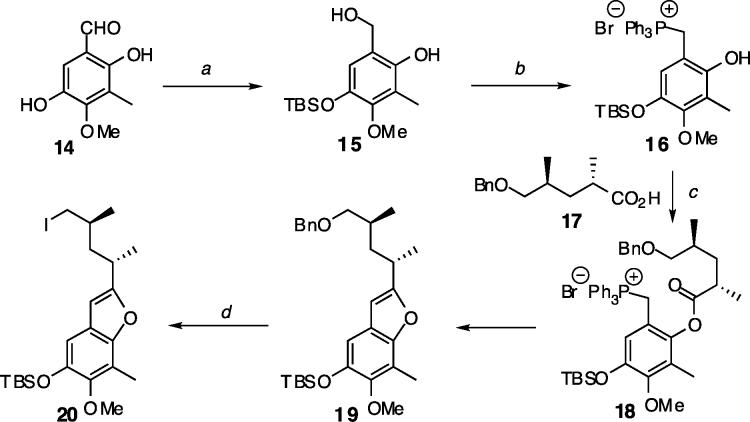
Scheme 3.
Synthesis of the Benzofuran Domaina
a Reagents and conditions: (a) (i) TBSCl, imidazole, DCM, (ii) Dibal-H, DCM, −78 °C, 95%; (b) Ph3P·HBr, CH3CN, 78%; (c) DCC/DMAP, DCM, then TEA, toluene, reflux, 93%; (d) (i) 10 mol % Pd/C, H2, EtOAc–CH3OH, 99%, (ii) I2/PPh3, imidazole, DCM, 96%.
Having defined a route to 5, we turned to the synthesis of the benzofuranyl domain using a two-stage condensation approach (Scheme 3).12 Thus, the phenolic phosphonium bromide 16 was prepared from the known aldehyde 1413 by selective silylation, reduction, and addition of HBr·PPh3.14 The sequential esterification and Wittig processes merged 16 and 1715 smoothly to give benzofuran 19 in high yield.12 Finally, removal of the benzyl group followed by iodination of the exposed hydroxyl group furnished the desired alkyl iodide 20.
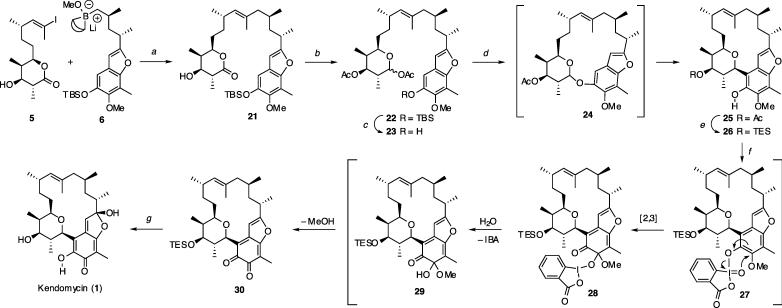
Scheme 4.
Suzuki–Miyaura Merger of the Key Fragments, Macroglycosidation, and Completion of the Total Synthesis of Kendomycin (1)a
a Reagents and conditions: (a) 4% PdCl2(dppf), 3 M aq K3PO4, Et2O–THF–DMF, 86%; (b) Dibal-H, toluene, then Ac2O, pyridine, 79%; (c) TBAF, THF, 91%; (d) SnCl4, 4 Å MS, CHCl 3, 40–70%; (e) (i) MeONa/MeOH, 87%, (ii) TESOTf, Et3N, DCM, 98%; (f) IBX, DMF, 62% (g) aq HF, CH3CN, 50%.
With the procurement of the two fragments, the feasibility of their union through C14–C15 bond formation was probed (Scheme 4). Under the Pd-catalyzed conditions,16 boranate 6, which was prepared from 20 by lithiation and transmetalation (t-BuLi, ether, then B-OMe-9-BBN), participated well in the cross-coupling reaction with iodide 5 to provide alkene 21 in excellent yield. To this intermediate containing all of the carbon atoms of kendomycin was imparted a glycosyl donor function by converting it into anomeric acetate 22, thus setting the stage for the macro aryl C-glycosidation. Our initial attempts to cyclize 22 under a variety of Friedel–Crafts conditions were unsuccessful, mainly leading to hydrolysis of the anomeric acetate.17 In contrast, the reaction employing phenol 23 as the substrate occurred smoothly to afford the desired macrocycle 25 as a single stereoisomer in 40–70% yield. As a nonpolar product was produced rapidly at −5 °C, which turned into 25 at room temperature over 12 h, this reaction appeared to proceed through facile formation of O-glycoside 24 and subsequent rearrangement to C-glycoside 25.18 After exchange of the C7 protecting groups, oxidative fashioning of the aryl core was achieved by careful treatment of 26 with IBX, which formed the unstable diketone 30.19 Monitoring this reaction by 1H NMR revealed the intermediacy of mixed ketal 28, which underwent slow hydrolysis to 29 and eventually to 30. The removal of the TES group and hydration of the ortho-quinone by the action of aqueous HF finally produced kendomycin (1). Synthetic kendomycin exhibited physical and spectroscopic characteristics (Rf, mp, [α]D, IR, 1H, 13C NMR, and HRMS) identical to those reported for the natural product.
In summary, the first total synthesis of kendomycin has been achieved. Notable features of the synthesis are high levels of convergence and stereocontrol in the assembly of key subunits, efficient establishment of the structural core by a macroglycosidation, and a novel oxidative hydration strategy to construct the ansa chromophore.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge Princeton University for startup funding. Y.Y. thanks FMC corporation for a graduate fellowship.
Footnotes
Supporting Information Available: Experimental details and spectral data for all new compounds. This material is available free of change via Internet at http://pubs.acs.org.
References
- 1.(a) Funahashi Y, Kawamura N, Ishimaru T. JP Patent 08231551 [A296090] 1996; (b) Su MH, Hosken MI, Hotovec BJ, Johnston TL. US Patent 5728727 [A980317] 1998
- 2.(a) Bode HB, Zeeck A. J. Chem. Soc., Perkin Trans. 2000;1:323. [Google Scholar]; (b) Bode HB, Zeeck A. J. Chem. Soc., Perkin Trans. 2000;1:2665. [Google Scholar]
- 3.Funayama S, Cordell GA. In: Studies in Natural Products Chemistry. Atta-ur-Rahman, editor. Vol. 23. Elsevier; New York: 2000. pp. 51–106. [Google Scholar]
- 4.(a) Martin HJ, Drescher M, Kaählig H, Schneider S, Mulzer J. Angew. Chem., Int. Ed. 2001;40:3186. doi: 10.1002/1521-3773(20010903)40:17<3186::AID-ANIE3186>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]; (b) Marques MMB, Pichlmair S, Martin HJ, Mulzer J. Synthesis. 2002:2766. [Google Scholar]; (c) Pichlmair S, Marques MMB, Green MP, Martin HJ, Mulzer J. Org. Lett. 2003;5:4657. doi: 10.1021/ol035846x. [DOI] [PubMed] [Google Scholar]; (d) Mulzer J, Pichlmair S, Green MP, Marques MMB, Martin HJ. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11980. doi: 10.1073/pnas.0401503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengoku T, Arimoto H, Uemura D. Chem. Commun. 2004:1220. doi: 10.1039/b402391a. [DOI] [PubMed] [Google Scholar]
- 6.(a) Jaramillo C, Knapp S. Synthesis. 1994:1. For reviews, see: [Google Scholar]; (b) Postema MHD. C-Glycoside Synthesis. CRC; Boca Raton, FL: 1995. [Google Scholar]
- 7.(a) Myers AG, Yang BH, Chen H, Gleason JL. J. Am. Chem. Soc. 1994;116:9361. [Google Scholar]; (b) Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J. Am. Chem. Soc. 1997;119:6496. [Google Scholar]
- 8.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;13:3769. [Google Scholar]
- 9.Dess DB, Martin JC. J. Org. Chem. 1983;48:4155. [Google Scholar]
- 10.(a) Evans DA, Clark JS, Metternich R, Novack VJ, Sheppard GS. J. Am. Chem. Soc. 1990;112:866. [Google Scholar]; (b) Evans DA, Cee VJ, Smith TE, Fitch DM, Cho PS. Angew. Chem., Int. Ed. 2000;39:2533. doi: 10.1002/1521-3773(20000717)39:14<2533::aid-anie2533>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (c) Evans DA, Fitch DM, Smith TE, Cee VJ. J. Am. Chem. Soc. 2000;122:10033. doi: 10.1002/1521-3773(20000717)39:14<2533::aid-anie2533>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (d) Ref 4b,d.
- 11.Semmelhack MF, Hooley RJ. Tetrahedron Lett. 2003;44:5737. [Google Scholar]
- 12.Hercouet A, Lecorre M. Tetrahedron Lett. 1979;20:2145. [Google Scholar]
- 13.Siddiqi SA, Heckrodt TJ. Z. Naturforsch. B. 2003;58:328. [Google Scholar]
- 14.Hercouet A, Lecorre M. Tetrahedron. 1981;37:2867. [Google Scholar]
- 15.Vong BG, Abraham S, Xiang AX, Theodorakis EA. Org. Lett. 2003;5:1617. doi: 10.1021/ol034243i. See Supporting Information for the synthesis acid 17. [DOI] [PubMed] [Google Scholar]
- 16.(a) Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]; (b) Marshall JA, Johns BA. J. Org. Chem. 1998;63:7885. doi: 10.1021/jo971900+. [DOI] [PubMed] [Google Scholar]
- 17.(a) Chen M, Guzei I, Rheingold AL, Gibson HW. Macromolecules. 1997;30:2516. For a rare example of macrocyclization via a Friedel–Crafts acylation reaction, see: [Google Scholar]; (b) Stoddart JF, Nepogodiev SA, Gattuso G. Chem. Rev. 1998;98:1919. doi: 10.1021/cr960133w. For a macro-O-glycosidation to prepare cyclodextrins, see: [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Katsuki M, Suzuki K. Tetrahedron Lett. 1988;29:6935. [Google Scholar]
- 19.(a) Magdziak D, Rodriguez AA, Van De Water RW, Pettus TRR. Org. Lett. 2002;4:285. doi: 10.1021/ol017068j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ozanne A, Pouységu L, Depernet D, François B, Quideau S. Org. Lett. 2003;5:2903. doi: 10.1021/ol0349965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.