Abstract
Multiply-transfused individuals are at higher risk for BM rejection. We show that whereas allosensitization resulted in the priming of both cellular and humoral immunity, preformed antibody was the major barrier to engraftment. The generation of cross-reactive alloantibody led to rejection of BM of a different MHC-disparate strain. Imaging studies indicated that antibody-mediated rejection was very rapid (< 3 hours) in primed recipients, while T-cell–mediated rejection in nonprimed mice took more than 6 days. Antibody-mediated BM rejection was not due to a defect in BM homing as rejection occurred despite direct intra-BM infusion of donor BM. Rejection was dependent upon host FcR+ cells. BM cells incubated with serum from primed mice were eliminated in nonprimed recipients, indicating that persistent exposure to high-titer antibody was not essential for rejection. High donor engraftment was achieved in a proportion of primed mice by mega-BM cell dose, in vivo T-cell depletion, and high-dose immunoglobulin infusion. The addition of splenectomy to this protocol only modestly added to the efficacy of this combination strategy. These data demonstrate both rapid alloantibody-mediated elimination of BM by host FcR+ cells and priming of host antidonor T cells and suggest a practical strategy to overcome engraftment barriers in primed individuals.
Introduction
Allosensitization can be a formidable barrier to bone marrow (BM) engraftment due to priming of the host's cellular and humoral immune responses.1–7 Despite the well-recognized hazard of antibody-mediated rejection in solid organ transplantation,8–15 donor BM rejection is generally attributed to cytolytic host antidonor T and natural killer (NK) cells that survive the conditioning regimen.16–23 However, antibody-mediated BM failure after allogeneic bone marrow transplantation (BMT) can occur either by antibody-dependent cell-mediated cytotoxicity or complement-mediated cytotoxicity.2,4–6 Preformed antibody present at the time of BM infusion is unaffected by standard transplantation conditioning regimens or T- or B-cell immunosuppressive or modulatory strategies given in the peritransplantation period. Although plasmapheresis, high-dose intravenous immunoglobulin, splenectomy, and immunoadsorption are commonly used in solid organ transplantation for the abrogation of alloantibody-mediated rejection,8–11,13,14,24–27 these strategies generally are not part of the BMT conditioning regimen.
We show that although allosensitization resulted in the priming of both the cellular and humoral arms of the immune response, preformed antibody was the major barrier to engraftment. A single priming event led to high, long-lived antibody levels that practically precluded waiting for the waning of antibody titer for successful BMT. Imaging studies indicated that antibody-mediated rejection of a moderate BM dose was nearly complete by 3 hours. Antibody-mediated rejection of donor BM in primed mice was dependent on a host FcR+ mechanism. Of significant clinical relevance, priming against one strain could result in the rejection of donor BM of a different strain. Furthermore, incubation of donor BM with serum from primed mice resulted in the destruction of the alloantibody-coated BM cells when transferred into a nonprimed recipient, suggesting that persistent exposure to endogenous high antibody titer was not requisite for antibody-mediated BM rejection. Despite the formidable barrier to engraftment, high levels of donor chimerism could be achieved in a proportion of primed, irradiated mice by a combination of high BM cell dose, in vivo T-cell depletion (TCD), and high-dose murine immunoglobulin (mIg).
Materials and methods
Mice
B10.BR (H2k), C57BL/6 (H2b) (termed B6), and B6 muMT mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6 muMT (formerly designated B6.129S2-Igh-6tm1cgn) (H2b) mice are B-cell deficient due to an immunoglobulin heavy chain defect. BALB/c (H2d) mice were purchased from the National Institutes of Health (Bethesda, MD). B6.129P2-Fcer1gtm1RavN12 (H2b) (termed B6.Fcer1g−/−) mice, deficient in the gamma chain subunit of the FcgRI, FcgRIII, FcgRIV, and FceRI receptors were purchased from Taconic (Hudson, NY). B6 green fluorescent protein (GFP) transgenic (Tg) mice were obtained from the laboratory of Dr Jonathan Serody and bred at the University of Minnesota. Mice were housed in a specific pathogen-free facility in microisolator cages and were used at 6 to 12 weeks of age.
Serum antibody detection
A flow cytometric assay published by Valujskikh et al28 was used for the detection of serum antibody in primed mice. Mice were primed by the intraperitoneal administration of 20 × 106 antigen-disparate splenocytes at various intervals from 7 days to 6 months prior to sera collection. Serum (100 μL; diluted at 1:10, 1:40, 1:160) was incubated for 1 hour with thymocytes (or BM cells) (106) of the priming strain (and in some cases, third-party strain). Cells were washed and incubated with FITC-conjugated goat anti–mouse Ig antibody (Pharmingen, San Diego, CA) for 1 hour, washed, and analyzed by flow cytometry. Controls for determination of serum antibody levels included thymocytes incubated with serum from nonprimed naive mice and thymocytes incubated with no serum.
Bone marrow transplantation (BMT)
For survival studies not involving imaging, B6, B6 muMT, or B6 Fcer1g−/− mice were irradiated with 6.0 Gy (a dose that permits a greater contribution by host T cells and allows for autologous recovery in the event of BM rejection) or 8.0 Gy (a dose that minimizes T-cell–mediated rejection; mice must engraft to avoid lethal aplasia) TBI by x-ray on day −1. BALB/c (or B10.BR) BM cells (range, 20-200 × 106 cells) were infused on day 0. Cohorts of mice were primed by the intraperitoneal administration of 20 × 106 BALB/c or B10.BR splenocytes at various times prior to transplantation. Where indicated, anti-CD8 (clone 2.43), anti-CD4 (clone GK1.5), and anti-NK1.1 (clone PK136) mAbs (400 μg each) were administered intraperitoneally on days −2, 1, 4, and 7 to deplete T and NK cells around the time of transplantation. One dose of mAb depleted more than 95% of its target cell (P.A.T., unpublished data, September 2000). Survival was monitored daily. Packed red blood cell volumes (PCVs) were monitored weekly in some experiments as a measure of BM aplasia. Donor chimerism of long-term survivors was documented by the phenotyping of peripheral blood leukocytes (PBLs) at 4 to 6 weeks and 3 months after transplantation. PBLs were stained with fluorochrome-conjugated antibodies (anti-CD8, -CD4, –MAC-1, -CD19, -H2b, -H2k, and -H2d, and isotype controls; Pharmingen) and analyzed using CellQuest software on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
For experiments designed to image the fate of B6 GFP+ BM under conditions of nonprimed rejection, primed rejection, or engraftment (Figure 4), BALB/c mice were irradiated on day −1 with 3.5 Gy by x-ray and infused with 20 × 106 T-cell–depleted B6 GFP+ BM cells on day 0 (conditions designed to ensure graft rejection in nonprimed mice). To ensure engraftment in a cohort of mice, anti-CD8, anti-CD4, and anti-NK1.1 (400 μg each) was administered on days −2, 1, 4, and 7. The primed cohort received 20 × 106 B6 splenocytes intraperitoneally on day −28. PBL phenotyping in survival experiments verified that untreated control and primed recipients uniformly rejected donor bone marrow. In contrast, all nonprimed mice receiving T- and NK-depleting antibodies engrafted with high levels of donor chimerism.
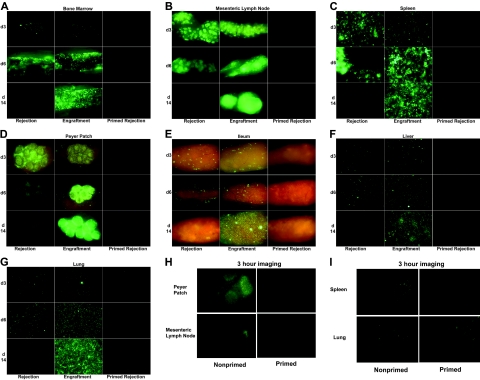
Figure 4.
Antibody-mediated rejection of donor BM in primed mice is far more rapid than T-cell–mediated rejection in naive mice. Shown are images of BALB/c mice irradiated with 3.5 Gy on day −1 and infused with 20 × 106 B6 GFP+ T-cell–depleted BM cells on day 0. (A-G) Left panels (rejection) indicate untreated mice that ultimately reject their grafts in a T-cell–dependent fashion. Middle panels (engraftment) indicate mice that received anti-CD4 and anti-CD8 mAbs in vivo around the time of transplantation to ensure long-term donor engraftment. Right panels (primed rejection) indicate mice primed with B6 splenocytes on day −28 that reject their donor BM grafts via preformed alloantibody. (H-I) Nonprimed and primed mice as indicated were imaged 3 hours after BMT. Representative images from 1 of 3 mice are shown. See “Materials and methods” for imaging details.
In vivo imaging
Images were taken with a Retiga Exi color camera and QCapture software (Qimaging, Burnaby, BC) mounted onto a Leica MZFLIII stereomicroscope using a GFP2 or a GFP/dsRED-bandpass filter and a 1.0× transfer lens (Leica Microsystems, Bannockburn, IL). Zoom factors from 3.5× to 10× were used for imaging on days 1 to 14 (3.5× for ileum and Peyer patch; 7.0× for femoral bone marrow cavity, liver, spleen, and kidney; 10.0× for lung) and 7× to 10× for imaging at 3 hours. Exposure times were optimized for each organ and identical times and settings were used for all mice imaged on any given day. To obtain optimal images, mice were killed and dissected for imaging but no tissue processing is required. Three mice per group were examined at each time point and results were reproduced in a second experiment (Figure 4A-G only). One experiment imaging mice at 3 hours was done (n = 3/group). Mice within a group yielded very similar results at each time point so a representative image is illustrated.
Incubation of donor BM with serum from naive or primed mice prior to BMT
BALB/c or B10.BR T-cell–depleted BM cells (20 × 106/mL) were incubated with a 1:20 dilution of serum obtained from either naive B6 mice or BALB/c-primed B6 mice for 1 hour on ice. (B6 serum donors were primed by intraperitoneal administration of 20 × 106 BALB/c splenocytes 28 days prior to serum collection.) After incubation, BM was washed 3 times prior to infusion of 20 × 106 donor BM into lethally irradiated (8.0 Gy—a dose chosen to minimize contribution of T-cell–mediated rejection), nonprimed B6 mice. The only alloantibody present is that which bound to the donor BM cells during the brief in vitro incubation prior to infusion.
Splenectomy and BMT
B6 recipients were primed on day −56 with 20 × 106 BALB/c splenocytes intraperitoneally. Splenectomy (splx) was performed on day −28 under pentobarbital anesthesia. An incision was made over the spleen, vessels were ligated at both ends, and the spleen was removed. The peritoneum was closed with nonabsorbable sutures and the skin closed with small staples. High-dose mouse Ig (20 mg/mouse; Rockland, Gilbertsville, PA) was administered intraperitoneally on day −15 and day −7 where indicated. Some mice were depleted of T cells by the administration of anti-CD4 and anti-CD8 mAbs on days −2, 0, 2, 4, and 7. Mice were irradiated with 6.0 Gy (a dose that permits T-cell–mediated rejection and allows for autologous recovery in the event of donor BM rejection) by x-ray on day −1 and infused with high-dose BM (100 × 106 cells) on day 0. To determine effect of splx and mIg on antibody levels, serum was taken just prior to splx (day −29) and again just prior to BMT (day −2).
Statistics
Survival data were analyzed by life-table methods and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. To assess engraftment data, group comparisons of percentage donor chimerism were analyzed by Student t test. Group comparisons of engraftment and survival rates were analyzed by chi-squared test. P values below .05 were considered significant in all tests.
Results
Alloantigen priming is a potent barrier to engraftment
Initial studies focused on the kinetics of priming. Cohorts of B6 mice were primed against BALB/c alloantigen, either 7, 14, or 28 days prior to transplantation by the intraperitoneal administration of BALB/c splenocytes. Mice were lethally irradiated and infused with BALB/c BM. All nonprimed mice survived and engrafted with an average of more than 95% donor chimerism (Figure 1A). In contrast, all primed mice, regardless of whether they were primed 7, 14, or 28 days prior, died of BM aplasia by 2 weeks after transplantation, similar to irradiated controls that did not undergo transplantation (Figure 1A, irradiation controls not shown). Additionally, rigorous host depletion of CD8+, CD4+, and NK+ cells failed to rescue primed mice from lethal BM aplasia, indicating that neither primed T cells nor NK cells surviving lethal irradiation were responsible for BM rejection. Increasing BM cell dose to 160 × 106 also failed to rescue primed mice from death (data not shown).
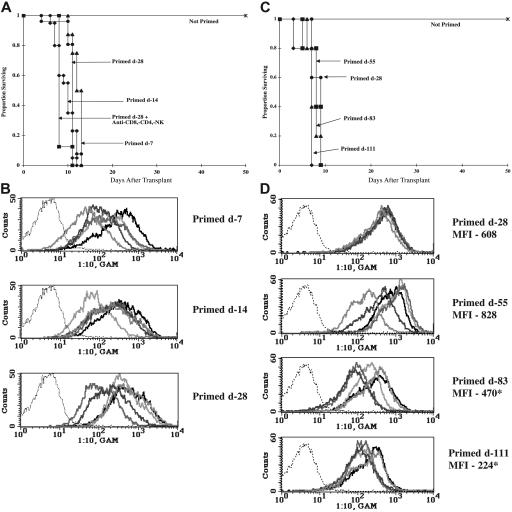
Figure 1.
Allosensitization results in antibody-mediated donor BM rejection. (A,C) B6 mice were lethally irradiated on day −1 (8.0 Gy) and infused with 20 × 106 (A) or 40 × 106 (C) BALB/c BM on day 0. Mice were primed at various times prior to BMT by the intraperitoneal administration of 20 × 106 BALB/c splenocytes. One group received anti-CD4, anti-CD8, and anti-NK mAbs around the time of transplantation (A). Survival is shown. (A) n = 16 to 26/group; data from 2 to 3 separate experiments with similar results were pooled. (C) n = 5/group. (B,D) Serum was collected from BALB/c-primed B6 mice after the indicated interval, diluted, and incubated with BALB/c thymocytes. Cells were washed and incubated with FITC-conjugated goat anti–mouse Ig Ab and analyzed by flow cytometry. Overlay histograms indicate alloantibody in diluted serum from 5 different mice binding to BALB/c thymocytes. Negative control of FITC-conjugate binding to thymocytes in absence of serum is indicated by the thin dotted line. Average mean fluorescent intensity (MFI) is listed for panel D. (D) *P < .05 versus primed day −28; n = 5. Each bold line represents a different mouse.
To detect the presence of anti-BALB/c antibody in primed B6 mice, an assay published by Valujskikh et al28 was used. Serum was collected 7, 14, or 28 days after priming, diluted, and incubated with BALB/c thymocytes (thymocytes have few Fc receptors thereby minimizing nonspecific binding). A second incubation with FITC-conjugated goat antimouse permitted the flow cytometric evaluation of serum antibody. As early as 7 days after priming, high levels of antibody were present in the serum of primed mice. A 1:10 serum dilution resulted in more than 95% positive binding in 4 of 5 mice at day 7, and all 5 mice at days 14 and 28 (Figure 1B). Even at a 1:160 dilution, serum from 11 of 15 primed mice bound more than 50% of BALB/c thymocytes (data not shown).
To determine how long one single priming event would preclude survival and engraftment, B6 mice were primed against BALB/c alloantigen and, after an interval of 28, 55, 83, or 111 days, were lethally irradiated and infused with BALB/c BM (Figure 1C). All primed mice, whether primed 28 or 111 days before BMT, died by 10 days after transplantation. Serum (1:10 dilution) collected just prior to BMT bound 97% to 99% of BALB/c thymocytes, indicating high levels of antidonor antibody in all mice (Figure 1D). The average mean fluorescent intensity (MFI) of serum binding to thymocytes generally declined over time, suggesting a gradual reduction in anti-BALB/c antibody levels albeit of insufficient magnitude to impact survival. At dilutions of 1:40, serum binding was high in all primed mice (97%, 96%, 81%, and 82% positive whether primed on days −28, −55, −83, or −111, respectively [data not shown]). Dilutions of 1:160 resulted in 80%, 84%, 42%, and 36% positive binding for mice primed on days −28, −55, −83, and −111, respectively (data not shown). In a subsequent experiment, sera collected 6 months after priming showed an average of 47% positive binding at a 1:160 dilution (n = 5, range of 20%-75% positive). These experiments indicated that a single priming event resulted in an early and long-lasting antibody response that would practically preclude waiting for waning antibody titer for successful transplantation.
Priming against one allogeneic strain can lead to the destruction of a third-party BM graft
To determine the degree of priming specificity, B6 mice were primed against either BALB/c or B10.BR alloantigen on day −28, lethally irradiated on day −1, and infused with either BALB/c or B10.BR T-cell–depleted BM on day 0. Antibody-mediated BM rejection was not entirely alloantigen specific as B6 mice primed against BALB/c also rejected B10.BR BM, albeit more slowly than BALB/c BM (Figure 2A). However, B6 mice primed against B10.BR rejected B10.BR BM but not BALB/c BM. All long-term survivors were more than 90% donor by PBL phenotyping at 3 months.
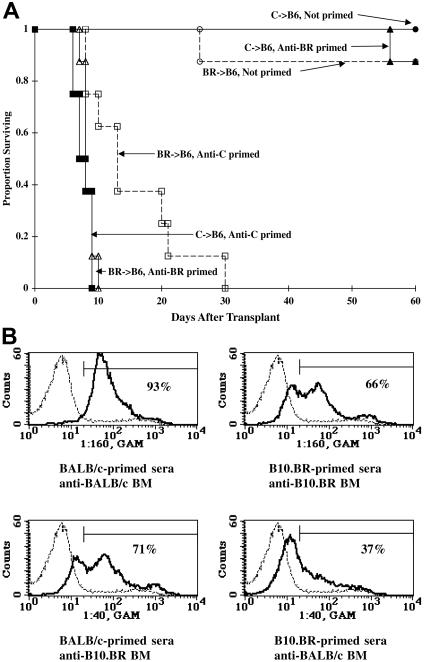
Figure 2.
Priming to one alloantigen can result in the elimination of third-party BM indicating nonspecificity in the priming response. (A) B6 mice were primed against BALB/c or B10.BR (BR) by the intraperitoneal administration of 20 × 106 splenocytes, lethally irradiated on day −1 (8.0 Gy), and infused with BALB/c or BR BM (20 × 106) on day 0. Survival is shown. n = 8/group. (B) Serum was pooled from BALB/c- or B10.BR-primed B6 mice (n = 5) and incubated with BALB/c or B10.BR BM. Histograms illustrate binding of serum antibody to BM of priming strain (top panels) and third-party strain (bottom panels). Percentage positive binding is given. Negative control is shown by thin dashed line.
Examination of serum from primed mice indicated that BALB/c priming resulted in a more vigorous and broadly cross-reactive antibody response than did B10.BR priming, perhaps due to the multiple minor antigen differences as well as MHC disparities that exist between BALB/c and B6 mice but not between B10.BR and B6 mice. Serum from BALB/c-primed mice bound 93% of BALB/c BM, whereas serum from B10.BR-primed mice bound 66% of B10.BR BM, suggesting less robust priming (Figure 2B top panels). With regard to third-party BM, serum from BALB/c-primed mice bound 71% of B10.BR BM, whereas serum from B10.BR-primed mice bound only 37% of BALB/c BM (Figure 2B bottom panels).
Preformed antibody, not T cells, is the major barrier to engraftment in primed mice
To further study the role of humoral immunity in primed rejection, engraftment was compared in primed B6 WT versus B6 muMT (Ig heavy chain deleted, B-cell deficient) mice that are unable to make antibody. Cohorts of B6 WT or B6 muMT mice were primed with BALB/c splenocytes 28 days prior to 6.0 Gy TBI and infusion with BALB/c BM, and survival and chimerism were monitored (Table 1). All nonprimed B6 WT and muMT control mice receiving 40 to 200 × 106 BM cells survived and engrafted (all > 95% donor by PBL phenotyping). Primed B6 WT mice receiving 40 × 106 BM cells died of BM aplasia. Although increasing the BM dose to 200 × 106 cells rescued 3 of 4 B6 WT mice from lethal aplasia, none of the 3 surviving mice had any evidence of donor chimerism. In contrast to the WT mice, all but one of the primed B-cell–deficient muMT mice survived, and all receiving a BM dose of at least 100 × 106 cells had donor chimerism levels more than 95% (Table 1). At a more modest BM cell dose (20 × 106), a role for T-cell–mediated primed rejection was uncovered in muMT mice. Whereas all 8 unprimed muMT mice receiving 20 × 106 BM cells engrafted, only 2 of 11 primed muMT mice had evidence of donor chimerism (Table 1). High BM cell dose abrogated primed rejection in muMT mice but not in WT mice, indicating that antibody, rather than primed T cells, was the major barrier to engraftment in primed WT mice.
Table 1.
In contrast with B6 WT, primed B-cell-deficient recipients engraft if given high donor BM cell doses
| Recipient and no. BM cells | Survival | No. engrafted | Mean % donor |
|---|---|---|---|
| B6 WT, 40 × 106 | |||
| Unprimed | 10/10 | 10/10 | 98 ± 2 |
| Primed | 0/10* | NA | NA |
| B6 WT, 200 × 106 | |||
| Unprimed | 4/4 | 4/4 | 100 ± 0 |
| Primed | 3/4 | 0/3* | 0 ± 0* |
| B6 muMT, 200 × 106 | |||
| Unprimed | 4/4 | 4/4 | 99 ± 1 |
| Primed | 4/4 | 4/4 | 100 ± 2 |
| B6 muMT, 100 × 106 | |||
| Unprimed | 4/4 | 4/4 | 98 ± 3 |
| Primed | 4/4 | 4/4 | 100 ± 0 |
| B6 muMT, 20 × 106 | |||
| Unprimed | 8/8 | 8/8 | 83 ± 11 |
| Primed | 11/12 | 2/11* | 16 ± 11* |
B6 WT or B6 muMT mice were primed on day −28 with 20 × 106 BALB/c splenocytes intraperitoneally, irradiated on day −1 (6.0 Gy), and given non-T-cell-depleted BALB/c BM cells at the indicated number on day 0. Survival was monitored. PBLs were phenotyped 6 weeks after transplantation for donor chimerism. All engrafting mice were high-level donor chimeras (> 90%). Shown is percentage of mean group donor chimerism ± 1 SEM.
NA indicates not available.
P < .01 versus nonprimed control at same BM cell dose.
If preformed antibody, per se, rather than primed B or T cells, was the major barrier to engraftment in the primed recipient, then a brief ex vivo incubation of donor BM with sera from mice primed against the donor alloantigen would effect elimination of the donor BM in a nonprimed recipient. To test this, BALB/c (or specificity control B10.BR) BM was incubated with diluted serum obtained from either naive B6 mice or BALB/c-primed B6 mice. After incubation, BM was washed thoroughly to remove free serum and unbound antibody and infused into lethally irradiated naive (ie, nonprimed) B6 mice.
Prior to infusion, an aliquot of BM was removed and incubated with FITC-conjugated goat anti–mouse Ig to determine the level of serum antibody that had bound to the donor BM. Incubation of BALB/c or B10.BR BM with serum obtained from unprimed, naive mice resulted in low-level binding likely due to the high percentage of FcR+ cells in BM (Figure 3A). As expected, incubation of BALB/c BM with serum obtained from BALB/c-primed B6 mice resulted in binding of more than 99% of BM cells. BALB/c-primed serum also bound B10.BR BM at low level.
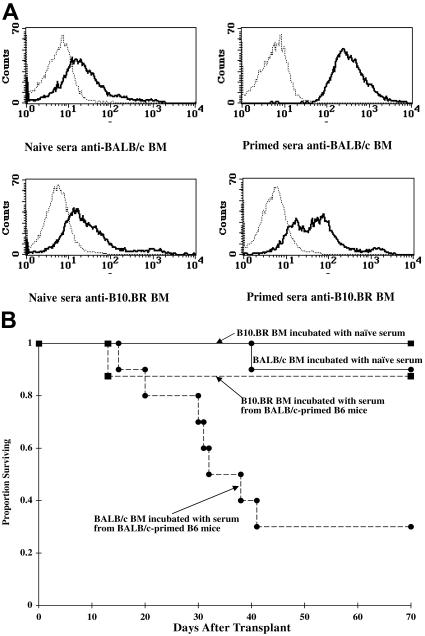
Figure 3.
The ex vivo incubation of donor BALB/c BM with serum from BALB/c-primed B6 mice results in the destruction of the antibody-coated donor BM cells in a nonprimed B6 recipient. (A) Serum was collected from naive or BALB/c-primed mice, diluted 1:20, and incubated with BALB/c or BR BM. BM was thoroughly washed. An aliquot was incubated with FITC-conjugated goat anti–mouse Ig Ab and analyzed by flow cytometry. Histograms indicate binding of serum alloantibody to BALB/c or BR BM. Negative control of FITC-conjugate binding to BM in absence of serum is indicated by the thin dotted line. (B) Nonprimed B6 mice were lethally irradiated (8.0 Gy) and infused with BALB/c or BR BM cells (10 × 106) that had been incubated with serum from naive or BALB/c-primed B6 mice as shown in panel A. Survival is shown. n = 8 to 10/group; BALB/c BM incubated with naive versus BALB/c-primed serum, P = .003. Results were reproduced in a second experiment.
The infusion of BALB/c BM that was incubated with serum from BALB/c-primed B6 mice resulted in lethal aplasia in 70% of recipients (Figure 3B, P = .003 vs incubation with serum from naive mice). Although the time to death was significantly slower than in primed recipients (compare Figure 3B with 1A,C), donor BM in primed recipients was continually exposed to high circulating levels of antibody, whereas the only antibody in this experiment was that which bound to the donor BM during the brief serum incubation prior to infusion. As further evidence that antibody-coated BALB/c BM was being eliminated, day 14 PCVs were much lower in recipients of primed serum-incubated BALB/c BM than in recipients of naive serum-incubated BM (average, 16.6% ± 3.4% vs 45.5% ± 2.4%, respectively; P < .001). Recipients of B10.BR BM had high survival and high day-14 PCVs regardless of whether BM was incubated with serum from naive mice or BALB/c-primed B6 mice, indicative of specificity under these experimental conditions of limited ex vivo exposure to serum antibody.
Elimination of donor BM by preformed antibody in the primed recipient is far more rapid than T-cell–mediated rejection in the nonprimed recipient
The kinetics of BM rejection in primed versus nonprimed mice is unknown. Imaging studies were performed to compare the kinetics of T-cell–mediated BM rejection in nonprimed mice to antibody-mediated BM rejection in primed mice. To image the fate of infused BM, the strain combination was switched to use B6 GFP donor BM and BALB/c recipient mice. Of 2 groups of BALB/c mice that were primed against B6 on day −28, one group received anti-CD4 and anti-CD8 mAbs and one did not. A nonprimed cohort received in vivo anti-CD4– and anti-CD8–depleting mAbs around the time of transplantation to ensure BM engraftment in one group of mice. BALB/c mice were sublethally irradiated (3.5 Gy TBI) to ensure that untreated, nonprimed mice would ultimately reject their BM graft. A moderate donor BM dose of 20 × 106 was chosen for imaging studies. Separate long-term chimerism studies substantiated the imaging model by verifying rejection in all primed and untreated nonprimed mice and high engraftment levels in all nonprimed mice receiving anti-CD4 and anti-CD8 mAbs (data not shown).
Initial imaging studies focused on days 1 (approximately 18 hours), 2, 3, 6, and 14 (Figure 4A-G and data not shown). Strikingly, antibody-mediated BM rejection in the primed mice was complete by 18 hours. Furthermore, primed mice, depleted of host T cells by the administration of anti-CD4 and anti-CD8 mAbs, also eliminated donor BM by 18 hours, indicating that acute BM rejection in primed mice was T-cell independent (data not shown). In contrast, T-cell–mediated rejection by nonprimed mice took more than 6 days. Untreated, nonprimed mice that would ultimately reject their BM graft showed equivalent numbers of GFP+ cells in most organs as engrafting mice on days 1, 2, and 3 (Figure 4 and data not shown). In fact, untreated mice destined to reject their BM graft had increased numbers of GFP+ cells in the spleen and Peyer patches on day 3 compared with mice that would ultimately accept their BM graft. By day 6, GFP+ BM was reduced in mesenteric LNs, spleen (see patchy distribution of BM loss in Figure 4C), Peyer patches, and gut-associated lymphoid tissue in the ileum in nonprimed graft-rejecting mice, consistent with BM rejection by host T cells that were likely primed in the lymphoid organs. In contrast to the reduction of donor BM in lymphoid organs by day 6, GFP BM was increased in femoral BM cavity, liver, and lung of rejecting, nonprimed mice. However, by day 14, donor BM was absent in all organs of rejecting, nonprimed mice. These data indicated that T-cell rejection occurred first in lymphoid organs and, only later, in BM and parenchymal organs. That this loss of donor graft was T-cell mediated was evident by the increasing GFP+ cell number in all organs in nonprimed mice that received anti-CD4 and anti-CD8 mAbs to ensure long-term stable engraftment.
Because antibody-mediated rejection of donor BM was complete by 18 hours, mice were imaged 3 hours after BMT. At this time, donor BM cells could be found in the Peyer patches, mesenteric LN, spleen, and lung of all 3 nonprimed mice (Figure 4H-I). In addition, 1 of 3 nonprimed mice had very few GFP+ cells in the inguinal LN and femoral BM cavity (data not shown). In contrast, donor BM was found in only the lung of 1 of 3 primed mice and in no other organs, suggesting hyperacute BM elimination prior to initial organ homing events.
We hypothesized that if donor BM cells could be delivered directly to the bone marrow cavity they might bypass early destruction by the RES system or a BM homing defect. To address this, BALB/c mice were primed against B6 alloantigen, lethally irradiated, and infused with B6 BM by either an intravenous or an intra-BM route of administration. Direct intra-BM infusion of donor BM did not abrogate rejection in primed recipients. Regardless of route of donor BM infusion, all primed mice died by 2 weeks (n = 5/group, data not shown). In contrast, all nonprimed controls, regardless of route of donor BM delivery, survived and were more than 99% donor by PBL phenotyping (data not shown).
Fc receptor–expressing cells are required for antibody-mediated elimination of donor BM in primed mice
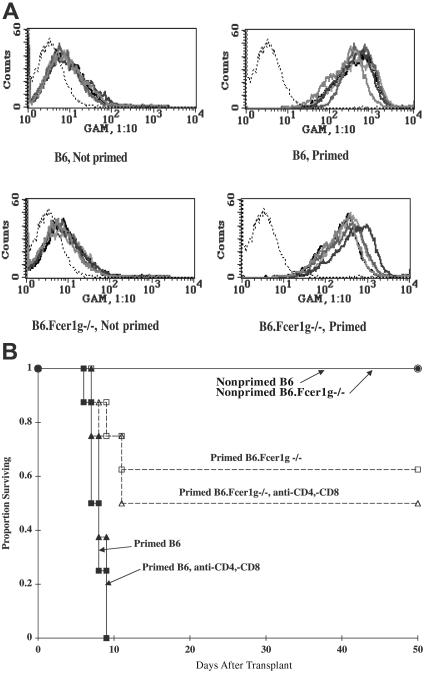
Although antigen specificity is determined by a small part of the variable region of an antibody, effector functions are determined by the Fc portion of the antibody.29,30 To examine the role of host FcR+ cells in antibody-mediated BM rejection, mice deficient in the gamma chain subunit of the FcγRI, FcγRIII, FcγRIV, and FcϵRI receptors (B6.Fcϵr1γ−/−) were primed against BALB/c, lethally irradiated, and infused with BALB/c BM. Despite having equivalent antibody levels to primed B6 WT mice, primed Fcϵr1γ−/− mice had a superior survival rate compared with primed WT mice (Figure 5A-B, 63% vs 0% survival, respectively; P < .001). In vivo TCD of primed WT or Fcϵr1γ−/− mice had no impact on survival in this experiment, indicating that the few deaths occurring in the Fcϵr1γ−/− mice were not the result of primed T-cell–mediated rejection. The residual activity might be due to complement-mediated lysis and/or phagocytic activity mediated by low-level FcgammaR1 expression.31
Figure 5.
Antibody-mediated BM rejection in the primed recipient is dependent on host FcR+ cells. B6 and B6.Fcϵr1γ−/− mice were primed against BALB/c on day −28, lethally irradiated (8.0 Gy) on day −1, and infused with 20 × 106 BALB/c T-cell–depleted BM cells on day 0. A cohort received anti-CD4 and anti-CD8 mAbs around the time of transplantation to ensure depletion of host T cells. (A) Overlay histograms illustrate equivalent serum alloantibody levels at time of BMT in primed Fcϵr1γ−/− as wild-type mice in a thymocyte-binding assay. Serum from nonprimed mice (histograms on the left) illustrates low-level binding. Histograms on the right illustrate high degree of thymocyte binding by serum from primed mice. n = 5/group. Negative control (no serum) is indicated by the thin dotted line. (B) Survival is shown. n = 10/group; P < .001 for primed B6 versus primed B6.Fcϵr1γ−/−. With the exception of the group of primed B6.FcϵrIγ−/− mice that received anti-CD4 and anti-CD8 mAbs, data were reproduced in a second experiment. Each bold line represents a different mouse.
Engraftment can be achieved by the combination of mega-BM cell doses, in vivo TCD, and high-dose murine Ig
The data indicated that preformed antibody was a formidable barrier to successful transplantation in the primed recipient. The near-complete elimination of a moderate BM cell dose by 3 hours after infusion allowed for no therapeutic window of intervention after transplantation. Waiting for antibody levels to decline might not be clinically feasible. A strategy of high BM cell dose, in vivo TCD, high-dose mIg, and splx was chosen for testing. Administration of depleting anti-CD4 and anti-CD8 mAbs was used to target T cells that would survive TBI conditioning. Our data indicated that a high BM cell dose abrogated primed T-cell rejection (Table 1). We hypothesized that in combination with other strategies, sufficient number of stem cells in a high BM cell dose might escape antibody-mediated elimination to permit engraftment. High-dose Ig, widely used in solid organ transplantation in highly sensitized individuals, has multiple immunomodulatory effects, including enhanced clearance of antibody, complement modulation, and inhibition of FcR-mediated clearance of cells.32 Splx was performed after priming but prior to transplantation in an attempt to reduce alloantibody levels and eliminate splenic RES clearance of antibody-coated BM cells that may occur despite FcR blockade with mIg. To test these strategies, B6 mice were primed on day −56 against BALB/c alloantigen. Splx was performed on cohorts of mice on day −28. High-dose mIg (20 mg/mouse) was administered on day −15 and day −7. Anti-CD4 and anti-CD8 mAbs were administered around the time of transplantation. Mice were irradiated on day −1 with 6.0 Gy, the minimum TBI dose that results in uniform lethal aplasia by 3 weeks if mice are not rescued with donor BM. Mice were infused with 100 × 106 BALB/c non–T-cell–depleted BM on day 0. As expected, all nonprimed mice survived and had high levels of donor engraftment (Table 2). Most primed control mice died by 2 weeks, and the 2 primed control mice that survived early lethal aplasia had no evidence of donor chimerism. Neither high-dose mIg nor TCD nor mIg combined with splx affected survival or engraftment. In contrast, the combinations of TCD and mIg and that of TCD, mIg, and splx resulted in significantly higher survival rates and high donor engraftment in 37.5% and 58.8% of recipients, respectively (Table 2, P < .01 vs primed control). All engrafting mice were more than 95% donor by PBL phenotyping at 4 weeks and at 6 months. Moreover, despite the infusion of high numbers of non–T-cell–depleted BM, mice had no weight loss or other clinical signs of GVHD (data not shown).
Table 2.
Engraftment can be achieved in donor-primed mice by the combination of high numbers of bone marrow cells, in vivo TCD, and megadose mIg with or without splenectomy
| Treatment group | Survival | Engraftment |
|---|---|---|
| Not primed | 16/16* | 16/16* |
| Primed | ||
| No treatment | 2/16 | 0/16 |
| Ig | 1/16 | 1/16 |
| TCD | 3/8 | 0/8 |
| TCD, Ig | 6/8* | 3/8* |
| Ig, Splx | 2/7 | 0/7 |
| TCD, Ig, Splx | 13/17* | 10/17* |
B6 recipients were primed on day −56 with 20 × 106 BALB/c splenocytes intraperitoneally. Splenectomy (Splx) was performed on day −28. Mouse Ig (20 mg) was administered intraperitoneally on day −15 and day −7. In vivo TCD was achieved by intraperitoneal administration of 400 μg anti-CD4 and anti-CD8 mAbs on days −2, 0, 2, 4, and 7. Mice were irradiated with 6.0 Gy on day −1 and infused with 100 × 106 BALB/c BM on day 0. All deaths occurred within 2 weeks after transplantation. All engrafted mice were more than 90% donor by peripheral blood leukocyte phenotyping 3 months after BMT.
P < .01 versus primed, no treatment (primed, TCD, Ig vs primed, TCD, Ig, Splx; P = .32.).
To determine the effect of mIg and mIg combined with splx on alloantibody levels, serum was obtained from mice 4 weeks after priming and then again just prior to irradiation. As expected, evaluation of serum 4 weeks after priming but prior to intervention strategies verified that all groups had equivalently high levels of antibody (not shown). Serum obtained just prior to transplantation demonstrated the effect of strategies on antibody level (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). A 1:10 serum dilution resulted in more than 99% positive binding in primed controls, mIg-treated primed mice, and mIg-treated, splenectomized primed mice, indicating high levels of sera antibody in all mice, although there was a significant reduction in MFI in serum from mIg-treated, splenectomized primed mice compared with primed controls. Serum antibody from control, mIg-treated, and mIg-treated, splenectomized mice bound 97%, 93%, and 86% of cells at a 1:40 serum dilution and 79%, 60%, and 41% of cells at a 1:160 serum dilution, respectively (Figure S1). Although these data indicated that mIg (especially in combination with splx) reduced antibody levels, the reduction was unlikely to be of sufficient magnitude to account for the increased survival and engraftment seen in mice when used in conjunction with in vivo TCD. The higher engraftment rate in TCD, mIg-treated mice was more likely due to other known effects of mIg, including inhibition of Fc receptor–mediated clearance of antibody-bound cells and possibly, of the complement cascade.
Discussion
More often used as therapy for hematologic malignancies, allogeneic BMT is also potentially curative for aplastic anemia and other hemoglobulinopathies. However, these patient populations often have been sensitized to alloantigens by multiple blood transfusions required for the palliative treatment of their disease that also may render them more resistant to BM engraftment-promotion strategies in the eventuality of allogeneic BMT.2,5 Increased rates of BM graft failure in sensitized patients have been attributed to priming of both the cellular and the humoral branches of the immune response. Although antibody-mediated acute rejection is a well-recognized problem in multiply-transfused individuals undergoing solid organ transplantation,5,8–10,12,15,33 it has been less well studied in the field of BMT.
Imaging data presented in this paper show for the first time that a moderate allogeneic BM dose was eliminated in the primed recipient by 3 hours after transplantation compared with more than 6 days in the naive recipient. These data graphically illustrate that preformed antibody is both the initial and most formidable barrier to successful BMT in the heavily allosensitized recipient. The speed of antibody-mediated BM rejection indicates there is no window of opportunity for clinical intervention after BMT and dictates the need for greater patient evaluation and more innovative therapeutic strategies prior to BMT, as standard conditioning and immunosuppressive agents given at the time of BMT are ineffective against preformed antibody.
Data indicating that host FcR+ cells played a major role in the elimination of donor BM in the primed recipient suggest a potential target for future preclinical studies.
ADCC, the cytotoxic destruction of antibody-coated target cells by FcR+ host cells, is triggered when antibody bound to the surface of a cell interacts with Fc receptors on NK cells or macrophages. Although NK cells are the most widely recognized mediators of ADCC, depletion of NK cells did not abrogate lethal aplasia in primed mice, indicating that other FcR+ cells are sufficient for this process (Figure 1A). Furthermore, primed host Ly49A transgenic (NK deficient) mice died of lethal aplasia with similar kinetics as primed WT mice, indicating that NK cells are not essential for antibody-mediated BM rejection (data not shown). In addition to directly targeted cytotoxicity, Fc-FcR engagement can destroy antibody-coated cells by engulfment and phagocytosis. Although the latter process should be absent in Fcϵr1γ−/− mice, recent work has demonstrated residual expression and function of the FcγR1 in these mice.31 Low-level activity of the FcγR1 could account for the lethality observed in some of the alloprimed Fcϵrγ1−/− mice. We cannot exclude a contribution of complement-mediated lysis in primed Fcϵrγ1−/− mice, although a primary role for complement-mediated lysis of antibody-coated BM cells seems unlikely as primed C3−/− mice died with similar kinetics as primed WT mice (data not shown).
Our data also indicate that continual exposure to high-titer circulating antibody is not required for the targeted elimination of antibody-coated donor BM. A brief ex vivo incubation of donor BM with serum from primed mice resulted in sufficient antibody coating of cells to mark them for destruction in nonprimed mice. Additionally, our data do not support approaches that might use intra-BM injection in allosensitized patients as direct intra-BM injection of donor BM did not rescue BM from destruction.
Of significant clinical relevance are those data that indicate that priming to one alloantigen can result in the elimination of donor BM of a different alloantigen (Figure 2A). BALB/c-primed B6 mice rejected third-party B10.BR BM and died of lethal aplasia. In contrast, B10.BR-primed B6 mice accepted BALB/c BM. In addition to being fully MHC disparate, B6 and BALB/c mice, unlike B6 and B10.BR mice, also differ at multiple minor antigens. As a result, BALB/c priming, but not B10.BR priming, of B6 mice resulted in a more vigorous and broadly cross-reactive antibody response that culminated in the rejection of third-party BM. These data imply that not only specific, but also cross-reactive or broadly reactive, alloantibodies found in a multiply-transfused individual might target that individual for BM rejection. Additionally, the highly proinflammatory milieu surrounding conditioning and transplantation may also contribute to the antigen-nonspecific activation of host macrophages and other FcR+ cells potentially increasing the efficiency of elimination of antibody-coated BM cells. Of potential clinical interest, although intraperitoneally and intravenously nonirradiated splenocytes appeared to result in equivalent priming, irradiated splenocytes were less effective at priming than nonirradiated splenocytes (P.A.T., unpublished data, November 2003).
Despite the formidable barrier presented by a single priming event, near-complete donor chimerism was achieved in a significant proportion of the mice by a combination of high BM cell number, high-dose mIg, and in vivo TCD. This triad of intervention perhaps along with pre-BMT plasmapheresis and immunoadsorption to reduce preformed antibody levels as used in allosensitized recipients of solid organ grafts should warrant consideration as strategies to specifically target antibody-mediated acute BM rejection in sensitized patients known to have antidonor reactive alloantibodies before BMT.
Supplementary Material
Acknowledgment
This work was supported by grants RO1 HL63452 (B.R.B.) and PO1 AI056299 (B.R.B.).
The authors thank Dr Thomas Waldschmidt (University of Iowa College of Medicine) for advice and critical review of paper.
Footnotes
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.A.T. designed, performed, and analyzed experiments and wrote the paper; M.J.E. conducted murine BMT experiments; M.M.R. and J.M.S. assisted with BMT, chimerism and serum binding assays, and imaging experiments; A.P.-M. offered advice about experimental design and imaging and edited the paper; J.S.S. provided advice, edited the paper, and provided mice; B.R.B. designed research, advised on experimental design, and edited the paper. M.J.E., M.M.R., and J.M.S. contributed equally to the paper and are listed alphabetically in the byline.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota Cancer Center and Department of Pediatrics, Division of BMT, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
- 1.Warren RP, Storb R, Weiden PL, Mickelson EM, Thomas ED. Direct and antibody-dependent cell-mediated cytotoxicity against HLA identical sibling lymphocytes: correlation with marrow graft rejections. Transplantation. 1976;22:631–635. [PubMed] [Google Scholar]
- 2.Anasetti C, Doney KC, Storb R, et al. Marrow transplantation for severe aplastic anemia: long-term outcome in fifty “untransfused” patients. Ann Intern Med. 1986;104:461–466. doi: 10.7326/0003-4819-104-4-461. [DOI] [PubMed] [Google Scholar]
- 3.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 4.Barge AJ, Johnson G, Witherspoon R, Torok-Storb B. Antibody-mediated marrow failure after allogeneic bone marrow transplantation. Blood. 1989;74:1477–1480. [PubMed] [Google Scholar]
- 5.Warren RP, Storb R, Weiden PL, Su PJ, Thomas ED. Lymphocyte-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity in patients with aplastic anemia: distinguishing transfusion-induced sensitization from possible immune-mediated aplastic anemia. Transplant Proc. 1981;13:245–247. [PubMed] [Google Scholar]
- 6.Colson YL, Schuchert MJ, Ildstad ST. The abrogation of allosensitization following the induction of mixed allogeneic chimerism. J Immunol. 2000;165:637–644. doi: 10.4049/jimmunol.165.2.637. [DOI] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–950. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 8.Glotz D, Haymann JP, Sansonetti N, et al. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg): a potential tool for transplantation of immunized patients. Transplantation. 1993;56:335–337. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Ross CN, Gaskin G, Gregor-Macgregor S, et al. Renal transplantation following immunoadsorption in highly sensitized recipients. Transplantation. 1993;55:785–789. doi: 10.1097/00007890-199304000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Tyan DB, Li VA, Czer L, Trento A, Jordan SC. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation. 1994;57:553–562. [PubMed] [Google Scholar]
- 11.Jordan SC, Quartel AW, Czer LS, et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation. 1998;66:800–805. doi: 10.1097/00007890-199809270-00017. [DOI] [PubMed] [Google Scholar]
- 12.Vongwiwatana A, Tasanarong A, Hidalgo LG, Halloran PF. The role of B cells and alloantibody in the host response to human organ allografts. Immunol Rev. 2003;196:197–218. doi: 10.1046/j.1600-065x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 13.Glotz D, Antoine C, Julia P, et al. Intravenous immunoglobulins and transplantation for patients with anti-HLA antibodies. Transpl Int. 2004;17:1–8. doi: 10.1007/s00147-003-0674-3. [DOI] [PubMed] [Google Scholar]
- 14.Morioka D, Sekido H, Kubota K, et al. Antibody-mediated rejection after adult ABO-incompatible liver transplantation remedied by gamma-globulin bolus infusion combined with plasmapheresis. Transplantation. 2004;78:1225–1228. doi: 10.1097/01.tp.0000137264.99113.2b. [DOI] [PubMed] [Google Scholar]
- 15.Takemoto SK, Zeevi A, Feng S, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz E, Lapidot T, Gozes D, Singer TS, Reisner Y. Abrogation of bone marrow allograft resistance in mice by increased total body irradiation correlates with eradication of host clonable T cells and alloreactive cytotoxic precursors. J Immunol. 1987;138:460–465. [PubMed] [Google Scholar]
- 17.Vallera DA, Blazar BR. T cell depletion for graft-versus-host-disease prophylaxis: a perspective on engraftment in mice and humans. Transplantation. 1989;47:751–760. doi: 10.1097/00007890-198905000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323:164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- 19.Cobbold S, Martin G, Waldmann H. Monoclonal antibodies for the prevention of graft-versus-host disease and marrow graft rejection: the depletion of T cell subsets in vitro and in vivo. Transplantation. 1986;42:239–247. doi: 10.1097/00007890-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Fleischhauer K, Kernan NA, O'Reilly RJ, Dupont B, Yang SY. Bone marrow-allograft rejection by T lymphocytes recognizing a single amino acid difference in HLA-B44. N Engl J Med. 1990;323:1818–1822. doi: 10.1056/NEJM199012273232607. [DOI] [PubMed] [Google Scholar]
- 21.Kernan NA, Flomenberg N, Dupont B, O'Reilly RJ. Graft rejection in recipients of T-cell-depleted HLA-nonidentical marrow transplants for leukemia: identification of host-derived antidonor allocytotoxic T lymphocytes. Transplantation. 1987;43:842–847. [PubMed] [Google Scholar]
- 22.Pei J, Akatsuka Y, Anasetti C, et al. Generation of HLA-C-specific cytotoxic T cells in association with marrow graft rejection: analysis of alloimmunity by T-cell cloning and testing of T-cell-receptor rearrangements. Biol Blood Marrow Transplant. 2001;7:378–383. doi: 10.1053/bbmt.2001.v7.pm11529487. [DOI] [PubMed] [Google Scholar]
- 23.Kraus AB, Shaffer J, Toh HC, et al. Early host CD8 T-cell recovery and sensitized anti-donor interleukin-2-producing and cytotoxic T-cell responses associated with marrow graft rejection following nonmyeloablative allogeneic bone marrow transplantation. Exp Hematol. 2003;31:609–621. doi: 10.1016/s0301-472x(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 25.Jordan SC, Vo AA, Nast CC, Tyan D. Use of high-dose human intravenous immunoglobulin therapy in sensitized patients awaiting transplantation: the Cedars-Sinai experience. Clin Transpl. 2003:193–198. [PubMed] [Google Scholar]
- 26.Jordan SC. Management of the highly HLA- sensitized patient: a novel role for intravenous gammaglobulin. Am J Transplant. 2002;2:691–692. doi: 10.1034/j.1600-6143.2002.20801.x. [DOI] [PubMed] [Google Scholar]
- 27.Jordan SC, Tyan D, Stablein D, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 28.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 29.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 30.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 31.Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 32.Knezevic-Maramica I, Kruskall MS. Intravenous immune globulins: an update for clinicians. Transfusion. 2003;43:1460–1480. doi: 10.1046/j.1537-2995.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 33.Hancock WW, Gao W, Shemmeri N, et al. Immunopathogenesis of accelerated allograft rejection in sensitized recipients: humoral and nonhumoral mechanisms. Transplantation. 2002;73:1392–1397. doi: 10.1097/00007890-200205150-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.