Abstract
Pleckstrin-2 is composed of 2 pleckstrin homology (PH) domains and a disheveled–Egl-10–pleckstrin (DEP) domain. A lipid-binding assay revealed that pleckstrin-2 binds with greatest affinity to D3 and D5 phosphoinositides. Pleckstrin-2 expressed in Jurkat T cells bound to the cellular membrane and enhanced actin-dependent spreading only after stimulation of the T-cell antigen receptor or the integrin α4β1. A pleckstrin-2 variant containing point mutations in both PH domains failed to associate with the Jurkat membrane and had no effect on spreading under the same conditions. Although still membrane bound, a pleckstrin-2 variant containing point mutations in the DEP domain demonstrated a decreased ability to induce membrane ruffles and spread. Pleckstrin-2 also colocalized with actin at the immune synapse and integrin clusters via its PH domains. Although pleckstrin-2 can bind to purified D3 and D5 phosphoinositides, the intracellular membrane association of pleckstrin-2 and cell spreading are dependent on D3 phosphoinositides, because these effects were disrupted by pharmacologic inhibition of phosphatidylinositol 3-kinase (PI3K). Our results indicate that pleckstrin-2 uses its modular domains to bind to membrane-associated phosphatidylinositols generated by PI3K, whereby it coordinates with the actin cytoskeleton in lymphocyte spreading and immune synapse formation.
Introduction
Pleckstrin-1, the platelet and leukocyte C kinase substrate, is the major substrate of protein kinase C (PKC) in platelets, monocytes, macrophages, lymphocytes, and granulocytes.1 Its 350 amino acid sequence can be divided into 3 motifs: PH domains at the amino- and carboxy-termini of the molecule and an intervening DEP domain.2–4 A short stretch of amino acids between the amino-terminal PH domain and the DEP domain contains 3 sites of phosphorylation (Ser113, Thr114, and Ser117) by PKC, which are essential for the function of pleckstrin-1.5–7
PH domains have been identified in approximately 252 other human proteins (Simple Modular Architecture Research Tool [SMART] database) and are found in many molecules involved in cellular signaling, cytoskeletal organization, membrane trafficking, and phospholipid modification.8 Previous results have suggested that PH domains mediate binding of their host proteins to certain phosphoinositides.9–11 Consistent with this hypothesis, nearly all PH domain-containing proteins require membrane association for their function in signal transduction, including pathways that contribute to cytoskeletal assembly, membrane budding, and fusion.4,7,12–15
DEP homology domains are present in numerous signaling proteins,3,16 including 67 found in the human genome (SMART database). The DEP domain is a protein module of approximately 100 amino acids, first found in the signaling proteins disheveled, Egl-10, and pleckstrin.3 The domain is also present in certain kinases, regulators of G-protein signaling proteins (RGSs), and Epac, the cyclic adenosine monophosphate-regulated guanine nucleotide exchange factor (GEF). DEP domains are present in proteins that regulate a broad range of cellular functions, from determination of planar-cell polarity in early embryonic development17 to highly specialized neuronal signaling in retinal photoreceptors.18 Although DEP domains are often described as being of unknown function, evidence is emerging that several signaling proteins may rely on their DEP domains for membrane association.18–24
We cloned a paralog of pleckstrin-1 that we termed pleckstrin-2.25 Pleckstrin-2 is a 353 amino acid protein that is 65% homologous and 39% identical to pleckstrin-1. Both paralogs contain a PH domain at the amino- and carboxy-termini flanking a central DEP domain. Although primary sequence and organizational homology are strong, differences between pleckstrin-1 and pleckstrin-2 do exist. For example, while pleckstrin-1 has 3 PKC phosphorylation sites, pleckstrin-2 appears to be a poor substrate for this kinase.25 In addition, expression of pleckstrin-1 is restricted to platelets and leukocytes, while pleckstrin-2 mRNA is present in a wide variety of nonhematopoietic cell types in addition to the bone marrow and the thymus.25
Pleckstrin-1 induces cell spreading and reorganization of the actin cytoskeleton.26,27 These pleckstrin-1–mediated effects are entirely regulated by its phosphorylation state. Because pleckstrin-2 does not appear to be a phosphoprotein, we reasoned that its interaction with its binding partners or intracellular localization regulates its activity. Therefore, to begin to understand the role of pleckstrin-2 and the function of its PH and DEP domains, we analyzed its ability to bind to specific phospholipids, its cellular distribution, and its effect on cell spreading or shape change in T-lymphocyte cell lines. We found that the PH domains of pleckstrin-2 bind membrane phospholipids, cluster integrins, and promote lamellipodia formation and ultimately cell spreading through a PI3K-dependent pathway. Furthermore, we demonstrate that pleckstrin-2 polarizes to the immune synapse via its PH domains and colocalizes with F-actin. We propose that the regional generation of PI3K products recruits pleckstrin-2 to discrete regions within the cellular membrane, allowing it to contribute to membrane extension, cell spreading, and immune synapse formation.
Materials and methods
Tissue culture and reagents
T-cell hybridoma 2B4 cells and the human acute leukemia T-cell line, Jurkat, were maintained in RPMI 1640 supplemented with penicillin/streptomycin and 10% FBS (Invitrogen Life Technologies, Carlsbad, CA). Human primary T-cell RNA was kindly provided by Dr James L. Riley of the Abramson Family Cancer Research Institute (University of Pennsylvania, Philadelphia).28 In selected experiments, cells were stimulated with fibronectin (Sigma-Aldrich, St Louis, MO) or the anti-CD3 monoclonal antibody, OKT3 (Mayo Clinic Pharmacy, Rochester, MN).
Generation of pleckstrin-2 mutants
The cDNA for wild-type pleckstrin-2 was previously described.25 The pleckstrin-2 cDNA truncation and point mutations were generated by polymerase chain reaction (PCR) splice-overlap-extension.29 These mutations include N13 N14 pleckstrin-2 (K13N K14N pleckstrin-2), N256 pleckstrin-2 (K256N pleckstrin-2), N13 N14 N256 pleckstrin-2, N-PH pleckstrin-2 (Leu6-Ala102), C-PH pleckstrin-2 (Val249-Leu352), K13 K14 N-PH pleckstrin-2, K256 C-PH pleckstrin-2, and N156 N157 N166 pleckstrin-2 (K156N K157N D166N pleckstrin-2). Wild-type or mutant pleckstrin-2 cDNA was cloned into either (1) pGEX2-TK (GE Healthcare Bio-Sciences, Piscataway, NJ) for the lipid-binding assay or (2) pEGFPC-1 (Clontech, Mountain View, CA) for expression of GFP-tagged fusion proteins in Jurkat or 2B4 cells.
Lipid-binding assay
Assays of lipid-binding specificity were performed as previously described.30 Phosphoinositides at 2 mg/mL in 1:1 chloroform-methanol (containing 0.1% HCl) were spotted (2 μL) onto nitrocellulose (MSI, Westborough, MA). After drying, nitrocellulose was blocked overnight at 4°C in Tris-buffered saline (TBS) containing 3% bovine serum albumin without detergent. GST fusion proteins were made in the pGEX-2TK vector that allowed the direct (γ-32P) ATP labeling by protein kinase A (PKA) of the fusion proteins in vitro. There are no predicted PKA phosphorylation sites within the pleckstrin-2 protein sequence. 32P-labeled GST fusion protein was then added to TBS/3% bovine serum albumin at a final concentration of 0.5 μg/mL, and this solution was used to probe the phosphoinositide-containing nitrocellulose for 30 minutes at room temperature. Results were viewed by standard autoradiograph techniques and quantified by a phosphorimager via the Image Quant Program (GE Healthcare Bio-Sciences). To determine the relative affinity of each protein for individual phospholipids, the quantities of 32P counts bound to each lipid were normalized for the activity (32P counts per microgram) of the radioactive probe.
Immunofluorescence and quantitation of cell spreading
Plasmids encoding for pleckstrin-2–GFP fusion proteins were electroporated into Jurkat cells or 2B4 cells as previously described.31 Direct fluorescence of transfected cells was visualized using a Nikon Microphot-SA microscope (Nikon, Tokyo, Japan), and images were captured using IP Lab Spectrum Image Analysis software for the Macintosh (BD Biosciences, Rockville, MD) and a Coolsnap FX-HQ camera. Jurkat cells were allowed to adhere and spread on glass slides coated with fibronectin (50 μg/mL) or OKT3 (5 μg/mL) for 30 minutes at 37°C. Cells were fixed with 4% paraformaldehyde, and for each experiment quantitation of footprint size of 50 of 120 cells was performed. All results shown represent the mean ± SEM of 3 independent experiments, with at least 50 cells analyzed in each experiment.
Quantitation of cell adhesion
Jurkat cells expressing the pleckstrin-2–GFP fusion proteins were plated on 96-well microtiter plates coated with fibronectin at 50 μg/mL and allowed to incubate for 30 minutes at 37°C. Cell adhesion was measured as described previously.32 The adherent cells were incubated for 1 hour at 25°C with 5 mM p-nitrophenyl phosphate (Sigma-Aldrich) in a buffer containing 0.1 M sodium citrate and 0.1% Triton X-100. The reaction was terminated by the addition of 100 μL 2N NaOH. The p-nitrophenol produced by the reaction was measured with a microplate reader (EL 800 Universal Microplate Reader; Bio-Tek Instruments, Winooski, VT) at 405 nm against a Jurkat-free blank. Results represent the mean ± SEM of 6 independent experiments, with 10 wells analyzed for each pleckstrin-2–GFP fusion protein.
Confocal microscopy
For live-cell imaging, Jurkat or 2B4 cells electroporated with GFP-pleckstrin constructs were cultured in RPMI media overnight and transferred, just prior to image collection, to Delta TC3 open-dish observation chambers coated with fibronectin (50 μg/mL) or OKT3 (5 μg/mL). The Delta TC3 open-dish system consists of individual 170 ± 10 μm–thick tissue-observation chambers and an objective heater to maintain cells at 37°C during the experiment (Bioptechs, Butler, PA). Images were acquired using an UtraVIEW-LCI spinning disk confocal microscope (Perkin-Elmer, Boston, MA) having a modified dual Nipkow disk (Yokogawa Electric, Tokyo, Japan) attached to a Nikon TE-300 inverted microscope and fitted with a 100× oil-immersion objective lens, NA 1.4 (Nikon). The GFP–pleckstrin-2 was excited using the 488 nm line of an argon laser (Melles Griot, Carlsbad, CA), and a 488 nm dichroic mirror transmitted the signal, which was then subsequently filtered by a 525/50 nm emission filter. Images were typically collected at 10-second intervals before and after the addition of 100 nM wortmannin, 1 μM latrunculin A, 10 μM cytochalasin D (Sigma-Aldrich), or 50 μM LY294002 (Calbiochem, San Diego, CA) using a Hamamatsu Orca-ER CCD camera (Hamamatsu Photonics, Hamamatsu, Japan).
For fixed-cell imaging, Jurkat cells expressing the GFP–pleckstrin-2 constructs were allowed to spread on glass slides coated with fibronectin (50 μg/mL) for 30 minutes at 37°C. The cells were fixed with 4% paraformaldehyde and incubated with a monoclonal antibody to the integrin β1 subunit (R&D Systems, Minneapolis, MN), and β1 was detected using a secondary antibody coupled to Texas Red (Invitrogen Molecular Probes, Carlsbad, CA). Colocalization of GFP–pleckstrin-2 and Texas Red–stained β1 integrin was measured by confocal microscopy. The integrin β1 subunit stained with Texas Red was excited using the 568 nm line of an argon laser (Melles Griot), and a 568 nm dichroic mirror transmitted the signal, which was then subsequently filtered by a 525/50 nm emission filter.
Total internal reflection fluorescence
Jurkat cells expressing the GFP–pleckstrin-2 constructs were allowed to spread on glass slides coated with fibronectin (50 μg/mL) for 30 minutes at 37°C. The cells were fixed with 4% paraformaldehyde and incubated with a monoclonal antibody to the integrin β1 subunit (R&D Systems). β1 was detected using a secondary antibody coupled to FITC (Invitrogen Molecular Probes). Objective-type total internal reflection fluorescence (TIRF) microscopy was performed on a modified Leica DMIRB microscope (Leica, Wetzlar, Germany) fitted with a Nikon 1.45 NA objective.33 Samples were illuminated through the rear port of the microscope using the 488 nm line of an argon laser (Melles Griot) focused on the back focal plane of the objective. Images were acquired with a Hamamatsu digital camera and Metamorph software (Universal Imaging, Downingtown, PA). A computer-controlled filter wheel (Sutter Instruments, Novato, CA) containing neutral density filters was placed in the beam path to allow for attenuation of the beam.
Conjugate assay
Conjugate assays were performed as described previously.34,35 Briefly, Raji B cells were stained with CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin; Invitrogen Molecular Probes) and incubated in the presence or absence of 2 μg/mL staphylococcal enterotoxin E (SEE; Toxin Technology, Sarasota, FL) for 1.5 hours, washed, and resuspended at 1 × 106 cells per millliliter in serumfree RPMI. Jurkat T cells expressing GFP alone or GFP fused to the amino-terminus of the various pleckstrin-2 constructs were resuspended at 1 × 106 cells per milliliter in serumfree RPMI. For conjugation, equal volumes of B and T cells were pelleted together and incubated at 37°C for 10 to 15 minutes prior to settling onto poly-l-lysine–coated coverslips. Following fixation, conjugates were labeled with rhodamine-phalloidin (Invitrogen Molecular Probes) to visualize F-actin. Cells were imaged on a Zeiss Axiovert 200M microscope (Carl Zeiss MicroImaging, Thornwood, NY) equipped with a Roper Coolsnap FX-HQ camera (Photometrics, Tucson, AZ). Nearest-neighbor deconvolution was performed using Slidebook v4.0 software (Intelligent Imaging Innovations, Denver, CO). Quantitation of protein polarization was determined by randomly selecting conjugates containing a green T cell contacting a blue B cell. Those conjugates showing a distinct polymerized band of the protein of interest at the T-cell–B-cell contact site were counted as polarized. All results shown represent the mean ± standard deviation of 3 independent experiments, with at least 50 conjugates scored in each experiment.
Results
Pleckstrin-2 shows highest affinity for purified phosphoinositides with D3 or D5 phosphates
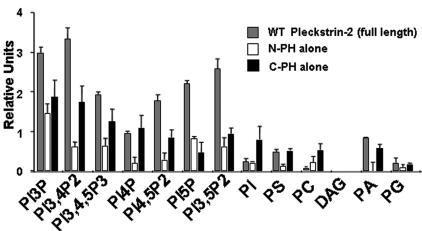
To define the lipid-binding specificity of pleckstrin-2, we examined the ability of full-length pleckstrin-2, and each of the individual PH domains of pleckstrin-2, to adhere to various phospholipids using a “dot blot” assay. This assay has previously confirmed the specificity of the PLC-δ1 PH domain for phosphatidylinositol 4,5-bisphosphate and the Grp1 PH domain for the PI3K product, phosphatidylinositol 3,4,5-trisphosphate.30 Consistent with studies of lipid binding using a pleckstrin-2 polypeptide containing a truncation of the C-terminal PH domain,36 full-length pleckstrin-2 displayed highest binding to phosphoinositides that have the phosphate in the D3 and D5 positions (Figure 1). Pleckstrin-2 did not bind to DAG, demonstrating that the inositol head group mediates this association.
Figure 1.
Pleckstrin-2 shows affinity for phosphoinositides with D3 phosphates. GST fusion proteins were made in the pEGX-2TK vector, which allowed the direct (γ-32P) ATP labeling by PKA of the fusion proteins in vitro. Results were viewed by standard autoradiograph techniques and quantified via the Image Quant Program. Relative affinity was calculated by quantitating bound 32P divided by specific activity of the radioactive probe. Shown is the mean ± SEM from 3 independent experiments.
To determine whether the individual PH domains of pleckstrin-2 had different lipid-binding specificity, we analyzed the lipid-binding characteristics of the isolated N-terminal and C-terminal PH domains. Both individual domains bound best to phosphoinositides with a D3 phosphate, but their affinity was reduced relative to the full-length protein. This demonstrated that both PH domains contribute to the phospholipid association of full-length pleckstrin-2. Point mutations within the presumed phospholipid-binding site of either the N-PH domain (N13 N14 pleckstrin-2) or the C-PH domain (N256 pleckstrin-2) completely abolished phospholipid binding of the individual PH domains in this assay (not shown). Similarly, simultaneous point mutations within both PH domains (N13 N14 N256 pleckstrin-2) eliminated the ability of the full-length protein to bind lipids (not shown).
Together, these data demonstrate that pleckstrin-2 has specificity for products of either PI3K or phosphatidylinositol 5-kinase (PI5K), implying that second messengers generated by either of these enzymes may regulate pleckstrin-2 localization. These data also suggest that the specific binding of pleckstrin-2 to these phospholipids may impact pleckstrin-2 function as an effector for either PI3K or PI5K. Notably, the dot blot assay does not discriminate well between low and high binding affinities. Because the phosphates in the D3 and D5 positions are both oriented in the same direction within the inositol ring, it is conceivable that pleckstrin-2 actually only prefers 1 of these 2 phosphate positions. This issue is further addressed in the data presented in Figure 6.
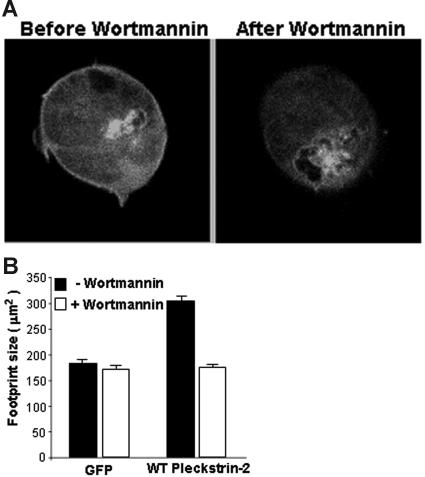
Figure 6.
Pleckstrin-2 membrane association and cell spreading are PI3K dependent. (A) Jurkat cells expressing GFP fused to the amino-terminus of wild-type pleckstrin-2 were plated on fibronectin. Real-time confocal imaging of live cells was performed. Shown is a cell expressing pleckstrin-2 before and after inhibition of PI3K with 100 nM wortmannin. This figure shows that wild-type pleckstrin-2 associates with membrane projections in cells spread on fibronectin, and this membrane association is rapidly (about 2 minutes) disrupted by the addition of wortmannin. (B) Jurkat cells expressing GFP (GFP) alone or GFP fused to the amino-terminus of wild-type pleckstrin-2 (WT pleckstrin-2) were plated on fibronectin. Where indicated, 100 nM wortmannin was added for 5 minutes to inhibit PI3K. Images were captured at 100× magnification and cell-footprint size was quantified using IP Lab imaging software. Shown is the mean ± SEM of 3 independent experiments.
Expression of pleckstrin-2 in Jurkat cells increases cell spreading upon stimulation of cell-surface receptors
Although an antibody specific for pleckstrin-2 is not available, Northern blots indicate that pleckstrin-2 is present in bone marrow and thymus.25 Therefore, we examined pleckstrin-2 expression in T cells. In Figure 2A we demonstrate that pleckstrin-2 mRNA is present in resting and activated primary human T cells. However, pleckstrin-2 mRNA is expressed in Jurkat T cells at barely detectable levels (Figure 2A, lane 3). This is similar to pleckstrin-1, which constitutes 1% of the total protein in primary T cells but is negligible in Jurkat T cells and other T-cell lines (not shown).
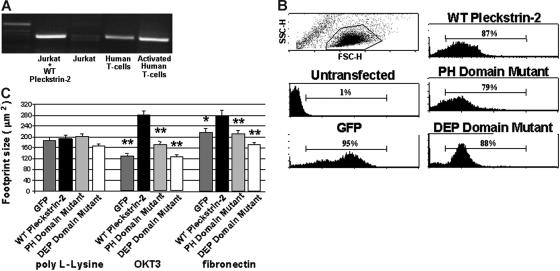
Figure 2.
Expression of pleckstrin-2 in Jurkat cells increases cell spreading upon stimulation of cell-surface receptors. (A) RT-PCR products using primers to pleckstrin-2 were derived from Jurkat cells and human T cells and analyzed by agarose electrophoresis. Lane 1, 1 kb ladder; lane 2, Jurkat cells transfected with wild-type (WT) pleckstrin-2; lane 3, untransfected Jurkat cells; lane 4, resting primary human T cells; lane 5, human T cells activated with antibodies to CD3 and CD28. (B) Flow cytometry was performed on Jurkat cells transfected with plasmids that direct the expression of GFP (GFP), GFP fused to the amino-terminus of pleckstrin-2 (WT pleckstrin-2), or GFP fused to the amino-terminus of pleckstrin-2 containing point mutations in both PH domains, N13 N14 N256 (PH domain mutant), or in the DEP domain, N156 N157 N166 (DEP domain mutant). (C) Jurkat cells expressing GFP alone, WT pleckstrin-2, the PH domain mutant, or the DEP domain mutant were plated on either poly-L-Lysine, OKT3, or fibronectin. Images were captured and cell-footprint size was quantified using IP Lab imaging software. Shown is the mean ± SEM of 3 independent experiments. The paired Student t test was performed of the transfectants compared with WT pleckstrin-2 on each matrix. *P < .05; **P < .005.
We reasoned that “adding back” pleckstrin-2 to Jurkat cells may give insights into its physiologic role within T cells. Transfection of wild-type pleckstrin-2 into Jurkat cells (Figure 2A, lane 2) demonstrates detectable reverse transcriptase (RT)–PCR products. Although measurement of mRNA by RT-PCR is not directly translatable to protein and thus cannot be quantitatively compared with that observed in primary human T cells (Figure 2A, lanes 4 and 5), the baseline levels of pleckstrin-2 mRNA in Jurkat T cells (Figure 2A, lane 3) suggest that these cells have little pleckstrin-2 protein expression, making it an appropriate system to add back pleckstrin-2. Flow cytometry of Jurkat cells stably transfected with GFP-tagged wild-type pleckstrin-2 or pleckstrin-2 containing point mutations in either both PH domains or the DEP domain was performed. The GFP–pleckstrin-2 expression levels of each cell type are demonstrated in Figure 2B. As demonstrated, each of the transfected pleckstrin-2 variants was expressed to the same level. Furthermore, overexpression of pleckstrin-2 in Jurkat cells did not influence mean forward scatter compared with untransfected cells and therefore did not grossly affect cell size in suspension (not shown).
Because expression of pleckstrin-1 enhances cell spreading within adherent cell lines,26,27 we analyzed whether pleckstrin-2 affected Jurkat T-cell spreading by quantifying the footprint sizes of Jurkat T cells overexpressing wild-type pleckstrin-2 or pleckstrin-2 mutants. We found that when pleckstrin-2–expressing Jurkat cells were nonspecifically adhered to poly-L-lysine–coated plates, there were only small changes in the cell-footprint size (Figure 2C). In contrast, wild-type pleckstrin-2 resulted in larger amounts of cell spreading of Jurkat cells plated upon either OKT3 (which activates the Jurkat T-cell antigen receptor) or fibronectin (which binds to the Jurkat integrin α4β1). Neither the double PH domain–mutated or the DEP domain–mutated variants of pleckstrin-2 had effects on spreading. These results imply that in these cells the PH domains cooperate with the DEP domain of pleckstrin-2 in creating a binding pocket for endogenous proteins essential in cell spreading.
Expressed pleckstrin-2 in Jurkat cells moves to the membrane upon stimulation of the T-cell receptor or α4β1
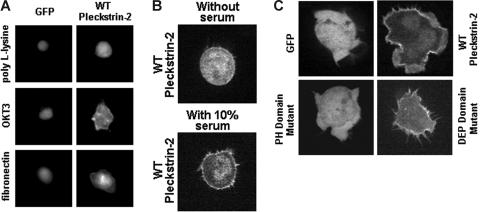
Pleckstrin-2 expressed in a variety of adherent cell lines is concentrated at the cell membrane, including the membrane of lamellipodia, ruffles, and other membrane protrusions.25 Interestingly, we found that pleckstrin-2 expressed in Jurkat cells is not concentrated at the cell membrane when the cells were observed in suspension or plated over a matrix that does not support Jurkat-cell adhesion, such as fibrinogen (not shown). Similarly, pleckstrin-2 was not bound to the cell membrane when the Jurkat cells were nonspecifically adhered to poly-L-lysine–coated coverslips (Figure 3A). We tested whether stimulation of the T-cell antigen receptor or the integrin α4β1 affected the intracellular localization of pleckstrin-2. As shown by the brighter rim on the cells shown in Figure 3A, specific engagement of the T-cell receptor induced the movement of pleckstrin-2 to the cell membrane. Plating the cells on fibronectin, which binds to α4β1, had a similar effect (Figure 3A, bottom row). However, this translocation is dependent upon the presence of serum, because it occurred only after the addition of 10% FBS to serum-starved Jurkat cells expressing wild-type pleckstrin-2 (Figure 3B).
Figure 3.
Expression of pleckstrin-2 in Jurkat cells localizes to the cellular membrane upon stimulation of cell-surface receptors and enhances lamellipodia formation. (A) Jurkat cells expressing GFP alone or GFP-labeled pleckstrin-2 variants were plated upon OKT3 or fibronectin. This figure shows that WT pleckstrin-2 associates with the cell membrane when cells are plated upon OKT3 or fibronectin. Images were captured at ×40 magnification using IP Lab imaging software. (B) Jurkat cells expressing GFP fused to the amino-terminus of wild-type pleckstrin-2 were plated on fibronectin. Real-time confocal imaging of live cells was performed. Shown is a cell expressing pleckstrin-2 before and after addition of 10% FBS. This figure shows that wild-type pleckstrin-2 associates with the membrane and membrane projections rapidly (about 30 seconds) with the addition of serum. Images were captured using an UtraVIEW-LCI confocal scanner at ×100 magnification. (C) Jurkat cells expressing GFP alone or GFP-labeled pleckstrin-2 variants were plated on OKT3. This figure demonstrates that membrane localization is dependent on the pleckstrin-2 PH domains. Similar to WT pleckstrin-2, the DEP domain mutant associates with the cell membrane upon stimulation by the T-cell receptor–activating antibody but does not form membrane ruffles. Images were captured using an UtraVIEW-LCI confocal scanner at × 100 magnification.
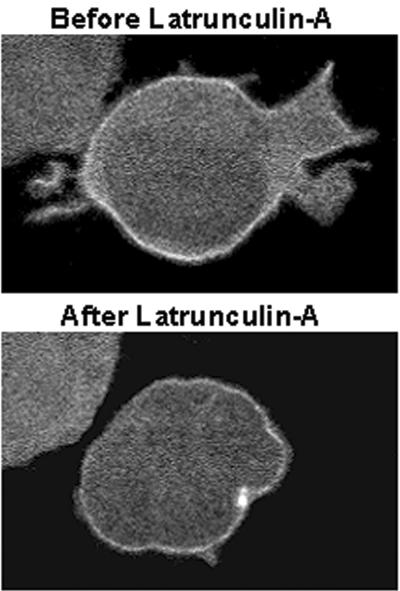
We previously reported that, compared with cells overexpressing pleckstrin-1, pleckstrin-2–expressing cells have more filopodia and large lamellipodia with ruffle formation.25 To determine whether the morphology of membrane projections is influenced by a particular pleckstrin-2 domain, we used real-time confocal imaging of GFP-labeled pleckstrin-2 in Jurkat cells. We observed that in Jurkat cells spread on OKT3 (Figure 3C) or fibronectin (not shown), both wild-type pleckstrin-2 and the DEP domain mutant become localized within membrane projections from the cell surface. Intriguingly, the DEP domain mutant was impaired in its ability to induce membrane ruffles and lamellipodia while filopodia formation remained intact. We questioned whether perturbation of the actin cytoskeleton influenced the intracellular distribution of pleckstrin-2. To address this issue, Jurkat cells expressing pleckstrin-2 were exposed to latrunculin A, a sponge toxin that sequesters G-actin and leads to the unopposed depolymerization of F-actin. As shown in Figure 4 and Video S1 (available on the Blood website; see the Supplemental Videos link at the top of the online article), latrunculin A rapidly disrupted lamellipodia and filopodia formation but did not interfere with the normal membrane distribution of pleckstrin-2. Cytochalasin D, a fungal metabolite that also leads to the unopposed depolymerization of F-actin by blocking of polymerization at the barbed end of actin filaments, similarly reduced membrane projections while the membrane localization of pleckstrin-2 remained intact (not shown).
Figure 4.
Cellular spreading of pleckstrin-2, but not membrane localization, is dependent on the actin cytoskeleton. Jurkat cells expressing GFP fused to the amino-terminus of wild-type pleckstrin-2 were plated on OKT3. When indicated, 1 μM final concentration latrunculin A was added to the chamber slide. Shown is a cell expressing pleckstrin-2 before and after inhibition of the actin cytoskeleton with latrunculin A for 60 seconds. This figure shows that wild-type pleckstrin-2 associates with membrane projections in cells spread on fibronectin, and this membrane localization remains despite the disruption in cellular spreading by the addition of latrunculin A. Similar results were seen when the actin cytoskeleton was disrupted with cytochalasin D. Images were captured using an UtraVIEW-LCI confocal scanner at × 100 magnification.
Taken together, these data demonstrate that in Jurkat cells, pleckstrin-2 translocates to the cell membrane and induces matrix-dependent lamellipodia formation and cell spreading in a T-cell receptor– or integrin-stimulated fashion. Because the distribution of pleckstrin-2 is not directly influenced by filamentous actin assembly, it is likely upstream to signaling pathways involved in F-actin assembly. We demonstrate that these signaling events require the contribution of all 3 pleckstrin-2 motifs.
Pleckstrin-2 expression in Jurkat cells enhances α4β1 clustering and adhesion to fibronectin
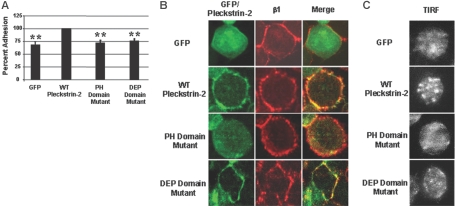
In general, the binding of T cells to fibronectin is very weak unless the cells are preactivated by stimulation through the T-cell receptor (inside-out signaling) or the addition of PMA. To further demonstrate that pleckstrin-2 is affecting integrin avidity/affinity in the absence of inside-out signaling, we demonstrate in Figure 5A that Jurkat cells expressing wild-type pleckstrin-2 are significantly more adherent to fibronectin compared with Jurkat cells expressing GFP alone, the double PH domain mutant, or the DEP domain mutant. We also demonstrate colocalization of pleckstrin-2 and α4β1 by confocal microscopy in Figure 5B. As expected, this colocalization is not seen in Jurkat cells expressing the double PH domain mutant. We used total internal reflection fluorescent (TIRF) imaging of Jurkat cells expressing each of the pleckstrin-2 constructs. Jurkat T cells expressing wild-type pleckstrin-2 show α4β1 clustering when plated on fibronectin. This degree of clustering is not seen in Jurkat cells expressing GFP alone or the double PH domain mutant (Figure 5C). There appear to be focal contacts in the Jurkat cells expressing the DEP domain mutant, but these appear to be quantitatively and qualitatively different from cells expressing wild-type pleckstrin-2. These results demonstrate that pleckstrin-2 colocalizes with α4β1 and, through a pathway dependent on its PH domains, enhances integrin clustering.
Figure 5.
Expression of pleckstrin-2 in Jurkat cells stimulates clustering of α4β1 and enhances adhesion to fibronectin. (A) Jurkat cells expressing GFP alone, WT pleckstrin-2, the PH domain mutant, or the DEP domain mutant were plated on fibronectin. Jurkat-cell adhesion was quantitated by measuring the activity of acid phosphatase 32. Shown is the mean ± SEM of 6 independent experiments. The paired Student t test was performed of the transfectants compared with WT pleckstrin-2. *P < .005 (B) GFP-fused pleckstrin-2 constructs (green) were plated on fibronectin, fixed, stained with a monoclonal antibody to the integrin β1 subunit (red), and analyzed by indirect immunofluorescence. The merger of the red and green fluorescence to yellow demonstrates that pleckstrin-2 colocalizes with β1. Simultaneous mutations in both PH domains prevented pleckstrin-2 from colocalizing with β1. Images were captured using an UtraVIEW-LCI confocal scanner at ×100 magnification. Data shown are representative of 3 independent experiments. (C) The same GFP-fused pleckstrin-2 constructs, stained with a monoclonal antibody to the integrin β1 subunit, were plated on fibronectin and analyzed using total internal reflection fluorescence (TIRF). Cells transfected with WT pleckstrin-2 demonstrate enhanced β1 clustering at the point of adhesion to fibronectin. Images were captured using a Hamamatsu digital camera and Metamorph software at × 60 magnification. Data shown are representative of 3 independent experiments.
Disruption of PI3K inhibits membrane association of pleckstrin-2
The biochemical data show an affinity of pleckstrin-2 for phospholipids with phosphates in the D3 or D5 position. However, the assay used does not discriminate well between low or high binding affinities. Activation of the T-cell receptor or ligation of integrins often results in membrane recruitment of proteins containing PH domains.10,37,38 Activation of these receptors leads to stimulation of PI3K and generation of D3 phosphoinositides while having relatively little effect on the concentration of D5 phosphoinositides. Furthermore, generation of D3 phosphoinositides by PI3K are generated in a time course comparable to that of the membrane binding of pleckstrin-2. If pleckstrin-2 prefers D3 phosphoinositides, we reasoned that pharmacologic inhibition of PI3K might disrupt the ability of pleckstrin-2 to associate with the cell membrane. Consistent with this hypothesis, using real-time confocal imaging of GFP-labeled pleckstrin-2 in Jurkat cells we found that approximately 2 minutes after the addition of the PI3K pharmacologic inhibitors, wortmannin (Figure 6A; Video S2) or LY294002 (not shown), the association of pleckstrin-2 with the cell membrane was disrupted. A similar PI3K-dependent localization of pleckstrin-2 was also observed with LY294002 in another T-cell line, 2B4 cells (Video S3), and in Cos 7-SH cells (not shown). Although our dot blot experiment (Figure 1) implies that pleckstrin-2 might bind to either D3 or D5 phosphoinositides, our experiments within cells suggest that D3 phospholipid products of PI3K are the preferred in vivo substrate.
Pleckstrin-2–induced spreading is PI3K dependent
We observed that mutations that disrupt the membrane association of pleckstrin-2 also disrupt cell spreading. Therefore, we reasoned that pharmacologic disruption of PI3K may not only affect the ability pleckstrin-2 to bind to the cell membrane but may also inhibit its ability to induce cell spreading. Jurkat cells expressing either GFP or GFP fused to pleckstrin-2 were plated on fibronectin. Cell-footprint size was quantitated in the presence or absence of a 5-minute exposure to wortmannin (Figure 6B). Pharmacologic inhibition of PI3K had no effect on the footprint size of the cells that expressed the control protein GFP. In contrast, expression of pleckstrin-2 increased the footprint size of the cell, and this effect was completely ablated by pharmacologic inhibition of PI3K. Figure 6 demonstrates that both the membrane association and cell spreading of pleckstrin-2 are PI3K dependent. This indicates that pleckstrin-2 is situated downstream of PI3K in a signaling pathway that contributes to cell spreading.
Pleckstrin-2 colocalizes with actin at the immune synapse
In response to the interaction with an appropriate antigen-presenting cell, T cells undergo dramatic shape changes to form a contact site enriched in F-actin and T-cell signaling molecules, termed the immune synapse, which is important to stabilize cell-cell adhesion and facilitate sustained signaling events.39,40 While much has been learned about upstream signaling pathways, the immediate effectors controlling actin dynamics are still poorly understood. Because our data implicate pleckstrin-2 in a signaling pathway downstream to PI3K and upstream to F-actin assembly upon T-cell receptor engagement, we speculated that pleckstrin-2 participates with signaling complexes localized at the immune synapse that contribute to the formation of stable F-actin structures.
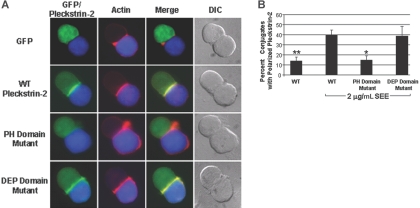
As a first step toward understanding the role of pleckstrin-2 in T-cell activation, we examined its distribution in T cells responding to antigen-presenting cells. As shown in Figure 7, pleckstrin-2 colocalized with newly polymerized F-actin at the immune synapse in Jurkat T cells interacting with superantigen-pulsed Raji B cells. Under the conditions studied here, both T-cell receptor and CD28 signaling are engaged by ligands on the Raji cells. Recruitment of pleckstrin-2 to the cell-cell contact site in the absence of antigen was minimal, as was the accumulation of F-actin under these conditions (data not shown). Pleckstrin-2 localization is dependent on its PH domains, but not the DEP domain, because the double PH domain mutant failed to associate with actin at the immune synapse. Thus, we demonstrate that T-cell activation induces recruitment of pleckstrin-2 to the immune synapse where it colocalizes with F-actin. These data suggest the involvement of pleckstrin-2 in mechanisms that link T-cell receptor signaling to actin cytoskeletal changes required for immune synapse formation.
Figure 7.
Pleckstrin-2 colocalizes with F-actin at the immune synapse. (A) Conjugates were formed between SEE-pulsed Raji B cells stained with CMAC-7 (blue) and Jurkat T cells transfected with the various GFP-fused pleckstrin-2 constructs (green). The fixed conjugates were then stained with rhodamine-phalloidin (red) to label F-actin and analyzed by indirect immunofluorescence at 63× magnification. The merger of the red and green fluorescence to yellow demonstrates that pleckstrin-2 colocalizes with actin at the immune synapse. Simultaneous mutations in both PH domains prevented pleckstrin-2 from colocalizing with actin. Note that the cells are plated on poly-L-lysine and do not demonstrate membrane localization of pleckstrin-2. Data shown are from 1 representative experiment of 3 independent experiments. (B) Colocalization of pleckstrin-2 and actin was quantified by randomly selecting conjugates containing a green T cell contacting a blue B cell. Shown is the mean ± standard deviation of 3 independent experiments. The paired Student t test was performed of WT without SEE stimulation or the pleckstin-2 mutants compared with WT pleckstrin-2. *P < .05; **P < .005.
Discussion
Signals from the environment modulate cellular shape and movement through large protein assemblies at the plasma membrane that link to the actin cytoskeleton. This includes regulation of proteins downstream from PI3K, such as the Ena/VASP protein family and the Arp2/3 complex that directly control actin polymerization.41,42 Both of these sets of proteins regulate cell protrusions such as lamellipodia and filopodia, which are important for cellular shape change and spreading. In this paper we identify distinct differences in the activation and signaling between pleckstrin-1 and pleckstrin-2 and demonstrate a novel mechanism by which pleckstrin-2, through the intracellular interactions of its individual domains, functions as a signal adaptor to modulate actin-dependent events.
Phosphorylation of pleckstrin-1 by PKC is an early marker of platelet and leukocyte activation and is a critical step in pleckstrin-1 activation.5,43 When expressed in cell lines, pleckstrin-1 causes rearrangements of the cytoskeleton and morphologic changes such as membrane ruffles and cell spreading. These effects are dependent on phosphorylation of pleckstrin and the N-terminal PH (N-PH) domain.7 In contrast, pleckstrin-2 lacks PKC substrate consensus sites and does not become phosphorylated after PMA stimulation of fibroblast lines.25 We have previously reported that pleckstrin-2 is capable of inducing actin changes within fibroblasts.25 We have now found that overexpression of pleckstrin-2 in Jurkat and 2B4 T cells induces adhesion and substrate-specific cell spreading through a pathway that requires the phospholipid binding pocket of both PH domains as well as the DEP domain. The different substrate specificity profile between pleckstrin-1 30 and pleckstrin-2 (Figure 1) and the requirement for PKC-mediated phosphorylation of pleckstrin-1 implies a distinct mechanism of activation and regulation between the 2 pleckstrin isoforms.
Our data demonstrate that in the presence of serum, pleckstrin-2 associates with the Jurkat-cell membrane and induces cell spreading, only after stimulation of the T-cell antigen receptor or α4β1, the fibronectin receptor. Furthermore, our results using live cells, along with our biochemical data, demonstrate an affinity of pleckstrin-2 for D3 phosphoinositides and provide both in vivo and in vitro evidence that pleckstrin-2 is downstream of PI3K. This implies that pleckstrin-2 is an effector for PI3K (an enzyme activated by both the T-cell antigen and integrin receptors) and suggests a model in which pleckstrin-2 is regulated by PI3K-generated membranebound phospholipids that recruit pleckstrin-2 to the cell membrane.
Signaling through either α4β1 or the T-cell receptor induces distinct morphologic changes and rearrangement of the actin cytoskeleton in Jurkat cells, many of which are mediated by PI3K.37 We have demonstrated that pleckstrin-2, as an effector of PI3K, enhances Jurkat-cell spreading through both α4β1 and the T-cell receptor. This spreading is indeed influenced by filamentous actin assembly. Krause et al have identified lamellipodin, a PH domain–containing protein that binds to and colocalizes with Ena/VASP proteins exclusively at the tips of filopodia and lamellipodia.42 The PH domain of lamellipodin binds specifically to phosphatidylinositol 4,5-bisphosphate and is sufficient for plasma membrane recruitment upon treatment with PDGF. Lamellipodin overexpression produces faster lamellipodial protrusions similar to those observed when Ena/VASP proteins are overexpressed or artificially targeted to the plasma membrane. In contrast, knockdown of lamellipodin expression results in impairment of lamellipodia formation, a phenotype more severe than that caused by loss of all Ena/VASP proteins. An important finding from our data is the attenuation of lamellipodia in the pleckstrin-2 DEP domain (Figure 3C). Therefore, we propose that in Jurkat cells pleckstrin-2 is situated similarly to lamellipodin, where it localizes to the membrane via its PH domains and interacts with the cytoskeletal machinery via its DEP domain to produce membrane protrusions in the form of lamellipodia.
SWAP-70 is another PH-domain–containing protein that has affinity for D3 phophoinositides. Like pleckstrin-2, SWAP-70 associates with the cell membrane and stimulates cytoskeletal changes through a signaling pathway involving PI3K products.44 Through a mechanism that is still to be fully defined, SWAP-70 promotes formation of the GTP-bound, activated form of the GTPase Rac, a central molecular switch in a variety of signaling pathways. The only close homolog of SWAP-70 is a protein called Def-6, SLAT, or IBP,45–47 which is reported to function in T-cell signaling.45,47 IBP is a novel guanine nucleotide exchange factor (GEF) for Rac1 and Cdc42 in T lymphocytes, which is recruited to the immune synapse upon engagement of the antigen receptor and demonstrates enhanced GEF activity by tyrosine phosphorylation and by binding newly generated phosphatidylinositol 3,4,5-trisphosphate.48 Interestingly, activation of Rac1 has been shown to result in the assembly of lamellipodia whereas activation of Cdc42 results in the assembly filopodia.49 We have demonstrated that pleckstrin-2–induced lamellipodia formation through α4β1 or the T-cell receptor is dependent upon its DEP domain. Although the structure of the DEP domain of pleckstrin-2 has been determined,50 a definitive ligand for this motif is unknown. We speculate that in lymphocytes, pleckstrin-2 cooperates with regulators of the actin cytoskeleton, such as Rac or Cdc42, via its DEP domain to transduce signals from α4β1 or the T-cell receptor into cytoskeletal dynamics and in turn plays a critical role in cellular spreading. We have previously demonstrated that dominant negative Rac1 will disrupt some of the actin changes induced by pleckstrin-1.26 We have not demonstrated any in vitro exchange activity for pleckstrin-1 or pleckstrin-2 (C.S.A, unpublished observation, May 2000), and this remains an area of active investigation in our laboratory.
It has been well documented that the productive interaction of a T cell with an antigen-presenting cell results in the profound reorganization of receptors and signaling molecules leading to the formation of the immune synapse.39 Remodeling of the actin cytoskeleton is fundamental to the achievement of this 3-dimensional structure. We now show that pleckstrin-2 is recruited to the immune synapse in a PH domain–specific manner. It is possible that the phospholipid binding pocket created by the PH domains enables the DEP domain to interact with downstream regulators of the actin cytoskeleton. Determining the interaction of the PH and DEP domains of pleckstrin-2 with the cellular membrane and cytoskeleton provides an important step toward understanding the role pleckstrin-2 in these cellular processes. Although our data suggest that pleckstrin-2 lies upstream of actin remodeling in cellular spreading and is recruited to the immune synapse, none of our mutants had a direct effect on actin assembly at the immune synapse. The role of the DEP domain in lamellipodia formation suggests that pleckstrin-2 may indeed have an effect on actin polymerization at the immune synapse, which may only be revealed on closer analysis of immune synapse architecture and the ability of T cells to respond to physiologic levels of antigen in vivo. It is also possible that the pleckstrin-2 DEP domain plays a more fundamental role in other actin-dependent T-cell processes, such as extravasation and migration. Because it is well established that costimulatory signaling via CD28 is required for optimal activation of PI3K at the immune synapse,51,52 another interesting question for future investigation will be the extent to which signaling via pleckstrin-2 is dependent on costimulatory interactions.
In conclusion, these experiments point to fundamental similarities and differences between the 2 pleckstrin isoforms. Both isoforms can induce membrane extensions and promote cellular spreading, but pleckstrin-1 is entirely regulated by its phosphorylation by PKC. Once phosphorylated, its amino-terminal PH domain can bind to many readily available phospholipids, thereby allowing pleckstrin-1 to associate with the cell membrane and promote cytoskeletal changes. In contrast, pleckstrin-2 is not regulated by its own phosphorylation but rather by the local generation of PI3K-phosphorylated phospholipids. We propose that in lymphocytes, the coordinated function of the individual pleckstrin-2 domains enables it to bind to membrane-associated phosphatidylinositols, such as those generated by PI3K, to organize the actin cytoskeleton and to promote lymphocyte spreading.
Supplementary Material
Acknowledgments
This work was supported in part by funds from the National Institutes of Health (NIH).
We gratefully acknowledge Qing-Min Chen for technical assistance and Alexa D. Davis for assistance with manuscript preparation. We thank the Wistar Institute Flow Cytometry Facility for help with the development of stable pleckstrin-2 transfectants. We also thank Lurong Lian and Xinsheng Chen for many helpful discussions.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.L.B. designed and performed research, analyzed data, and wrote the paper; W.T.K., Y.W., E.M.B., P.K., E.L.W., and R.S.M. performed research and analyzed data; and E.K.W., E.M.O., J.K.B., G.A.K., M.J.B., and C.S.A. offered advice on experimental design, data analysis, and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles S. Abrams, Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA 19104; e-mail: abrams@mail.med.upenn.edu.
References
- 1.Haslam RJ, Lynham JA, Fox JE. Effects of collagen, ionophore A23187 and prostaglandin E1 on the phosphorylation of specific proteins in blood platelets. Biochem J. 1979;178:397–406. doi: 10.1042/bj1780397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyers M, Rachubinski RA, Stewart MI, et al. Molecular cloning and expression of the major protein kinase C substrate of platelets. Nature. 1988;333:470–473. doi: 10.1038/333470a0. [DOI] [PubMed] [Google Scholar]
- 3.Ponting CP, Bork P. Pleckstrin's repeat performance: a novel domain in G-protein signaling? Trends Biochem Sci. 1996;21:245–246. [PubMed] [Google Scholar]
- 4.Lemmon MA, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 5.Abrams CS, Zhao W, Belmonte E, Brass LF. Protein kinase C regulates pleckstrin by phosphorylation of sites adjacent to the N-terminal pleckstrin homology domain. J Biol Chem. 1995;270:23317–23321. doi: 10.1074/jbc.270.40.23317. [DOI] [PubMed] [Google Scholar]
- 6.Abrams CS, Zhang J, Downes CP, Tang X, Zhao W, Rittenhouse SE. Phosphopleckstrin inhibits gbetagamma-activable platelet phosphatidylinositol-4,5-bisphosphate 3-kinase. J Biol Chem. 1996;271:25192–25197. doi: 10.1074/jbc.271.41.25192. [DOI] [PubMed] [Google Scholar]
- 7.Ma AD, Brass LF, Abrams CS. Pleckstrin associates with plasma membranes and induces the formation of membrane projections: requirements for phosphorylation and the NH2-terminal PH domain. J Cell Biol. 1997;136:1071–1079. doi: 10.1083/jcb.136.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemmon MA, Ferguson KM. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J. 2000;350(pt 1):1–18. [PMC free article] [PubMed] [Google Scholar]
- 9.Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature. 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 10.Lemmon MA, Ferguson KM. Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem Soc Trans. 2001;29:377–384. doi: 10.1042/bst0290377. [DOI] [PubMed] [Google Scholar]
- 11.Balla T, Bondeva T, Varnai P. How accurately can we image inositol lipids in living cells? Trends Pharmacol Sci. 2000;21:238–241. doi: 10.1016/s0165-6147(00)01500-5. [DOI] [PubMed] [Google Scholar]
- 12.Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Luby-Phelps K, Das B, et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 14.Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinasegammaa Rac guanosine exchange factor and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 16.Burchett SA. Regulators of G protein signaling: a bestiary of modular protein binding domains. J Neurochem. 2000;75:1335–1351. doi: 10.1046/j.1471-4159.2000.0751335.x. [DOI] [PubMed] [Google Scholar]
- 17.Sokol S. A role for Wnts in morpho-genesis and tissue polarity. Nat Cell Biol. 2000;2:E124–E125. doi: 10.1038/35017136. [DOI] [PubMed] [Google Scholar]
- 18.Lishko PV, Martemyanov KA, Hopp JA, Arshavsky VY. Specific binding of RGS9-Gbeta 5L to protein anchor in photoreceptor membranes greatly enhances its catalytic activity. J Biol Chem. 2002;277:24376–24381. doi: 10.1074/jbc.M203237200. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman GA, Garrison TR, Dohlman HG. Endoproteolytic processing of Sst2, a multidomain regulator of G protein signaling in yeast. J Biol Chem. 2000;275:37533–37541. doi: 10.1074/jbc.M005751200. [DOI] [PubMed] [Google Scholar]
- 22.Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9–1. Proc Natl Acad Sci U S A. 2002;99:9755–9760. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patikoglou GA, Koelle MR. An N-terminal region of Caenorhabditis elegans RGS proteins EGL-10 and EAT-16 directs inhibition of G(alpha)o versus G(alpha)q signaling. J Biol Chem. 2002;277:47004–47013. doi: 10.1074/jbc.M208186200. [DOI] [PubMed] [Google Scholar]
- 24.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 25.Hu MH, Bauman EM, Roll RL, Yeilding N, Abrams CS. Pleckstrin 2, a widely expressed paralog of pleckstrin involved in actin rearrangement. J Biol Chem. 1999;274:21515–21518. doi: 10.1074/jbc.274.31.21515. [DOI] [PubMed] [Google Scholar]
- 26.Ma AD, Abrams CS. Pleckstrin induces cytoskeletal reorganization via a Rac-dependent pathway. J Biol Chem. 1999;274:28730–28735. doi: 10.1074/jbc.274.40.28730. [DOI] [PubMed] [Google Scholar]
- 27.Roll RL, Bauman EM, Bennett JS, Abrams CS. Phosphorylated pleckstrin induces cell spreading via an integrin-dependent pathway. J Cell Biol. 2000;150:1461–1466. doi: 10.1083/jcb.150.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley JL, Mao M, Kobayashi S, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 30.Kavran JM, Klein DE, Lee A, et al. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 31.Boerth NJ, Judd BA, Koretzky GA. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J Biol Chem. 2000;275:5143–5152. doi: 10.1074/jbc.275.7.5143. [DOI] [PubMed] [Google Scholar]
- 32.Bellavite P, Andrioli G, Guzzo P, et al. A colorimetric method for the measurement of platelet adhesion in microtiter plates. Anal Biochem. 1994;216:444–450. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
- 33.Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–774. doi: 10.1034/j.1600-0854.2001.21104.x. [DOI] [PubMed] [Google Scholar]
- 34.Cannon JL, Labno CM, Bosco G, et al. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 35.Gomez TS, Hamann MJ, McCarney S, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 36.Dowler S, Currie RA, Campbell DG, et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyduk SJ, Cybulsky MI. Alpha 4 integrin signaling activates phosphatidylinositol 3-kinase and stimulates T cell adhesion to intercellular adhesion molecule-1 to a similar extent as CD3, but induces a distinct rearrangement of the actin cytoskeleton. J Immunol. 2002;168:696–704. doi: 10.4049/jimmunol.168.2.696. [DOI] [PubMed] [Google Scholar]
- 38.Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 39.Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 40.Kupfer A, Kupfer H. Imaging immune cell interactions and functions: SMACs and the immunological synapse. Semin Immunol. 2003;15:295–300. doi: 10.1016/j.smim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Lafuente EM, van Puijenbroek AA, Krause M, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Krause M, Leslie JD, Stewart M, et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7:571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Tyers M, Haslam RJ, Rachubinski RA, Harley CB. Molecular analysis of pleckstrin: the major protein kinase C substrate of platelets. J Cell Biochem. 1989;40:133–145. doi: 10.1002/jcb.240400202. [DOI] [PubMed] [Google Scholar]
- 44.Shinohara M, Terada Y, Iwamatsu A, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Lee A, Hu C, et al. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 46.Hotfilder M, Baxendale S, Cross MA, Sablitzky F. Def-2, -3, -6 and -8, novel mouse genes differentially expressed in the haemopoietic system. Br J Haematol. 1999;106:335–344. doi: 10.1046/j.1365-2141.1999.01551.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y, Bi K, Kitamura R, et al. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 48.Gupta S, Fanzo JC, Hu C, et al. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J Biol Chem. 2003;278:43541–43549. doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 49.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 50.Civera C, Simon B, Stier G, Sattler M, Macias MJ. Structure and dynamics of the human pleckstrin DEP domain: distinct molecular features of a novel DEP domain subfamily. Proteins. 2005;58:354–366. doi: 10.1002/prot.20320. [DOI] [PubMed] [Google Scholar]
- 51.Pages F, Ragueneau M, Rottapel R, et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 52.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.