Abstract
Second mitochondria–derived activator of caspases (Smac) promotes apoptosis via activation of caspases. Here we show that a low-molecular-weight Smac mimetic LBW242 induces apoptosis in multiple myeloma (MM) cells resistant to conventional and bortezomib therapies. Examination of purified patient MM cells demonstrated similar results, without significant cytotoxicity against normal lymphocytes and bone marrow stromal cells (BMSCs). Importantly, LBW242 abrogates paracrine MM cell growth triggered by their adherence to BMSCs and overcomes MM cell growth and drug-resistance conferred by interleukin-6 or insulinlike growth factor-1. Overexpression of Bcl-2 similarly does not affect LBW242-induced cytotoxicity. Mechanistic studies show that LBW242-induced apoptosis in MM cells is associated with activation of caspase-8, caspase-9, and caspase-3, followed by PARP cleavage. In human MM xenograft mouse models, LBW242 is well tolerated, inhibits tumor growth, and prolongs survival. Importantly, combining LBW242 with novel agents, including tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) or the proteasome inhibitors bortezomib and NPI-0052, as well as with the conventional anti-MM agent melphalan, induces additive/synergistic anti-MM activity. Our study therefore provides the rationale for clinical protocols evaluating LBW242, alone and together with other anti-MM agents, to improve patient outcome in MM.
Introduction
Tumor cell responses to chemotherapy include growth arrest and apoptosis.1 Stress-induced apoptosis2–4 is associated with alterations in mitochondrial membrane permeabilization and release of several apoptogenic factors, such as cytochrome c (cyto c),5,6 Smac/DIABLO,7,8 AIF,9 EndoG,10 and HtrA2/Omi.11 Once released into the cytosol, these mitochondrial proteins trigger both caspase-dependent (by cyto c or second mitochondria–derived activator of caspases [Smac]) and caspase-independent (by AIF or Endo-G) apoptosis. Conversely, inhibitors of apoptosis proteins (IAPs) block the enzymatic activity of caspases that mediate cell death,12,13 and overexpression of IAPs confers chemoresistance in various tumor types.14–16
Recent studies have shown that the mitochondrial apoptotic protein Smac can abrogate the protective function of IAPs, such as X-linked inhibitor of apoptosis (XIAP).7,8 XIAP is the most potent caspase inhibitor among IAPs and binds with initiator caspase-9 and executioner caspases 3 and 7 through its BIR3 and BIR2 domains, respectively.17,18 Stress stimuli trigger the release of Smac from mitochondria into the cytosol, where it binds to and eliminates its inhibitory effect on caspase-9, thereby resulting in activation of caspase-9–mediated apoptotic signaling cascade.7,19–21 These findings suggest the potential clinical utility of Smac mimetics to trigger apoptosis and overcome drug resistance conferred by IAPs.
There are several additional rationales for using Smac mimetic as a potential therapy. First, the cell-permeable Smac peptides, when combined with chemotherapy, inhibited tumor growth in vivo with little toxicity in mice.22–25 Second, our prior studies have established that Smac release is critical during most anti–multiple myeloma (MM) agent–induced apoptosis, and that dysfunctional Smac release may, in part, contribute to the development of drug resistance.26 Third, defects in the mitochondrial apoptotic machinery includes up-regulated antiapoptotic protein Bcl-2, which inhibits Smac activity.26 Smac mimetics have the ability to circumvent the requirement for mitochondrial processing and release of Smac, thereby potentially triggering apoptosis even in Bcl-2–overexpressing MM cells. Fourth, MM cells have constitutively activated NF-κB growth/survival signaling,27–31 and a Smac mimetic was shown to potentiate apoptosis in TNF-α–treated cells despite NF-κB activation.32 Thus, Smac agonists are promising candidates as novel cytotoxic therapies in MM.
Several groups have succeeded in developing potent, small-molecular-weight Smac mimetic compounds with high affinity for the BIR3 domain of IAPs at pharmacologically achievable concentrations.32–36 Our prior studies have shown that various anti-MM agents down-regulate IAPs;37 however, whether or not direct inhibition of XIAP by a Smac mimetic could trigger apoptosis in these cells is undefined.
In the present study, we characterized the effects of the Smac mimetic LBW242, a small drug-like molecule, against MM cell lines and primary patient cells resistant to conventional therapies. Both our in vitro and in vivo xenograft model studies suggest that LBW242 can inhibit the growth of MM cells and overcome drug resistance, setting the stage for clinical trials of this novel therapeutic to improve patient outcome in MM.
Materials and methods
Cell culture and reagents
MM.1S, MM.1R, RPMI-8226, doxorubicin (Dox)–resistant (Dox-40), U266, and OPM2 human MM cell lines were maintained as previously described.38 MM cells were freshly isolated from patients relapsing after multiple prior therapies, including dexamethasone (Dex), melphalan, thalidomide, or bortezomib. Tumor cells were purified by CD138+ selection39 using the Auto MACS magnetic cell sorter (Miltenyi Biotec Inc, Auburn, CA). Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki protocol. The cell viability of tumor cells was within a range of 82% to 94% after 4 to 5 days of culture ex vivo. Informed consent was obtained from all patients in accordance with the Helsinki protocol. Cells were treated with LBW242 (Novartis, Cambridge, MA), bortezomib (Millennium Pharmaceuticals, Cambridge, MA), NPI-0052 (Nereus Pharmaceuticals Inc, San Diego, CA), Dex (Sigma, St Louis, MO), melphalan, recombinant tumor necrosis factor–related apoptosis-inducing ligand (rTRAIL), recombinant interleukin-6 (rIL-6), or recombinant insulinlike growth factor-1 (rIGF-1) (all from Calbiochem, San Diego, CA). Peripheral blood mononuclear cells (PBMNCs) or CD34+ bone marrow (BM) cells from normal healthy donors were maintained in culture medium (viability, 90%-95%), as previously described.38 PBMNCs were stimulated by exposing cells to 5 μg/mL PHA and 500 U/mL IL-2 for 48 hours in the presence or absence of LBW242.
XIAP (BIR3) binding assay
The fluorescence resonance energy transfer (FRET) assay is performed in solution: compounds (LBW242, Smac 7mer, or LCV843) were preincubated with GST-BIR3 (12.5 nM) for 30 minutes, followed by incubation for another 30 minutes with biotin-Smac (3.5 nM), europium-labeled strepavidin beads (1 nM), and APC-labeled anti-GST (25 nM) antibody. The 384-well Opti-plate (Perkin Elmer Life Sciences, Wellesley, MA) was read after incubating for 60 minutes using an Envision multilabel reader (Perkin Elmer; excitation and emission filters, Europium 615 nM and APC 665 nM, and optical module Lance Eu/APC Dual 452). The IC50 at which 50% of GST-BIR3 is released from biotinylated Smac were determined using the XL-fit4 program (IDBS, Burlington, MA).
Cell viability, apoptosis and transfection assays
Cell viability was assessed by MTT (3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide; Chemicon International Inc, Temecula, CA) assay, according to the manufacturer's instructions, and as previously described.39,40 Cell Death Detection ELISAplus was used to quantitate cell death, as per the manufacturer's instructions (Roche Applied Sciences, Indianapolis, IN). Bcl-2 or the empty (neo) constructs (Upstate Biotechnologies, Lake Placid, NY) were transfected into MM.1S cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The baseline viability of the transfected cells was greater than 90%. Stable clones were selected after incubation in growth medium containing G418 (500 μg/mL; Life Technologies; Stratagene, La Jolla, CA), as previously described.41
Effect of Smac mimetic on paracrine MM cell growth in the BM
The growth of MM cells triggered by adhesion to MM patient-derived BMSCs (viable cell range, 90%-95%) was assessed, as previously described.42,43
Transwell cell migration assay
Cell migration was assayed using a modified Boyden chamber, as previously described.44
Western blotting
Western blot analysis was performed as previously described.45 Immunoblot analysis was performed using antibodies to caspase-8, caspase-9, caspase-3, or cleaved caspase-3 (Cell Signaling, Beverly, MA), and tubulin (Sigma, St Louis, MO) as well as poly (ADP ribose) polymerase (PARP), XIAP, Smac, or Bcl-2 (BD Bioscience Pharmingen, San Diego, CA). Blots were then developed by enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL).
Human plasmacytoma xenograft model
All experiments involving animals were approved by an institutional Animal Care and Use Committee. For the animal study, LBW242 was dissolved in acetate buffer (pH 4.6) and 6.0 N HCl at a final concentration of 10 mg/mL. The xenograft tumor model was performed as previously described.46 CB-17 severe combined immunodeficiency (SCID) mice (n = 14; Taconic, Gemantown, NY) were subcutaneously inoculated in the interscapular area with 2.5 × 106 MM.1S cells in 100 μL RPMI-1640 medium. When tumors were measurable at approximately 3 weeks after MM cell injection, mice were treated for 14 consecutive days with either vehicle alone or LBW242 (35 mg/kg) orally. Tumor size was measured every other day in 2D using a caliper; tumor volume was calculated using the formula V = 0.5 a × b2, where “a” and “b” are the long and short diameters of the tumor, respectively. Animals were killed when their tumors reached 2 cm3. Survival was evaluated from the first day of treatment until death.
Statistical analysis
The Wilcoxon signed rank test was performed to compare proliferation in untreated and treated patient cells, and the Jonchkeere-Terpstra (J-T) trend test was performed for measuring the viability of lymphocytes and cell lines resistant to conventional therapy. Statistical significance of differences observed in LBW242-treated mice compared with control groups was determined using a Student t test. The minimal level of significance was a P value below .05. The tumor growth inhibition and survival were determined using the SigmaPlot analysis software (Systat Software, San Jose, CA). Isobologram analysis was performed using the CalcuSyn software program (Biosoft, Ferguson, MO, and Cambridge, United Kingdom). A combination index (CI) less than 1.0 indicates synergism, and a CI of 1 indicates additive activity.47
Results
Chemical structure and XIAP (BIR3) binding activity of Smac mimetic LBW242
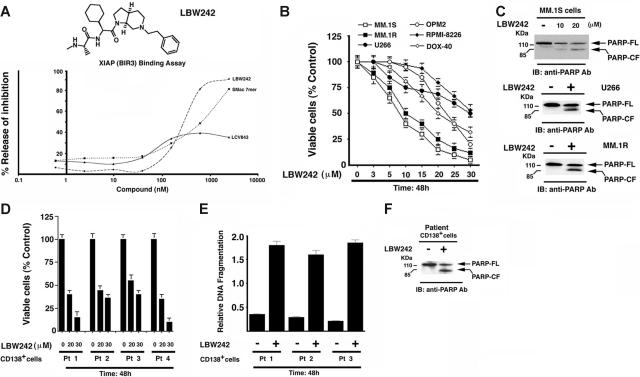
The structure-activity relationship (SAR) studies underlying the development of LBW242 have been described.33 The functional specificity of LBW242 was determined using a FRET-based competition assay. In this assay, the ability of LBW242 to compete with biotinylated Smac for occupancy of the XIAP BIR3 binding pocket is assessed (“Materials and methods”). LBW242 (IC50, 280 nM) was slightly more potent than Smac 7mer (IC50, 422 nM; AnaSpec, San Jose, CA) (Figure 1A). Importantly, LCV843, a compound lacking BIR3-binding activity (IC50 > 10 000 nM), did not significantly inhibit the interaction between XIAP-BIR3 and biotinylated Smac (Figure 1A).
Figure 1.
Effects of LBW242 on MM cells. (A) Chemical structure and XIAP (BIR3) binding activity of LBW242. (B) LBW242 inhibits growth and triggers apoptosis in human MM cells. MTT assays were performed after incubation of various human MM cell lines with indicated concentrations of LBW242 for 48 hours. Data represent means ± SD from 3 independent experiments. (C) Total protein lysates from LBW242-treated MM.1S, U266, or MM.1R MM cells were subjected to immunoblot analysis with anti-PARP antibodies (Abs). Blots shown are representative from 2 independent experiments. (D-F) Purified patient MM cells (CD138+) were treated for 48 hours with LBW242 and then analyzed for viability (MTT assays) and apoptosis (DNA fragmentation and PARP cleavage assays). Data represent means ± SD of triplicate samples.
LBW242 inhibits growth and triggers apoptosis in MM cells
Based on these findings, we next directly examined whether LBW242 affects the viability of MM cells. LBW242 treatment (48 hours) of MM cell lines (MM.1S, U266, RPMI-8226, and OPM2), as well as those resistant to conventional anti-MM agents, Dex (MM.1R) and Dox (Dox-40), induces a dose-dependent significant (P < .005; n = 3) decrease in viability in all cell lines (IC50 range, 8-30 μM; Figure 1B). To determine whether LBW242-induced decrease in MM cell viability is due to apoptosis, we examined proteolytic cleavage of PARP enzyme,48,49 a hallmark of apoptosis. MM cell lines (MM.1S, U266 and MM.1R) were treated for 48 hours with LBW242 at their respective IC50, harvested, and analyzed for PARP cleavage. LBW242 triggered apoptosis in all MM cell lines, as evidenced by PARP cleavage (Figure 1C). No cytostatic or necrotic effects of LBW242 were observed in LBW242-treated MM.1S cells (data not shown). To determine whether LBW242 similarly affects purified patient MM cells, tumor cells (viable cells, 85%-95%) from 5 patients with MM relapsing after multiple prior therapies, including Dex (patient no. 1), bortezomib (patient nos. 2 and 3), and thalidomide (patient no. 4) were treated for 48 hours with LBW242 (20 and 30 μM) and then analyzed for viability. A significant decrease in viability of all patient MM cells was noted (P < .005; Figure 1D). Importantly, 2 of 4 patients studied were refractory to bortezomib therapy, and 2 were resistant to thalidomide and Dex therapies. Patients were considered refractory to bortezomib therapy when they have relapsed with progressive disease after bortezomib therapy. Moreover, the LBW242-triggered decrease in viability of MM patient cells was due to apoptosis, as evidenced by DNA fragmentation (Figure 1E; P < .005, n = 2) and PARP cleavage (Figure 1F). Taken together, these findings suggest that LBW242 induces apoptosis and overcomes drug resistance in MM cells.
Effects of LBW242 on normal lymphocytes
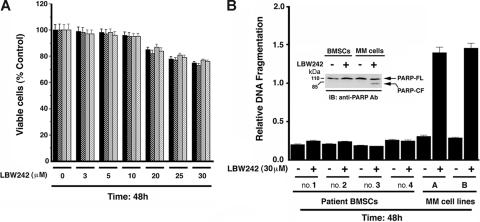
Normal lymphocytes from 4 healthy donors (viable cells, 90%-95%) were treated with LBW242 (3-30 μM) and analyzed for viability. LBW242 does not significantly decrease the viability of normal lymphocytes at concentrations between 0 and 10 μM (P = .22 from J-T trend test; Figure 2A). Furthermore, treatment of PHA/IL-2–stimulated PBMNCs only decreased viability at high doses (21% ± 1.2% at 40 μM). Higher concentrations of LBW242 decrease viability of lymphocytes by 20% ± 2.1%, suggesting that normal cells are not completely refractory to LBW242. Additionally, examination of CD34+ BM cells showed little, if any, decrease in their viability in response to treatment with LBW242 (data not shown).
Figure 2.
Effect of LBW242 on normal lymphocytes and MM patient BMSCs. (A) Lymphocytes from 4 healthy donors were treated with indicated concentrations of LBW242 and then analyzed for viability. Data are means ± SD of 3 independent experiments (P = .25 from J-T test for trend). (B) BMSCs from 4 patients with MM were treated with indicated concentrations of LBW242 for 48 hours and analyzed for apoptosis by DNA fragmentation assays. Data are means ± SD of 3 independent experiments (P < .005). As a positive control, MM cell lines (A, MM.1S; B, MM.1R) were treated with LBW242 and similarly analyzed for apoptosis. Inset shows total protein extracts from untreated and LBW242-treated BMSCs or patient MM cells when subjected to immunoblotting with anti-PARP Abs.
LBW242 inhibits paracrine MM growth triggered by adherence to BMSCs
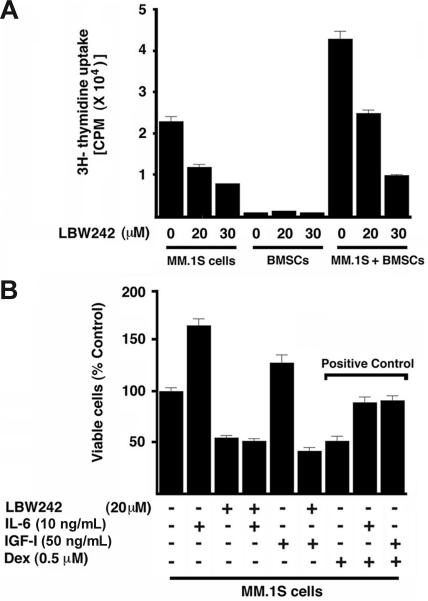
The interaction of MM cells with bone marrow stromal cells (BMSCs) induces cytokine secretion, which mediates paracrine growth of MM cells as well as protects against drug-induced apoptosis.27,42,50 We therefore next examined whether LBW242 affects the viability of BMSCs. LBW242 (30 μM) treatment of BMSCs (patient nos. 1-4) for 48 hours does not induce apoptosis, as evidenced by DNA fragmentation (Figure 2B) and PARP cleavage analysis (Figure 2B; inset). As a positive control, apoptosis was noted in MM.1S (A) and MM.1R (B) cells (Figure 2B; inset showing PARP cleavage). MM cells were then cultured with or without BMSCs, in the presence or absence of LBW242. Adherence of tumor cells to BMSCs induces growth, as evidenced by increased [3H]-thymidine uptake of MM.1S cells (1.9-fold increase; P < .05; Figure 3A). Importantly, LBW242 inhibits the adhesion-induced growth of MM cells in a dose-dependent manner (P < .05). These findings indicate that LBW242 retains antitumor activity against MM cells even in the protective BM microenvironment.
Figure 3.
LBW242 overcomes the protective effects of MM BM microenvironment. (A) MM.1S cells were cultured in BMSCs coated or noncoated plates for 48 hours in the presence of medium alone or with the indicated concentrations of LBW242. DNA synthesis was assessed by [3H]-thymidine uptake assay, and data represent means of triplicate cultures; bars, SD. (B) MM.1S cells were treated for 48 hours with the indicated concentrations of LBW242 or Dex, in the presence or absence of rhIL-6 or rhIGF-1, and then analyzed for viability. Shown are means ± SD of 3 independent experiments (P < .05 for all samples).
LBW242 overcomes human IL-6 and IGF-1–mediated antiapoptotic effects
Both IL-6 and IGF-1 induce growth, up-regulate expression of cIAPs, and protect against chemotherapy-induced apoptosis in MM cells in vitro.27,51–57 We therefore next asked whether IL-6 or IGF-1 affects LBW242-induced apoptosis in MM cells. Neither IL-6 nor IGF-1 block LBW242-triggered cytotoxicity in MM.1S cells (Figure 3B). Similar results were obtained with pretreatment or cotreatment of MM.1S cells with IL-6 and LBW242. As a positive control,57,58 both IL-6 and IGF-1 block Dex-induced decreases in MM.1S cell viability. These findings demonstrate that LBW242, unlike Dex, overcomes the cytoprotective effects of the BM microenvironment due both to adhesion of MM cells to BMSCs and induction of cytokines.
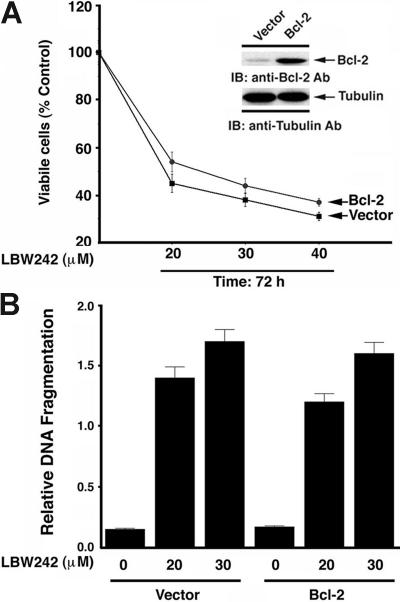
LBW242 overcomes Bcl-2–mediated cytoprotective effects
Besides the BM milieu, drug resistance in MM cells is also conferred by various intracellular factors. For example, Bcl-2 confers resistance to conventional therapies in MM cells.26,59 Bcl-2 inhibits apoptosis by blocking the release of cyto c and smac/DIABLO from mitochondria.6,60 We therefore next examined whether ectopic expression of Bcl-2 in MM.1S cells affects responsiveness to LBW242. Vector- or Bcl-2–transfected MM.1S cells41 were treated with LBW242 (20, 30, and 40 μM) for 72 hours, and then analyzed for viability. LBW242 significantly decreased cell viability in both cell types (P < .005; Figure 4A). Western blot analysis showed markedly enhanced Bcl-2 protein levels in Bcl-2–transfected MM.1S cells compared with empty vector–transfected MM.1S cells (Figure 4A; inset). Moreover, LBW242 triggered apoptosis in both vector and Bcl-2–transfected MM.1S cells, as evidenced by DNA fragmentation (Figure 4B; P < .005). These data demonstrate the ability of LBW242 to overcome the cytoprotective effects of Bcl-2 in MM cells.
Figure 4.
LBW242 decreases survival in Bcl-2–overexpressing MM.1S cells. (A) Empty vector– or Bcl-2–transfected MM.1S cells were treated for 72 hours with LBW242 at the indicated concentrations and then analyzed for viability. Results are means ± SD of 2 independent experiments. Inset shows Western blot of Bcl-2 protein levels. (B) Vector- or Bcl-2–transfected MM.1S cells were treated for 48 hours with LBW242 at the indicated concentrations, and analyzed for apoptosis by DNA fragmentation assays (mean ± SD; n = 2). The percentage of specific cell death was calculated by subtracting the percentage of spontaneous apoptosis of the relevant controls from the total percentage of cell death.
LBW242 blocks VEGF-induced migration of MM cells
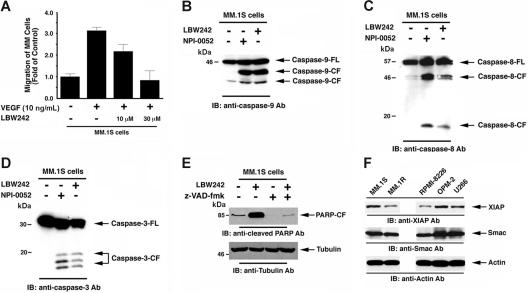
Vascular endothelial growth factor (VEGF) is elevated in the MM BM microenvironment, and our studies showed that VEGF triggers migration and growth of MM cells, as well as angiogenesis in MM.44 We next therefore examined whether LBW242 affects VEGF-triggered MM cell migration. Results demonstrate that VEGF alone markedly increases MM.1S cell migration, and that LBW242 significantly (P < .05) inhibits VEGF-dependent MM cell migration (Figure 5A). Importantly, LBW242 at the concentrations used in migration assays did not affect survival of MM cells (viability > 95%) as assessed by MTT assay (data not shown). These findings indicate that LBW242 may negatively regulate homing of MM cells to the BM, as well as their egress into the peripheral blood.
Figure 5.
LBW242-induced signal transduction in MM cells. (A) LBW242 inhibits VEGF-induced migration of MM cells. Growth factor–deprived MM.1S cells were either pretreated with indicated concentrations of LBW242 or left untreated. Cells were then plated on a fibronectin-coated polycarbonate membrane in a modified Boyden chamber and exposed for 4 hours to VEGF (5 ng/mL) in the lower chamber. Cells on the lower part of the membrane were then counted with a Coulter counter ZBII (mean ± SD; n = 2). (B-D) LBW242 induces both extrinsic and intrinsic apoptotic signaling MM.1S cells were treated with LBW242 (10 μM) for 48 hours or NPI-0052 (7 nM) for 24 hours and harvested; the total protein extracts were subjected to immunoblot analysis with anti–caspase-9 (B), caspase-8 (C), or caspase-3 (D) Abs. Lysates from NPI-0052–treated MM.1S MM cells served a positive control. (E) MM.1S cells were treated with LBW242 in the presence or absence of z-VAD-fmk for 24 hours and harvested; total protein extracts were subjected to immunoblot analysis with anticleaved PARP Abs. (F) Total protein lysates from MM.1S, MM.1R, RPMI-8226, OPM2, and U266 MM cells were subjected to immunoblot analysis with anti-XIAP (top panel), anti-Smac (middle panel), or antiactin (bottom panel) Abs.
Mechanisms mediating anti-MM activity of Smac mimetic
Apoptosis is associated with activity of aspartate-specific cysteine proteases or caspases (cysteinyl, aspartate-specific proteases), which can either inactivate or activate target substrates such as PARP by proteolytic cleavage.48 Initiator caspases undergo autocatalytic processing, and then cleave and activate the downstream executioner caspases that orchestrate cell death.48 Genetic and biochemical evidence indicates that apoptosis proceeds by 2 major cell-death pathways: an intrinsic pathway that involves mitochondrial membrane permeabilization and release of several apoptogenic factors, followed by caspase-9 activation; and an extrinsic apoptotic signaling pathway that occurs via caspase-8 activation.2 Both caspase-8 and caspase-9 activate downstream caspase-3.2
We next examined whether LBW242 triggers extrinsic and/or intrinsic apoptotic signaling pathways. Our results show that LBW242 induces activation of caspase-9, casapse-8, and caspase-3 (Figure 5B-D). LBW242 eliminates the inhibitory effect of XIAP on caspase-9, thereby resulting in activation of caspase-9–mediated apoptotic cascade;7,33 in concert with these findings, our data show that LBW242 induces caspase-9 activation in MM cells. Interestingly, LBW242 also triggers caspase-8 activation, which is consistent with a previous report.61 We therefore examined whether LBW242-triggered caspase-8 activation is occurring through a caspase-9–mediated signaling pathway. Pretreatment of MM.1S cells with a caspase-9–specific inhibitor (LEHD-fmk) showed only modest effects (15%-20% decrease) on caspase-8 activity, along with similar decreases in both PARP cleavage and viability (data not shown). These results suggest that LBW242-induced caspase-8 activation is not completely dependent on caspase-9 activation. Further genetic studies using dominant-negative or siRNA strategies will define the precise mechanisms mediating LBW242-triggered apoptosis in MM cells. Nonetheless, our data suggest that Smac mimetic LBW242 may facilitate cross-talk between the intrinsic and extrinsic apoptotic signaling pathways. Importantly, pretreatment of MM.1S cells with a pancaspase inhibitor (z-VAD-fmk) significantly blocked LBW242-induced apoptosis, as evidenced by abrogation of PARP cleavage (Figure 5E). These data suggest that LBW242-induced apoptosis is mediated by the activation of a caspase cascade.
We next examined whether XIAP, Smac, or Bcl-2 protein levels in MM cell lines correlate with sensitivity to LBW242. As seen in Figure 5F, XIAP expression varied among the cell lines, and Smac was similarly expressed in all cell lines. Bcl-2 regulates apoptotic signaling upstream of smac/cyto c release, and our data show that overexpression of Bcl-2 does not block LBW242-induced MM cell death. Thus, although Bcl-2 expression is variable among the cell lines (data not shown), this does not affect the downstream apoptotic events triggered by Smac mimetic LBW242. These findings, together with the data in Figure 1B, suggest that (1) XIAP and/or Smac protein levels do not correlate to observed sensitivity to LBW242 in MM cell lines; and (2) other factors may be involved in mediating LBW242-triggered cell death in MM cells.
LBW242 inhibits human MM cell growth in vivo in a murine model
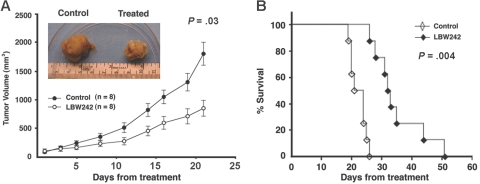
Having shown that LBW242 induces apoptosis in MM cells in vitro, we next examined the in vivo efficacy of LBW242 using our human plasmacytoma xenograft mouse model.39,46 Treatment of tumor-bearing mice with LBW242 (n = 8), but not vehicle alone (n = 6), significantly inhibits MM tumor growth (P = .03; Figure 6A; inset). As seen in Figure 6B, there was also significantly prolonged survival in treated animals versus controls (P = .004): the median overall survival was 30 days in the control group and 42.75 days in the LBW242-treated group. LBW242 (35 mg/kg orally) was well tolerated by mice, without significant weight loss (data not shown). Moreover, no neurologic changes were observed even after 3 to 4 weeks of LBW242 treatment. These data demonstrate the potent anti-MM activity of LBW242 in vivo at the dose that is well tolerated in a murine model, confirming our in vitro data and further supporting the early clinical promise of LBW242 in the treatment of MM. Importantly, LBW242 is orally bioavailable and efficacious.
Figure 6.
LBW242 inhibits human plasmacytoma growth and prolongs survival in immune-deficient beige-nude-xid (BNX) mice. (A) Kinetics of myeloma xenograft growth. Mice (n = 8) received LBW242 (35 mg/kg, orally) for 2 weeks daily. A significant delay in tumor growth in LBW242-treated mice was noted compared with vehicle-treated control mice (n = 6; P = .03). Points indicate mean; bars, SE. Inset photographs show the tumors excised from control (vehicle) and LBW242-treated mice. (B) Daily treatment was started on day 1, when tumors were measurable. Mice were treated with LBW242 or with vehicle alone for 2 weeks daily. Survival was evaluated from the first day of treatment until death using the SigmaPlot analysis software.
Combined treatment with LBW242 and TRAIL, melphalan, or Dex induces additive anti-MM activity
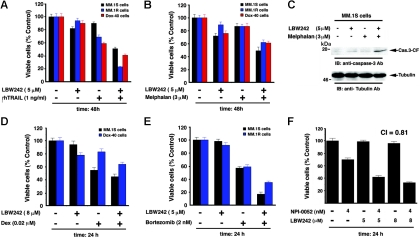
Because our data suggest potent single-agent antitumor activity of LBW242 in MM cells, we next examined whether it can be combined with other conventional and novel drugs to enhance cytotoxicity in these cells. Prior studies showed that Smac peptides sensitize human cancer cells to apoptosis when combined with chemotherapeutic agent– or TNF-related apoptosis, inducing ligand (TRAIL).22–25,32 We therefore first examined whether combined LBW242 and TRAIL trigger similar synergistic/additive antitumor activity in the MM cell system. MM.1S MM cells were treated with both LBW242 and TRAIL and analyzed for viability. LBW242 plus TRAIL triggered additive anti-MM activity, evidenced by a significant decrease in viability of MM.1S cells (Figure 7A; shown are representative results from minimally toxic and maximally additive concentrations of each agent). Isobologram analysis confirmed additive anti-MM activity of LBW242 and TRAIL (CI = 1.0; P < .05; n = 3). We further examined whether LBW242 plus TRAIL induces similar responses in the drug-resistant MM cell lines MM.1R and Dox-40. LBW242 plus TRAIL induced synergistic cell death in both MM.1R and Dox-40 cells (CI = .78 and .82, respectively; Figure 7A). These data suggest that LBW242 plus TRAIL exerts additive or synergistic anti-MM activity in MM cell lines. Nevertheless, our findings are consistent with a previous study showing enhanced antitumor activity of combined IAP mimetic and TRAIL.32 Importantly, our prior study showing that TRAIL induces apoptosis in MM,62 together with our present findings, suggest a novel therapeutic approach combining LBW242 and TRAIL in the treatment of MM.
Figure 7.
Combination of low doses of LBW242 with anti-MM agents induces additive/synergistic MM cell death. (A). MM.1S, MM.1R, or Dox-40 MM cells were treated with indicated doses of LBW242, rhTRAIL, or LBW242 plus rhTRAIL and assessed for viability using MTT assays (mean ± SD; n = 3, P < .005). (B) MM.1S, MM.1R, or Dox-40 cells were treated with LBW242, melphalan, or LBW242 plus melphalan and assessed for viability (mean ± SD; n = 3, P < .005). (C) MM.1S cells were treated with LBW242, melphalan, or LBW242 plus melphalan for 24 hours, and total protein lysates were analyzed for caspase-3 cleavage by Western blot analysis. (D) MM.1S or MM.1R cells were treated with LBW242, Dex, or LBW242 plus Dex for 24 hours and assessed for viability (mean ± SD; n = 3, P < .006). (E) MM.1S or MM.1R cells were treated with LBW242, bortezomib, or LBW242 plus bortezomib for 24 hours and assessed for viability (mean ± SD; n = 3, P < .005). (F) MM.1S cells were treated with LBW242, NPI-0052, or LBW242 plus NPI-0052 for 24 hours and assessed for viability (mean ± SD; n = 3, P < .004).
To further address this issue, we combined LBW242 with melphalan, an agent commonly used to treat MM.63 MM.1S cells were treated with melphalan (3 μM) and LBW242 (5 μM), followed by analysis for viability. LBW242 significantly enhances the anti-MM activity of melphalan (CI = 1.0; P < .05; n = 3) (Figure 7B; shown are the representative results from minimally toxic and maximally additive concentrations of each agent). Combined LBW242 plus melphalan also induced additive anti-MM activity in drug-resistant MM cell lines (ie, MM.1R and Dox-40 [CI = 1.0 for both cell lines]; Figure 7B; data not shown for Dox-40). Similar results were obtained using purified patient MM cells without any significant affect on the viability of normal lymphocytes (data not shown). Furthermore, combined low doses of LBW242 plus melphan triggered more robust activation of caspase-3 and PARP cleavage than either agent alone at these low doses (Figure 7C; data not shown). No significant decrease in the viability of PHA/IL-2–stimulated normal PBMNCs was noted in response to treatment with combined LBW242 and melphalan (data not shown). LBW242 also enhances the anti-MM activity of Dex in MM.1S and Dox-40 cells (Figure 7D; CI = 1).
The combination of LBW242 with agents that inhibit the proteasome results in synergistic anti-MM activity
Bortezomib is US Food and Drug Administration (FDA)–approved for the treatment of MM; however, prolonged treatment can be associated with toxicity.64 We therefore next examined whether combining LBW242 with bortezomib would allow for the use of lower doses of bortezomib. Dex-sensitive MM.1S and Dex-resistant MM.1R MM cells were treated with both LBW242 and bortezomib and analyzed for the viability. As seen in Figure 7E, the combination of LBW242 and bortezomib induced synergistic anti-MM activity in both MM.1S and MM.1R cells (CI = .78 and .83, respectively). No significant decrease in the viability of PHA/IL-2–stimulated normal PBMNCs was noted in response to treatment with combined LBW242 and bortezomib.
Our recent study showed that the novel proteasome inhibitor NPI-0052 is a potent inducer of apoptosis in MM cells.45 We therefore similarly examined the combined effect of LBW242 and NPI-0052 on MM cell viability. As with bortezomib, a combination of Smac mimetic with NPI-0052 triggered synergistic MM cell death (CI < .81; Figure 7F). Taken together, these data suggest that LBW242 enhances the antitumor activity of both conventional and novel anti-MM agents, which may allow for use of lower doses and thereby reduce toxicities.
Discussion
Collectively, our study shows the following: (1) Smac mimetic LBW242 induces apoptosis in MM cells resistant to conventional and bortezomib therapies, without affecting normal lymphocyte viability at clinically achievable concentrations; (2) LBW242 does not affect MM-derived BMSC viability; (3) LBW242 overcomes MM BMSC adhesion–induced growth and cytoprotective effects; (4) LBW242 inhibits growth of MM cells, even in the presence of MM growth factors such as IL-6 or IGF-1; (5) LBW242 overcomes drug resistance conferred by the antiapoptotic protein Bcl-2; (6) LBW242 blocks VEGF-induced migration of MM cells, confirming its antiangiogenic activity; (7) LBW242-induced apoptosis in MM cells is associated with activation of caspase-8, caspase-9, and caspase-3; (8) combinations of low doses of LBW242 and melphalan, Dex, bortezomib, or NPI-0052 trigger additive/synergistic anti-MM activity; and finally, (9) LBW242 is orally bioavailable and efficacious—LBW242 inhibits MM tumor growth in vivo, as well as prolongs survival. Reports that synthetic Smac peptides enhance the apoptotic activity of chemotherapeutic agents,22,24,65,66 coupled with our present findings, provide the framework for clinical trials of Smac mimetic LBW242, either alone or in combination with other anti-MM agents, to enhance efficacy, reduce toxicity, and overcome drug resistance in patients with recurring/refractory MM.
Acknowledgments
Supported by National Institute of Health (NIH) grants CA 50947, CA 78373, and CA10070; a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); the Department of Veterans Affairs Merit Review Awards Research Service (N.C.M.); the Multiple Myeloma Research Foundation (D.C.); and The Myeloma Research Fund.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: D.C. designed experiments, analyzed data, and wrote the manuscript; P.N., P.T., and M.F. performed animal studies; M.V., K.P., T.H., M.T., and N.R. performed research and analyzed data; C.M. and N.M. provided valuable reagents; P.R. and N.M. provided blood samples; L.Z. provided LBW242; and K.C.A. critically evaluated and wrote the manuscript.
D.C. and P.N. contributed equally to this work.
Conflict-of-interest statement: Some of the authors (L.Z. and M.T.) are employees of a company (Novartis Institute of Biomedical Research) whose products were used in this research.
Correspondence: Kenneth C. Anderson, M557, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.
References
- 1.Willie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 2.Bossy-Wetzel E, Green DR. Apoptosis: checkpoint at the mitochondrial frontier. Mutat Res. 1999;434:243–251. doi: 10.1016/s0921-8777(99)00032-4. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 4.Dalton WS. Targeting the mitochondria: an exciting new approach to myeloma therapy [commentary]. Clin Cancer Res. 2002;8:3643–3645. [PubMed] [Google Scholar]
- 5.Kluck RM, Kashibatta S. A lively meeting of a deathly topic. Apoptosis. 1997;2:337–342. doi: 10.1023/a:1026449422844. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Liu X, Balla K, et al. Prevention of apoptosis by Bcl-2-release of cytochrome-c from the mitochondria blocked. Science. 1997;275:1129–1136. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 7.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 8.Verhagen AM, Ekert PG, Pakusch M, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 9.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 10.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 12.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 13.Harada H, Grant S. Apoptosis regulators. Rev Clin Exp Hematol. 2003;7:117–138. [PubMed] [Google Scholar]
- 14.Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa T, Suzuki K, Sakamoto C, et al. Expression of the inhibitor of apoptosis (IAP) family members in human neutrophils: up-regulation of cIAP2 by granulocyte colony-stimulating factor and overexpression of cIAP2 in chronic neutrophilic leukemia. Blood. 2003;101:1164–1171. doi: 10.1182/blood-2002-05-1505. [DOI] [PubMed] [Google Scholar]
- 16.Krajewska M, Krajewski S, Banares S, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–4925. [PubMed] [Google Scholar]
- 17.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 18.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 19.Wu G, Chai J, Suber TL, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Sun C, Olejniczak ET, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasula SM, Hegde R, Saleh A, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 22.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 23.Pardo OE, Lesay A, Arcaro A, et al. Fibroblast growth factor 2-mediated translational control of IAPs blocks mitochondrial release of Smac/DIABLO and apoptosis in small cell lung cancer cells. Mol Cell Biol. 2003;23:7600–7610. doi: 10.1128/MCB.23.21.7600-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Mashima T, Sato S, et al. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 2003;63:831–837. [PubMed] [Google Scholar]
- 25.Mizukawa K, Kawamura A, Sasayama T, et al. Synthetic Smac peptide enhances the effect of etoposide-induced apoptosis in human glioblastoma cell lines. J Neurooncol. 2006;77:247–255. doi: 10.1007/s11060-005-9045-5. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan D, Anderson KC. Mechanisms of cell death and survival in multiple myeloma (MM): therapeutic implications. Apoptosis. 2003;8:337–343. doi: 10.1023/a:1024164700094. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 28.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;28:28. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 29.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 30.Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications. Blood. 2002;99:4079–4086. doi: 10.1182/blood.v99.11.4079. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiades CS, Mitsiades N, Poulaki V, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Straub C, Zawel L. Development of peptidomimetics trageting IAPs. Int J Peptide Res Ther. 2006;12:21–32. doi: 10.1007/s10989-005-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oost TK, Sun C, Armstrong RC, et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–4426. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 35.Park CM, Sun C, Olejniczak ET, et al. Non-peptidic small molecule inhibitors of XIAP. Bioorg Med Chem Lett. 2005;15:771–775. doi: 10.1016/j.bmcl.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Sun H, Nikolovska-Coleska Z, Chen J, et al. Structure-based design, synthesis and biochemical testing of novel and potent Smac peptido-mimetics. Bioorg Med Chem Lett. 2005;15:793–797. doi: 10.1016/j.bmcl.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–937. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 38.Chauhan D, Li G, Podar K, et al. Targeting mitochondria to overcome conventional and bortezomib/proteasome inhibitor PS-341 resistance in multiple myeloma (MM) cells. Blood. 2004;104:2458–2466. doi: 10.1182/blood-2004-02-0547. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan D, Catley L, Hideshima T, et al. 2-Methoxyestradiol overcomes drug resistance in multiple myeloma cells. Blood. 2002;100:2187–2194. doi: 10.1182/blood-2002-02-0376. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell SS, Nicholson B, Teisan S, Lam KS, Potts BC. Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J Nat Prod. 2004;67:1400–1402. doi: 10.1021/np049970g. [DOI] [PubMed] [Google Scholar]
- 41.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 43.Hideshima T, Akiyama M, Hayashi T, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood. 2003;101:703–705. doi: 10.1182/blood-2002-06-1874. [DOI] [PubMed] [Google Scholar]
- 44.Podar K, Tai YT, Lin BK, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKCalpha activation. J Biol Chem. 2002;277:7875–7881. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- 45.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 46.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 47.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 49.Oberhammer FA, Hochegger K, Froschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994;126:827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson KC. Moving disease biology from the lab to the clinic. Cancer. 2003;97:796–801. doi: 10.1002/cncr.11137. [DOI] [PubMed] [Google Scholar]
- 51.Hardin J, MacLeod S, Grigorieva I, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 52.Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. Br J Haematol. 1997;97:429–440. doi: 10.1046/j.1365-2141.1997.592708.x. [DOI] [PubMed] [Google Scholar]
- 53.Rowley M, Liu P, Van Ness B. Heterogeneity in therapeutic response of genetically altered myeloma cell lines to interleukin 6, dexamethasone, doxorubicin, and melphalan. Blood. 2000;96:3175–3180. [PubMed] [Google Scholar]
- 54.Jelinek DF, Witzig TE, Arendt BK. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J Immunol. 1997;159:487–496. [PubMed] [Google Scholar]
- 55.Chauhan D, Kharbanda S, Ogata A, et al. Interleukin-6 inhibits Fas-induced apoptosis and stress-activated protein kinase activation in multiple myeloma cells. Blood. 1997;89:227–234. [PubMed] [Google Scholar]
- 56.Chauhan D, Hideshima T, Rosen S, Reed JC, Kharbanda S, Anderson KC. Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2001;276:24453–24456. doi: 10.1074/jbc.C100074200. [DOI] [PubMed] [Google Scholar]
- 57.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma other hematologic malignancies and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 58.Chauhan D, Pandey P, Hideshima T, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 59.Gazitt Y, Fey V, Thomas C, Alvarez R. Bcl-2 overexpression is associated with resistance to dexamethasone, but not melphalan, in multiple myeloma cells. Int J Oncol. 1998;13:397–405. doi: 10.3892/ijo.13.2.397. [DOI] [PubMed] [Google Scholar]
- 60.Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20:6627–6636. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 62.Mitsiades CS, Treon SP, Mitsiades N, et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98:795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 63.Chauhan D, Pandey P, Ogata A, et al. Cytochrome-c dependent and independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 64.Chauhan D, Hideshima T, Anderson KC. Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol. 2005;45:465–476. doi: 10.1146/annurev.pharmtox.45.120403.100037. [DOI] [PubMed] [Google Scholar]
- 65.Guo F, Nimmanapalli R, Paranawithana S, et al. Ectopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood. 2002;99:3419–3426. doi: 10.1182/blood.v99.9.3419. [DOI] [PubMed] [Google Scholar]
- 66.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277:44236–44243. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]