Abstract
Background
Although the effectiveness of statins is well established, analyses of spontaneous adverse event reports have recently questioned the safety of rosuvastatin.
Methods and Results
We evaluated the risks and benefits of rosuvastatin and compared it with other statins presently on the market. Information was obtained from a search of medical and scientific literature that produced 3001 entries, of which 591 publications containing particularly relevant data were identified, and from the US Food and Drug Administration (FDA) Adverse Events Reporting System (AERS) and Spontaneous Reporting System through June 30, 2004. For the AERS data and to control for overreporting in the first postmarketing year and the effect on reporting due to the withdrawal of cerivastatin in 2001, we used the rate of a given adverse event among all adverse events as a measure of risk. We found that adverse effects of rosuvastatin in skeletal muscle, liver, and kidney function did not substantially differ in frequency from those reported for those of other statins in the market in 2004, except for the uncommon development of a mild form of presumably “tubular” proteinuria at doses of 40 mg/day or greater. In contrast, cerivastatin had significantly higher rates of myopathy and rhabdomyolysis than rosuvastatin's, but there was no additional effect on renal failure beyond that mediated through rhabdomyolysis. From our literature review, we found that rosuvastatin reduces abnormal lipids on a milligram-per-milligram comparison more than atorvastatin.
Conclusion
We conclude that rosuvastatin at approved doses incurs no greater risk for adverse events than other marketed statins, except for a mild form of tubular proteinuria when doses at or above the maximum recommended levels (≥ 40 mg/day) were administered. Its risk-benefit ratio is acceptable when compared with other statins on the market in 2006.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eyes only or for possible publication via email: glundberg@medscape.net
Condensed Abstract
This study evaluated the safety of rosuvastatin. Information was obtained from a search of medical and scientific literature, and from the US Food and Drug Administration (FDA) Adverse Events Reporting System (AERS) and Spontaneous Reporting System through June 30, 2004. We found that adverse effects of rosuvastatin in skeletal muscle, liver, and kidney function did not substantially differ in frequency from those reported for other statins, except for the uncommon development of a mild form of presumably “tubular” proteinuria at doses of 40 mg/day or greater. We conclude that rosuvastatin at approved doses incurs no greater risk for adverse events than other marketed statins.
Introduction
Two previous analyses of the FDA database of the Adverse Events Reporting System (AERS) have questioned the safety of rosuvastatin. Allegations were made that patients taking low doses of rosuvastatin were at greater risk of developing serious kidney damage, kidney failure, and rhabdomyolysis than those taking other statins.[1] These concerns were expressed in a Public Citizen petition to the FDA on March 4, 2004 calling for its removal from the market. That petition was denied after an FDA review of all available evidence.[2]
The issue of rosuvastatin safety has been raised again in the recent report by Alsheikh-Ali and colleagues,[3] which concluded that rosuvastatin was significantly more likely to be associated with the composite adverse endpoints of rhabdomyolysis, proteinuria, nephropathy, or renal failure on the basis of analysis of the FDA's spontaneous adverse event reports data. It has been emphasized that although spontaneous adverse event reports are useful for identifying signals of drug toxicity, they are also subject to multiple limitations and biases.[4] It is well accepted that adverse event report rates rise during the first years after launch, reflecting increased exposure and heightened awareness and interest in identifying the adverse event profile of a new drug. For a safe drug, the spontaneous adverse event reports decline thereafter, due to decreased reporting as adverse events are appropriately characterized and methods to control them are established (the “Weber effect”). In this context, when recognition of adverse events for a class of related drugs takes place over multiple calendar years, comparisons at years when drugs were first launched are biased toward higher rates for those drugs introduced later. Furthermore, drugs introduced after a member of the class has been withdrawn are likely to be subject to overreporting of adverse events due to enhanced awareness on the part of physicians and patients.[3] Pooling different endpoints, as done by Alsheikh-Ali and colleagues,[3] further compounds the impact of these limitations.
We present an independent analysis of the FDA's spontaneous adverse event data with an approach to control for reporting biases. To compensate for overreporting of adverse events for the more recently introduced statins, we used the total number of reports of adverse events as the denominator for the rates of adverse events of interest (eg, rhabdomyolysis). If both serious and nonserious adverse events are overreported by the same factor for a newly introduced statin relative to the existing ones, our analysis will provide valid comparisons. In contrast, analysis of rates with prescriptions as the denominator will be biased toward higher rates for the newly introduced statins. In addition, we present data on the efficacy of rosuvastatin to reduce abnormal lipids.
Methods
Adverse Event Data: Sources and Definitions
A medical and scientific literature search related to the safety of rosuvastatin and other statins produced 3001 entries, of which 591 publications containing particularly relevant data were identified. Rosuvastatin was first marketed in The Netherlands on November 6, 2002 and on July 19, 2003 in the United States. Up to June 2004, global market exposure was estimated to be 1.8 million patients and 7.7 million prescriptions.
Information was obtained from the FDA AERS and Spontaneous Reporting System through June 30, 2004. These data, compiled since 1969, are now contained in the AERS database, with the MedDRA (Medical Dictionary for Regulatory Activity) 7.0 dictionary for reaction terms. Our analysis, which was done in collaboration with DrugLogic, Inc. (Reston, Virginia), used Qscan-FDA, a DrugLogic software database system.
Direct MedDRA preferred terms (PTs), eg, “rhabdomyolysis,” were used as well as logical groupings of terms permitted by Qscan. The terms that were used represent groupings of PTs relating to renal disease, muscle disease, and liver toxicity, designed by team members with appropriate expertise. Care was taken to differentiate event reports from cases; case counts with multiple events received a single entry.
The analysis encompassed data on atorvastatin (Lipitor), simvastatin (Zocor), pravastatin (Pravachol), lovastatin (Mevacor), fluvastatin (Lescol), cerivastatin (Baycol), and rosuvastatin (Crestor) to determine relationships between adverse event report rates with rosuvastatin as the reference. Because cerivastatin was withdrawn from the market in 2001, it played the role of a “positive control,” with the other statins playing the role of “equivalence controls” in the analysis.
Because the statins were introduced into the market at different times spanning the period from 1987 to 2003, comparisons of adverse events at the years when the statins were launched are subject to different levels of recognition of adverse events. Therefore, our analysis compared rosuvastatin with the other statins concurrently in the year from July 2003 to June 2004 when rosuvastatin was introduced in the market. In addition, the adverse events reported for rosuvastatin from July 2003 to June 2004 were compared with those for the other statins in each of the previous 4 years between July 1999 and June 2003. Even though cerivastatin was withdrawn from the market in August 2001, adverse events continued to be reported to the FDA, up to the final year of this analysis.
Statistical Methods
The key data for a particular adverse event (eg, rhabdomyolysis) in a given period were the number of reports for that event, denoted by a1, a2, a3, a4, a5, and a6 for atorvastatin, simvastatin, pravastatin, lovastatin, fluvastatin, and cerivastatin, respectively; and a0 for rosuvastatin. The corresponding data for the total number of reports for all adverse event categories in the same given period are denoted by n1, n2, n3, n4, n5, n6, and n0, respectively. The simplest measure of frequency of an adverse event is given as the ratio (a/n) × 100, representing the percentage of a given adverse event among all adverse events reported for a given statin in a given period.
To compare the statins with rosuvastatin as the reference, we use the rate ratio (RRi = [ai/ni]/[a0/n0]) so that RR0 for rosuvastatin is 1. When RRi is greater than 1, the percentage of an adverse event of interest among all adverse event reports for a statin will have had more adverse event reports of interest than rosuvastatin, whereas if RRi is less than 1, it will have had less. Determination of RRi being significantly different than 1 was accomplished by the score test under the assumption that ai followed a Poisson distribution with expected value directly proportional to ni.
Results
Clinical Safety of Rosuvastatin
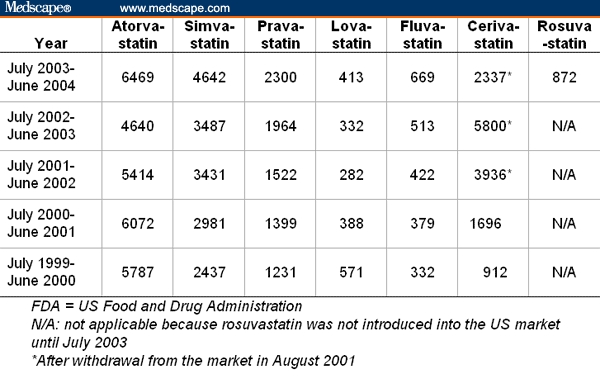
Table 1 provides the denominators for our analysis of the FDA AERS database. They are the total number of adverse events reported to the FDA for each of the 7 statins of interest and in each of the 5 calendar years from July 1999 to June 2004. The substantial increase of the total number of adverse events for cerivastatin in the 2 years after its withdrawal in August 2001 illustrates the overreporting of spontaneous reports to the FDA due to enhanced awareness, and thus imposes the need to control for overreporting in the analysis. This is the primary reason why we use the total number of reports of adverse events as the denominator for our analysis comparing the frequency of specific adverse events between statins.
Table 1.
Total Number of Adverse Events Reported to the FDA for Statins in Each Year for the Period From July 1999 to June 2000
Adverse Muscle Events
Data from the spontaneous event reports in the FDA AERS database were the primary source for this analysis. However, other data sets were examined as well.
In data obtained from controlled clinical trials, the overall frequency of adverse events associated with rosuvastatin was similar to all other statins (except cerivastatin), namely, 2.5% to 6% of patients complained of muscle symptoms; myopathy was reported in 0.03%; and rhabdomyolysis was rarely mentioned in patients taking rosuvastatin at doses of 40 mg/day or less.[5–13] We also analyzed data collected during the period from drug launch to the end of the most recent postmarketing period available to us (November 6, 2004). Patients were stratified into 3 categories: those with muscle symptoms alone (myalgia), those with myopathy (myalgia plus a significantly elevated creatine kinase [CK] > 10 × upper limit of normal [ULN]), and those with rhabdomyolysis.
Data obtained for the Periodic Safety Report Updates (PSUR) sent to the FDA from AstraZeneca dated February 2003 to December 4, 2004 and included in an FDA Advisory Committee Briefing Document prepared by AstraZeneca[14] were also reviewed and demonstrated that rosuvastatin, even at the highest approved dosage, was associated with a lower prevalence of reports of rhabdomyolysis than other statins, including cerivastatin.[15] The prevalence of rhabdomyolysis at the highest approved dose of rosuvastatin, 40 mg/day, was similar to those reported for the highest approved doses of lovastatin and simvastatin. At lower doses the prevalence of reports of rhabdomyolysis was comparable to those of other statins at similar dose levels. Only 28 of 83 “serious” cases of muscle disease met the American College of Cardiology/American Heart Association (ACC/AHA) criteria for rhabdomyolysis (serum CPK>10 ULN) and even fewer (19 of 83) met the FDA definition (serum CPK >10,000 units), and there were no deaths in the reporting period.[10–15]
With the FDA AERS data, for each year from June 1999 to July 2004, Table 2 shows the ratios of the rates of myopathy of statins relative to the rate of 1.38% myopathies among the 872 total adverse events of rosuvastatin reported in the July 2003-June 2004 period. Multiplication of 1.38% by the table entries provides the rate of myopathy for a given statin in a given year (eg, 1.38 × 1.47 = 2.03% was the rate of myopathy for atorvastatin in July 2003-June 2004). The rates for other statins were higher than rosuvastatin's rate in all except 4 instances (indicated by shaded areas in Table 2). Cerivastatin's ratios were the highest and were all significantly (P < .001) higher than 1 (ie, rates higher than rosuvastatin's). Of the 7 ratios that were significantly (P < .05) different from 1 for statins other than cerivastatin, 6 were greater than 1 (ie, rates higher than rosuvastatin's).
Table 2.
Ratios of Rates of Myopathy* Among All Adverse Events Reported on Statins Relative to the Rate of 1.38% (= 12 of 872) for Rosuvastatin From July 2003 to June 2004
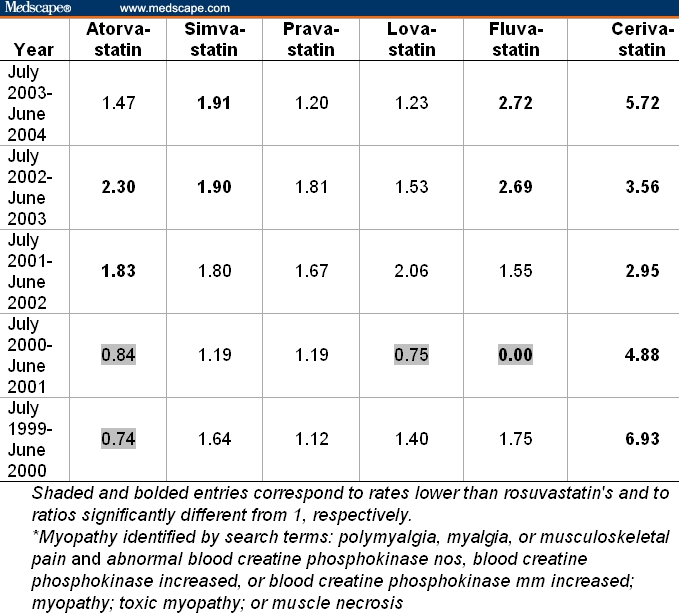
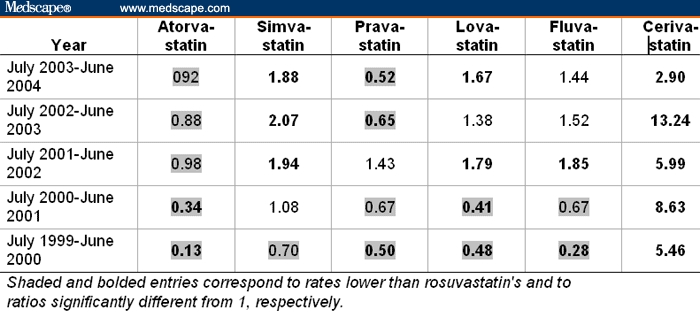
Table 3 shows the ratios of the rates of rhabdomyolysis of statins relative to the rate of 4.36%, which rosuvastatin had among all the 872 total adverse events reported in the July 2003-June 2004 period. Results for rhabdomyolysis show rosuvastatin lying in the middle of rates for the other statins. Specifically, excluding cerivastatin, 14 (56%) of the 25 comparisons had lower rates than rosuvastatin, and among the 14 comparisons that reached statistical significance, 8 (57%) comparisons had lower rates than rosuvastatin. During the 2 years before its withdrawal in 2001, cerivastatin's rates of rhabdomyolysis were dramatically (P < .001) higher than rosuvastatin's rates, 5.46-fold and 8.63-fold higher than the rate reported for rosuvastatin. They continued to rise over the 2 years following the withdrawal of cerivastatin, reaching a 13.24-fold rate (ie, 57.73% = 4.36% × 13.24) in the period from July 2002 to June 2003.
Table 3.
Ratios of Rates of Rhabdomyolysis Among All Adverse Events Reported on Statins Relative to the Rate of 4.36% (= 38 of 872) for Rosuvastatin From July 2003 to June 2004
Adverse Renal Events
We first determined the incidence of acute renal failure associated with rhabdomyolysis in subjects receiving 5- to 40-mg/day doses of rosuvastatin that were included in the clinical trial database.[14] Five patients developed renal failure, none of which were judged to have been drug-associated. Six cases receiving rosuvastatin 80 mg/day (not marketed) developed acute renal failure, 4 of which were attributable to rhabdomyolysis.
As a surrogate for renal injury, changes in serum creatinine levels (and calculated values for the estimated glomerular filtration rate [GFR]) were also analyzed. Serum creatinine levels showed little change from baseline in subjects receiving approved doses of rosuvastatin, as was the case for other statins. Subjects receiving the nonmarketed 80-mg/day dose did experience small increases in serum creatinine, and 2 episodes of acute renal failure (unrelated to rhabdomyolysis) were also observed in a total of 146 patients.[16]
Controlled clinical trials of rosuvastatin also showed that proteinuria, presumed to be of tubular origin, was associated with doses of 40 mg/day or higher. Proportions of rosuvastatin-treated subjects developing proteinuria at any time during follow-up ranged from 0.5% (comparable to placebo, 0.6%) at a dose of 5 mg/day to 2.8% at a dose of 40 mg/day and 16.2% at 80 mg/day (the nonmarketed dose). The maximum proportion observed for the other statins was 1.1% at doses of 20 mg/day of pravastatin or simvastatin. Proteinuria in patients receiving 80 mg/day of rosuvastatin was reversible, decreasing from 16.2% of patients continuing to receive 80 mg/day to 3.2% of those back-titrated to 40 mg/day. However, even in the latter group, the prevalence of proteinuria (1.0% to 1.6%) remained higher than observed in those receiving the 5- to 20-mg/day doses.
Long-term (> 96 weeks of treatment) observations indicated that the proportion of patients with proteinuria at last follow-up was actually lower than the proportion that developed it at any time during the trial.[14] Analyses indicated that the composition of urine proteins was consistent with a tubular form of proteinuria. No other evidence of proximal tubule malfunction was observed, and more than 90% of the samples with proteinuria did not contain myoglobin.
Among 10,449 patients enrolled in controlled trials, 2% (211) were reported to have developed hematuria detected by a dipstick test while receiving rosuvastatin. No dose dependency was evident. The highest prevalence in the comparator statins was 2.8% for simvastatin at 80 mg/day. Patients developing proteinuria appeared to be more likely to also develop hematuria.
Findings were similar in a review of global cumulative spontaneous reports of rates with MedDRA PTs of “renal failure,” “renal failure acute,” and “renal failure chronic” (with or without rhabdomyolysis) as reported in the PSUR data supplied by AstraZeneca. Table 4 depicts the spontaneous reporting rates of renal failure, proteinuria, or hematuria per million prescriptions filled and per million patients stratified by dosage of rosuvastatin (5-40 mg/day). A somewhat higher rate of renal failure events (with or without concomitant rhabdomyolysis) was noted for the highest dosage of rosuvastatin used (40 mg/day) compared with lower doses (5-20 mg/day) (2.0-5.7 times; Table 4). However, by weighted average the total spontaneous reporting rates of renal failure for all dosages of rosuvastatin combined were 4.9 per million prescriptions filled and 16.5 per million patients exposed (data not shown in Table 4). Thus, analysis of the spontaneous adverse event reports did not reveal any overall evidence of renal toxicity, independent of rhabdomyolysis, for rosuvastatin or other statins sufficient to cause acute renal failure or renal impairment.
Table 4.
Global Cumulative Spontaneous Reporting Rates for Renal Adverse Events for Rosuvastatin
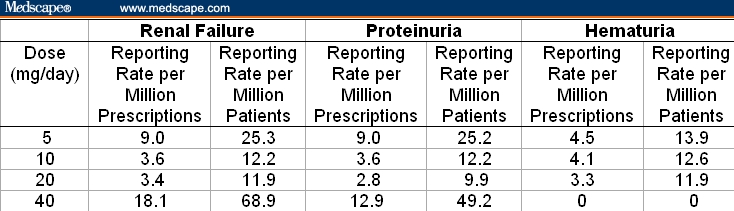
Cumulative spontaneous global reporting rates for hematuria were 3.3-4.5 per million prescriptions filled and 11.9-13.9 per million patients receiving doses of rosuvastatin between 5 and 20 mg/day. These event rates for hematuria are well below that observed in screening studies applied to the population as a whole (about 3%).
Global spontaneous cumulative reporting rates for proteinuria (defined by MedDRA PTs “albumin, urine,” “microalbuminuria,” “protein, urine,” and “proteinuria”) in patients receiving rosuvastatin (Table 4) were variable, but did indicate that rosuvastatin, at doses of 40 mg/day, is associated with a 1.4-4.6 times greater rate of proteinuria (per million prescriptions or patients) compared with lower doses (5-20 mg/day). This observation is quite consistent with data obtained from premarketing clinical trials, which showed a similar association.[5]
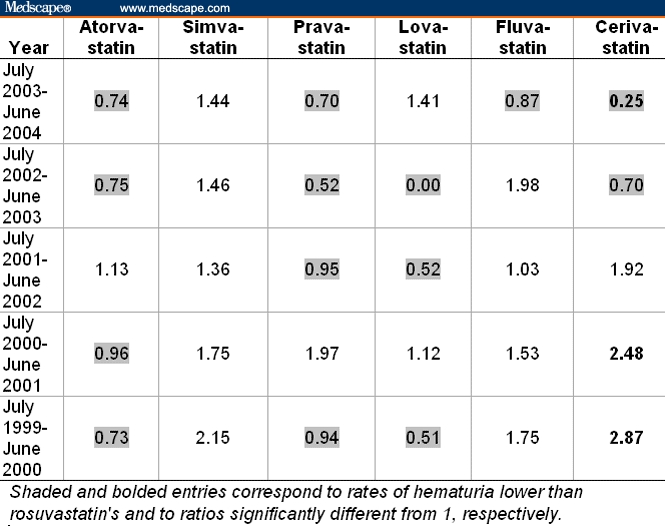
Analysis of the FDA AERS data with the MedDRA PTs “renal failure acute,” “renal failure not otherwise stated (nos),” or “renal impairment nos” revealed that report rates for rosuvastatin from July 2003 to June 2004 were comparable to those for other statins in the same year and previous 4 years (Table 5). Excluding the data for cerivastatin, the rates for the other statins were significantly different from those for rosuvastatin in only 7 out of 25 instances, 2 higher and 5 lower. In contrast, during the 2 years preceding its withdrawal, the rates for cerivastatin were 1.98- to 2.33-fold higher (P < .001) than rosuvastatin's rate. The rate for cerivastatin continued to increase after its withdrawal in August 2001, reaching a rate of 48.8% (= 9.67 × 5.05%) in 2002-2003 and only declining in 2003-2004, 3 years after its withdrawal.
Table 5.
Ratios of Rates of Renal Failure* (Including Patients With Concomitant Rhabdomyolysis) Among All Adverse Events Reported on Statins Relative to the Rate of 5.05% (= 44 of 872) for Rosuvastatin From July 2003 to June 2004
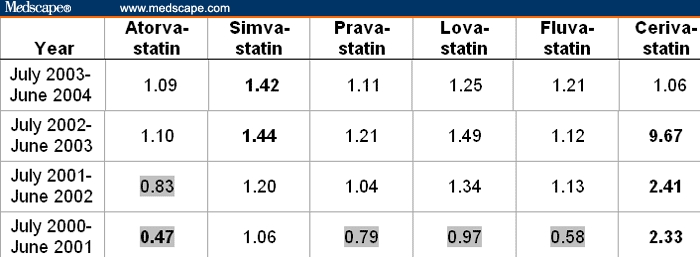
Table 6 shows data for the same group of renal failure but excludes those reports associated with rhabdomyolysis. In this instance, renal adverse events represented 3.21% of all adverse events of rosuvastatin during the July 2003-June 2004 period. The large majority (25 of 30) of reports for other statins were not significantly different (displayed in regular font in Table 6) from the rosuvastatin reference rate. The impact of the well-known association of cerivastatin with rhabdomyolysis (Table 3) is evident in the decline in the cerivastatin reports (Table 5 vs Table 6) when rhabdomyolysis is excluded; thus, cerivastatin's renal toxicities are mediated through rhabdomyolysis. However, when rhabdomyolysis due to the 0.8-mg dose and the deleterious interaction with gemfibrozil (which alone or in combination accounts for about 38% of all reports to the FDA), are eliminated, then the safety profile of cerivastatin approximates that of the other marketed statins.
Table 6.
Ratios of Rates of Renal Failure* Minus Rhabdomyolysis Among All Adverse Events Reported on Statins Relative to the Rate of 3.21% (= 28 of 872) for Rosuvastatin From July 2003 to June 2004
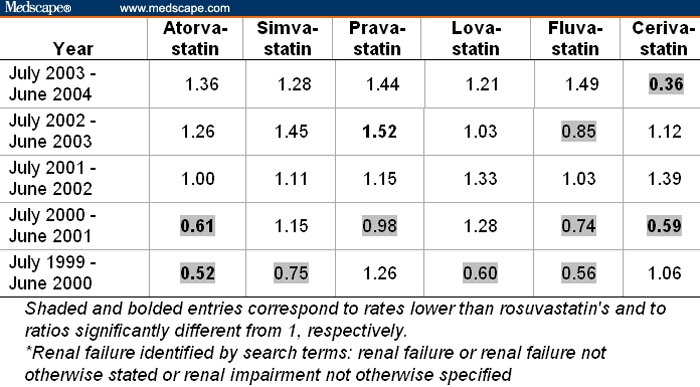
Table 7 summarizes the data for “proteinuria.” Of note, albeit a very low rate of 0.57% (= 5 of 872) of proteinuria for rosuvastatin, among the 30 comparisons, 27 indicated an even lower rate of proteinuria (displayed as shaded entries in Table 7) than that observed with rosuvastatin. This analysis does not contain information with regard to dosage of rosuvastatin or the comparator statin. The pattern seen in these ratio-of-rates comparisons with cumulative global spontaneous reporting rates for proteinuria is consistent with prior observations from premarketing clinical trials.
Table 7.
Ratios of Rates of Proteinuria Among All Adverse Events Reported on Statins Relative to the Rate of 0.57% (= 5 of 872) for Rosuvastatin From July 2003 to June 2004
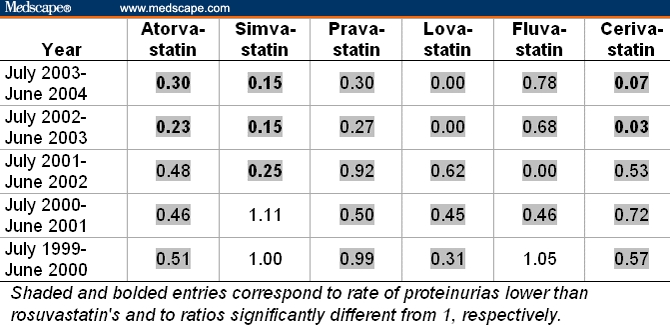
Table 8 summarizes the spontaneous report data for “hematuria.” Here, none of the rates for the other statins, except for cerivastatin, were significantly different from the rosuvastatin reference value of 0.69%; 12 were lower and 13 were higher than rosuvastatin's. In the 2 years preceding its withdrawal, cerivastatin showed significant (P < .05) elevations of “hematuria” relative to rosuvastatin.
Table 8.
Ratios of Rates of Hematuria Among All Adverse Events Reported on Statins Relative to the Rate of 0.69% (= 6 of 872) for Rosuvastatin From July 2003 to June 2004
Liver Adverse Events
Although some patients taking rosuvastatin may experience a slight rise in serum transaminase levels several weeks after therapy is begun, the rise is generally transient and self-limiting.[17] On the basis of evidence from clinical trials, the frequency of transaminase elevation was similar for all available statins and was related to drug dose. Rosuvastatin up to 20 mg/day showed an incidence of persistent transaminase elevation similar to placebo (ie, from 0.2% to 0.4%). Increases of rosuvastatin to 40 mg/day raised the incidence to about 1%, and a nonmarketed dose of 80 mg/day further increases the rate to a level between 1.9% and 2.7%.[10]
Shepherd and colleagues[10] assessed the safety and tolerability of rosuvastatin with data from 12,400 patients (representing 12,212 patient-years of continuous exposure) who received 20-40 mg/day of the drug in a multinational phase 2/3 program, up to August 2003. The occurrence of clinically significant transaminase increases was low and similar across rosuvastatin doses: 0.5% of 1317 patients taking 5 mg/day, 0.1% of 7726 taking 10 mg/day, 0.1% of 3882 taking 20 mg/day, and 0.3% of 3957 taking 40 mg/day. In most cases, the elevations were transient and resolved or improved with continued treatment, with or without downward titration in dose. Clinically significant transaminase elevation occurred in the same proportion of patients (0.2%) in each statin group. Rosuvastatin, 5-40 mg/day, showed changes in transaminase levels similar to those seen with 10-80 mg/day of atorvastatin, 10-80 mg/day of simvastatin, and 10-40 mg/day of pravastatin.[10]
Efficacy of Rosuvastatin
Effects on Plasma Low-Density Lipoprotein Cholesterol
Studies on more than 20,000 patients with various forms of dyslipidemia showed that rosuvastatin and atorvastatin are significantly more effective in reducing plasma low-density lipoprotein (LDL) levels than other statins currently on the market.[5–9] In numerous equal-dose comparisons over the entire range of FDA-approved doses, ie, 5-40 mg/day for rosuvastatin and 5-80 mg/day for atorvastatin, rosuvastatin reduced plasma LDL 3-12% (average 8.5±2.6%) more than atorvastatin (see Table 9).[5,10–15]
Table 9.
Comparison of Rosuvastatin With Atorvastatin: Mean Percentage of Reduction in LDL Induced at Their Maximum FDA-Approved Doses of 40 mg/day for Rosuvastatin and 80 mg/day for Atorvastatin
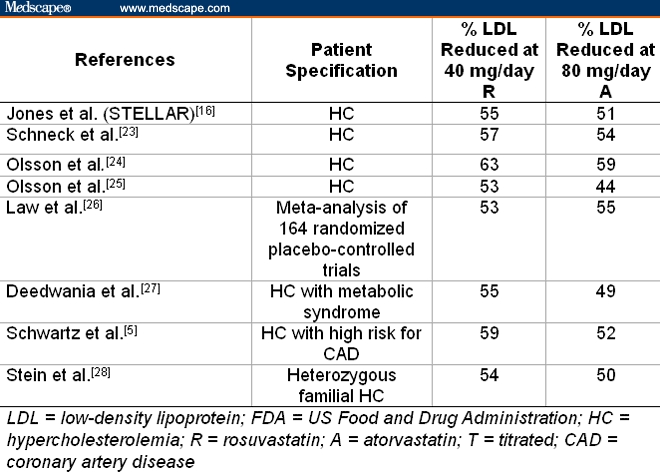
In addition, rosuvastatin, followed by atorvastatin, enabled patients to achieve National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) cholesterol goals (Table 10) better than other statins.[18–22]
Table 10.
Comparison of Rosuvastatin With Atorvastatin: Difference in Percentage of Patients Reaching Their Cholesterol Goals at Equal Doses
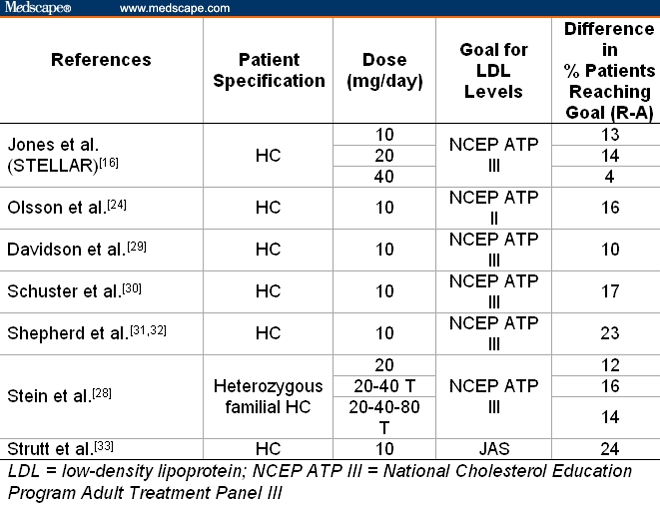
Discussion
Major Findings
Adverse skeletal muscle effects caused by rosuvastatin at currently recommended doses of 5-40 mg/day are rare and do not significantly differ in frequency from those reported for other currently marketed statins. Rosuvastatin does not pose a greater risk for rhabdomyolysis than other currently marketed statins.
In clinical trials, long-term negative effects of rosuvastatin on renal function have not been observed, even though a majority of the enrolled subjects (estimated as 52%) had some degree of preexisting renal impairment. The evidence reviewed does not suggest that use of rosuvastatin, at approved dosage, is causally related to the development of acute renal failure or chronic renal disease. Reported rates of renal failure were low compared with those found in epidemiologic studies in defined geographic populations, and have been stable over time since the launch of rosuvastatin.[15] A suggestion that higher spontaneous reporting rates of renal failure were associated with the highest approved dosage level (40 mg/day) was not corroborated when the rates were compared with other marketed statins (all dosage levels combined). On the other hand, a mild form of proteinuria, presumably of tubular rather than glomerular origin, was observed to occur more frequently with rosuvastatin than with other marketed statins, most likely seen when rosuvastatin was administered in high dosage (40 mg/day and above). This proteinuria did not appear to have any short- or long-term adverse effect on renal function and tended to be transient rather than persistent. No consistent evidence of hematuria as an adverse event of rosuvastatin compared with other stains was observed.
Some patients taking rosuvastatin experience a slight rise in serum transaminase levels several weeks after starting therapy; this is generally transient and self-limiting.[17] Elevations greater than 3 x ULN occur in about 0.5% to 2% of all patients taking statins. Most often, these are also self-limited, and in more than half of patients, the transaminases return to normal within 1 week. In almost all patients, they are normal within 4-6 weeks after discontinuation of treatment, and reports of prolonged elevations after treatment is stopped are rare.
Comparison With Other Published Reports
Our conclusions are at variance with those reported by Alsheikh-Ali and colleagues.[3] We propose that the difference between their conclusions and ours may be attributable to substantial differences in methodology used to analyze the FDA AERS database. Specifically, with rates of adverse events of interest among total number of prescriptions as the denominator, their analysis did not control for overreporting bias in total adverse event reports, which was likely to be even more pronounced for severe adverse events,[3] for rosuvastatin in the first postmarketing year. It is also important that this time frame was 2 years after cerivastatin had been withdrawn from the market because of high report rates of rhabdomyolysis. Indeed, for simvastatin, pravastatin, and atorvastatin, their analysis showed that rates for all adverse events declined from over 200 reports per million prescriptions in their respective first years (1992, 1992, and 1997, respectively) to less than 50 between October 2003 and September 2004. Considering that the rosuvastatin reports were all made during its first year of marketing, a similar decline over time would be expected.
Our analysis measured frequency by calculating the proportion of an adverse event of interest against the total number of adverse events reported for a given drug. It quantified comparisons between drugs by determining relative rates for statins with rosuvastatin as the reference. On the other hand, our analysis was performed without adjustment for significant adverse publicity, such as Public Citizen letters, editorials, and requests for withdrawal to the FDA, as well as wide media coverage and commentary on rosuvastatin. In addition, the 2001 withdrawal of cerivastatin heightened awareness of the potential for adverse events of rosuvastatin. This caused a measurable increase (a factor of 2 or more) in the reporting rate in the first postmarketing year for rosuvastatin relative to the other statins, as seen in the data of Alsheikh-Ali and colleagues[3] in which reports in the first postmarketing year for rosuvastatin were over 500 per million prescriptions, compared with approximately 285, 250, 205, and 250 per million prescriptions for simvastatin, pravastatin, atorvastatin, and cerivastatin, respectively. Had this bias and the one described in the previous paragraph been taken into account in the analysis by Alsheikh-Ali and colleagues,[3] their conclusions would likely have been consistent with the results of our analysis. To overcome limitations of the adverse events data resulting from spontaneous reports, we used the percentage among all reports of an adverse event of interest as the measure of frequency to compare statins' adverse event profiles via relative rates with rosuvastatin as the reference. An alternative method compares the report rate for a given statin with the background rate defined by report rates for all statins except the given statin. In the pharmacoepidemiology literature, this measure is called the proportional rate ratio (PRR). Its calculation rests on an assumption of similar reporting rates for all statins used to define the background rate. Ratios of PRR of a statin to rosuvastatin's PRR were practically identical to those reported here (data not shown), and so were the inferences and conclusions.
Efficacy
When compared on a dosage basis, rosuvastatin appears to be highly potent in achieving beneficial clinical endpoints for lipid management. The greater efficacy means that low doses can be used effectively alone, often as low as 5 mg/day, or, if needed, in combination with other lipid-lowering agents with less risk for potential side effects associated with drug-drug interactions that occur with other statins.
Conclusion
After careful and independent evaluation of all available published information and unpublished data supplied by AstraZeneca, we conclude that rosuvastatin, compared with the other marketed statins, incurs no greater risk for adverse events, except for the uncommon development of a mild form of tubular proteinuria at higher dosages (40 mg/day and higher) and has an efficacy equal to or exceeding atorvastatin on a milligram-per-milligram basis. This analysis reinforces the comprehensive review by the FDA on March 11, 2005.[2] Although the FDA report also addressed the fact that the frequency of myopathy was greater with the use of 80 mg/day of rosuvastatin, we did not include data on this dose because they is not marketed.
Acknowledgments
We thank Drs. Robert Croy, Tanyel Kiziltepe, and Michael DeMott for their valuable contributions to the analysis, and Ms. Laura Trudel for her assistance in manuscript preparation.
Funding Information
This manuscript was funded by a grant from AstraZeneca Pharmaceuticals, LP.
Disclaimer
AstraZeneca did not have control over the conduct of the assessment or the content and conclusions of the analysis.
The decision to submit a manuscript describing the results of the analysis was made independently by Science Partners after the analysis was complete. AstraZeneca played no role in the preparation of this manuscript.
Contributor Information
Douglas P. Zipes, Pharmacology, and Toxicology; Krannert Institute of Cardiology, Indiana University School of Medicine, Indianapolis, Indiana. Email: dzipes@iupui.edu.
Nathan J. Zvaifler, University of California, San Diego, School of Medicine, La Jolla, California.
Richard J. Glassock, David Geffen School of Medicine at UCLA. Email: glassock@cox.net.
Sid Gilman, University of Michigan Medical School, Ann Arbor, Michigan. Email: sgilman@umich.edu.
Alvaro Muñoz, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Victor Gogolak, DrugLogic, Reston, Virginia. Email: vgogolak@druglogic.com.
Leon Gordis, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Peter C. Dedon, Massachusetts Institute of Technology, Cambridge, Massachusetts.
Frederick P. Guengerich, Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, Tennessee. Email: f.guengerich@vanderbilt.edu.
Stephen I. Wasserman, University of California, San Diego, School of Medicine, La Jolla, California.
Joseph L. Witztum, University of California, San Diego, School of Medicine, La Jolla, California.
Gerald N. Wogan, Massachusetts Institute of Technology, Cambridge, Massachusetts.
References
- 1.Wolfe SM. Dangers of rosuvastatin identified before and after FDA approval. Lancet. 2004;363:2189–2190. doi: 10.1016/S0140-6736(04)16513-6. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. doi: 10.3109/15360288.2015.1037530. Re Docket No. 2004-P-0013/CP1. March 11, 2005. Available at: http://www.fda.gov/ohrms/dockets/dockets/04p0113/04p-0113-pdn0001.pdf Accessed June 6, 2006. [DOI] [PubMed]
- 3.Alsheikh-Ali AA, Ambrose MS, Kuvin JT, et al. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111:3051–3057. doi: 10.1161/CIRCULATIONAHA.105.555482. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. The issue of statin safety: where do we stand? Circulation. 2005;111:3016–3019. doi: 10.1161/CIRCULATIONAHA.105.557652. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Bolognese MA, Tremblay BP, et al. Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trial. Am Heart J. 2004;148:e4. doi: 10.1016/j.ahj.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Brewer HB., Jr Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol. 2003;92:23K–29K. doi: 10.1016/s0002-9149(03)00779-3. [DOI] [PubMed] [Google Scholar]
- 7.Evans M, Roberts A, Davies S, et al. Medical lipid-regulating therapy: current evidence, ongoing trials and future developments. Drugs. 2004;64:1181–1196. doi: 10.2165/00003495-200464110-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–297. doi: 10.2165/00129784-200404050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Scott LJ, Curran MP, Figgitt DP. Rosuvastatin: a review of its use in the management of dyslipidemia. Am J Cardiovasc Drugs. 2004;4:117–138. doi: 10.2165/00129784-200404020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Hunninghake DB, Stein EA, et al. Safety of rosuvastatin. Am J Cardiol. 2004;94:882–888. doi: 10.1016/j.amjcard.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JW. Rosuvastatin in the management of hyperlipidemia. Clin Ther. 2004;26:1368–1387. doi: 10.1016/j.clinthera.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Safety. 2004;3:547–557. doi: 10.1517/14740338.3.6.547. [DOI] [PubMed] [Google Scholar]
- 13.Barry M. Rosuvastatin-warfarin drug interaction. Lancet. 2004;363:328. doi: 10.1016/S0140-6736(03)15396-2. [DOI] [PubMed] [Google Scholar]
- 14. Anonymous. AstraZeneca FDA Advisory Committee Briefing Document. 2003.
- 15. Anonymous. AstraZeneca Periodic Safety Update Report for Crestor. 2004.
- 16.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 17.Tolman KG. Defining patient risks from expanded preventive therapies. Am J Cardiol. 2000;85:15E–19E. doi: 10.1016/s0002-9149(00)00946-2. [DOI] [PubMed] [Google Scholar]
- 18.Brewer HB., Jr Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol. 2003;92:23K–29K. doi: 10.1016/s0002-9149(03)00779-3. [DOI] [PubMed] [Google Scholar]
- 19.Kendrach MG, Kelly FM. Approximate equivalent rosuvastatin doses for temporary statin interchange programs. Ann Pharmacother. 2004;38:1286–1292. doi: 10.1345/aph.1D391. [DOI] [PubMed] [Google Scholar]
- 20.Schuster H. Rosuvastatin – a highly effective new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor: review of clinical trial data at 10-40 mg doses in dyslipidemic patients. Cardiol. 2003;99:126–139. doi: 10.1159/000070669. [DOI] [PubMed] [Google Scholar]
- 21.Cheng AYY, Leiter LA. Rosuvastatin. J Drug Eval. 2004;2:41–55. [Google Scholar]
- 22.Cheng-Lai A. Rosuvastatin: a new HMG-CoA reductase inhibitor for the treatment of hypercholesterolemia. Heart Dis. 2003;5:72–78. doi: 10.1097/01.HDX.0000050417.89309.F8. [DOI] [PubMed] [Google Scholar]
- 23.Schneck DW, Knopp RH, Ballantyne CM, McPherson R, Chitra RR, Simonson SG. Comparative effects of rosuvastatin and atorvastatin across their dose ranges in patients with hypercholesterolemia and without active arterial disease. Am J Cardiol. 2003;91:33–41. doi: 10.1016/s0002-9149(02)02994-6. [DOI] [PubMed] [Google Scholar]
- 24.Olsson AG, Istad H, Luurila O, et al. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am Heart J. 2002;144:1044–1051. doi: 10.1067/mhj.2002.128049. [DOI] [PubMed] [Google Scholar]
- 25.Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol. 2001;88:504–508. doi: 10.1016/s0002-9149(01)01727-1. [DOI] [PubMed] [Google Scholar]
- 26.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–1427. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deedwania PC, Hunninghake DB, Bays H. Effects of lipid-altering treatment in diabetes mellitus and the metabolic syndrome. Am J Cardiol. 2004;93:18C–26C. doi: 10.1016/j.amjcard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 2003;92:1287–1293. doi: 10.1016/j.amjcard.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Davidson M, Ma P, Stein EA, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol. 2002;89:268–275. doi: 10.1016/s0002-9149(01)02226-3. [DOI] [PubMed] [Google Scholar]
- 30.Schuster H, Barter PJ, Stender S, et al. Effects of switching statins on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147:705–713. doi: 10.1016/j.ahj.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG. Guidelines for lowering lipids to reduce coronary artery disease risk: a comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals. Atherosclerosis Suppl. 2004;5:115–121. doi: 10.1016/j.atherosclerosissup.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG. Guidelines for lowering lipids to reduce coronary artery disease risk: a comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals. Am J Cardiol. 2003;91:11C–17C. doi: 10.1016/s0002-9149(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 33.Strutt K, Caplan R, Hutchison H, Dane A, Blasetto J. More Western hypercholesterolemic patients achieve Japan Atherosclerosis Society LDL-C goals with rosuvastatin therapy than with atorvastatin, pravastatin, or simvastatin therapy. Circ J. 2004;68:107–113. doi: 10.1253/circj.68.107. [DOI] [PubMed] [Google Scholar]


