Abstract and Introduction
Abstract
There is now a clear causal relationship between symptomatic gastroesophageal reflux and esophageal adenocarcinoma (Lagergren et al, 1999). The risk factor is now identified as Barrett's metaplasia (Solaymani et al, 2004). Chronic reflux results in Barrett's metaplastic change, and the route to carcinoma is a stepwise progression, through dysplasia to invasive carcinoma (Jankowski et al, 2000). Earlier-stage disease is found in patients undergoing surveillance and is the major predictor of survival following surgery (Fountoulakis et al, 2004). Screening and surveillance by endoscopic biopsy regimen has profound implications for the allocation of healthcare resources and the provision of clinical services. Screening a high-risk group such as men with gastroesophageal reflux disease (GERD) will result in the detection of more patients with Barrett's esophagus, many of whom are asymptomatic. Once detected, questions remain as to surveillance intervals and the current methodology for surveillance. There are profound challenges with the accurate endoscopic and pathologic detection and categorization of Barrett's metaplasia, dysplasia, and, indeed, cancer. New endoscopic detection methods are being investigated to improve the diagnosis and definition of the premalignant phenotype. The detection of dysplasia requires increased surveillance and usually intervention either endoscopically or with surgery.
Introduction
Barrett's esophagus is a pathophysiologic condition affecting the gastroesophageal junction and lower esophagus. It is clear that in some patients it proceeds to the development of adenocarcinoma of the lower esophagus (Figure 1).
Barrett's-associated esophageal adenocarcinoma is the fastest rising cancer in the developed world.[1] Thirty years ago, esophageal adenocarcinoma was a rare malignancy, and now, more than 5000 cases occur each year in the United Kingdom alone. The current rate of increase in the incidence of esophageal adenocarcinoma is exceeding that of any other cancer, including melanoma, lymphoma, and small-cell lung cancer. This continuing rise in the annual incidence[2,3] is matched by little change in the effect of treatment on mortality. The median survival was 0.75 years (1973–1977) and has only improved to 0.9 years (1993–1999).[4] Various risk factors for progression to cancer have been identified, and Barrett's represents an increasing problem for white men in England and Scotland.[5,6] Currently, 0.5%–1.0% of adults with Barrett's metaplasia will progress to cancer. The annual conversion to adenocarcinoma for patients with long-segment disease (> 3 cm of Barrett's metaplasia) is 1% in the United Kingdom. There also appears to be regional variation, with lower rates in the United States and some other Western countries.[6] It is estimated that as many as 1%–2% of the adult population in the United States may be at risk, with up to 20% suffering from chronic reflux symptoms. In 2002, there were 6000 deaths from esophageal adenocarcinoma, but this figure is expected to rise with time.[7] Despite the fact that this condition is seen in family clusters, no obvious heritable genetic factors appear evident. If this trend continues, there may be little prospect of altering the impact modern medicine will have on this disease.
A profound clinical dilemma remains in that most patients with Barrett's esophagus will die due to other diseases. The overall mortality rate of patients with Barrett's esophagus is similar to that of the general population. Mortality from esophageal cancer is clearly increased, but a large epidemiologic study indicated that only 4.7% of patients die as a result of this malignancy.[8] These data indicate that although patients progress to cancer through Barrett's esophagus, most patients have comorbid disease such that the diagnosis of Barrett's esophagus does not appear to influence overall mortality, despite the fact that deaths from esophageal cancer are greatly increased.
Although Norman Barrett described the condition and its neoplastic potential several decades ago, the mechanisms of damage, repair, and transformation are still the subject of intense research. It is generally accepted that the normal squamous lower esophageal epithelium is injured by chronic duodeno-gastroesophageal reflux. In some patients, repair is effected in this abnormal environment by columnar intestinal and gastric cells instead of the native squamous cells.[5] Three distinct types of columnar metaplasia are recognized. The most common is the intestinal form, and this is the most likely to undergo malignant transformation to adenocarcinoma. The other 2 types, cardiac and fundic, are difficult to distinguish from gastric mucosa at these sites.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eyes only or for possible publication via email: glundberg@medscape.net
Current Situation: The Clinical Value of Surveillance and Screening
A meta-analysis that identified 46 papers evaluating 11,056 Barrett's patients with 42,880 patient-years of follow-up is in progress and is being prepared for publication (P. Moayyedi, personal communication, January 2006). A current meta-analysis of papers from the United Kingdom[9] suggests the cancer conversion rate is about 1% per year (95% CI = 0.67 to 1.39%), again without any statistical evidence of publication bias. These findings are consistent with the fact that the United Kingdom has the highest incidence of esophageal adenocarcinoma worldwide.
The prognosis for symptomatic and advanced esophageal adenocarcinoma in patients who are suitable for radical surgical intervention is very poor, with a median survival of 1 year and a 5-year survival rate of 10%.[10] The poor survival rate has promoted the development of surveillance programs and guidelines recommending that patients with Barrett's esophagus undergo upper gastrointestinal endoscopy every 2–3 years to detect esophageal adenocarcinoma early, when the prognosis is much more favorable.[11,12] Lymph node metastases were seen in 18% of patients with surveillance-detected cancer compared with in 56% of patients with symptoms. Also, surveillance-detected cancers were at a significantly earlier stage than symptomatic cancers. This translated into significantly better survival rates: 88% (surveillance) vs 67% (symptomatic) at 1 year and 80% (surveillance) vs 31% (symptomatic) at 3 years.[13] These observations suggest that patients enrolled in surveillance programs have a better actuarial survival and have helped define the current recommendations for surveillance.[14,15] These are very weak data in epidemiologic terms. Patients who will enter surveillance programs may be different from those who present with advanced esophageal adenocarcinoma. They are often younger and generally in better health. Thus, the value of surveillance is still subject to considerable debate and much controversy.[16–18] It is therefore important to emphasize that no studies to date have confirmed that the mortality from esophageal cancer is decreased by endoscopic surveillance in Barrett's metaplasia.
There remains the question of whether the actual incidence of Barrett's esophagus is rising. This could partly explain the observed increase in adenocarcinoma. A systematic review has demonstrated an increased rate of 0.08% per year from 1980–1996.[12] The 6- to 8-fold increase in cancer is mirrored by a 6-fold increase in Barrett's esophagus in the past 15 years. The mean age at diagnosis of Barrett's esophagus is 62 years, and the marked increase in incidence occurs in the population of patients older than 40–50 years, with 65% of Barrett's patients being male. The transformation to cancer appears to be associated with male sex, with a possible protective effect of female sex.[12]
Nevertheless, there is some evidence to support the view that surveillance is of no overall benefit. The results of a study conducted by Macdonald and colleagues[19] at a large center in the United Kingdom suggested this view. They found that there was no benefit in the survival of patients with Barrett's esophagus as a result of repeated endoscopic surveillance. One patient died as a result of surgery, and few cancers were detected at a stage that was beneficial. However, only 409 patients were observed. It is possible that many of the patients enrolled in this study from Leicester may not have had Barrett's esophagus, but rather a large hiatal hernia. This type of cohort study reduces the number of possible biases, but does not eliminate the problem and, in particular, there are concerns about healthy volunteers, and lead-time and length-time bias.[20]
In an effort to apply the current weak nonrandomized data available, various models have been developed to provide an incremental cost-effectiveness ratio of Barrett's surveillance under ideal assumptions. Decision analysis models have suggested that endoscopic surveillance every 2 years costs approximately $30,000 per life-year saved,[21–23] although all reports noted this was very sensitive to the assumptions used in the model and that cost-effectiveness was very sensitive to the risk for development of esophageal adenocarcinoma, as well as to the efficacy of surveillance.
Clinical Implications: Problems of Definition and Diagnosis
Accurate diagnosis is very dependent on the endoscopist clearly identifying the site of the biopsy as being in the gastric cardia, hiatal hernia, or the esophagus. The endoscopic problem is that the anatomy and position of the gastroesophageal junction is difficult to define. There is a lack of a universally accepted and reproducible set of criteria to endoscopically identify the cardia of the stomach from the distal esophagus. During endoscopy, it is important to identify certain key anatomic landmarks to allow some delineation of abnormal columnar-lined esophagus. The squamo-columnar junction is usually visible as the pale squamous epithelium merges into redder columnar mucosa (Figure 2). The precise location of the gastroesophageal junction is hard to identify clinically. At present, it is defined endoscopically as the level of the most proximal gastric fold. Some patients with a hiatal hernia have defective and weak lower esophageal sphincters, and, therefore, there is no clear-cut demarcation as the clinician enters the stomach with the endoscope. The proximal margin of the gastric folds must be determined when the distal esophagus is minimally inflated. Overinflation will flatten and obscure all the gastric folds. If the squamocolumnar and gastroesophageal junction coincide, the entire esophagus is lined with squamous mucosa. When the squamocolumnar junction is proximal to the gastroesophageal junction, there is a columnar-lined segment or Barrett's esophagus (Figure 2). Pathology can offer a clear indication of esophageal origin when an esophageal gland or, more commonly (eg, in a biopsy sample), a duct from these glands is seen. The depth of biopsy required makes these findings unusual,[12] as the depth of endoscopic biopsy is usually too shallow. The debate has deepened as cytokeratin immunoreactivity (CK7/CK20 pattern) may be able to differentiate the intestinal metaplasia associated with Barrett's esophagus from that associated with Helicobacter pylori gastritis.[24,25] The important risk factors for the development of adenocarcinoma within a segment of Barrett's esophagus are age greater than 45 years, male sex, long segments (> 8 cm), long duration of reflux history, early onset of reflux, and the presence of ulceration or stricture.[5] The endoscopic assessment of the length of Barrett's esophagus is unreliable unless rigorous attention is paid to the above guidance. Even experienced units have found a mean difference between endoscopic and histologic length of 1 cm (range, 0–5 cm). The histologic length was assessed using biopsy to establish the presence of intestinal metaplasia within columnar epithelium. The length of the histologic segment of Barrett's was 36% greater than the endoscopic length.[26]
Figure 2.
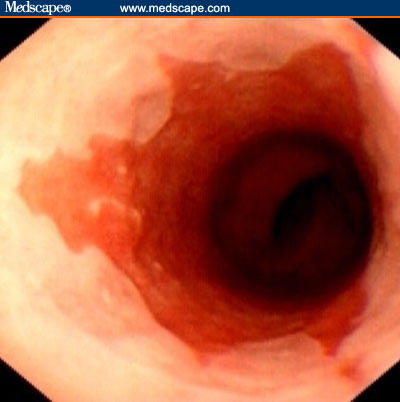
Endoscopic image of Barrett's esophagus with pale squamous epithelium merging into the darker columnar-lined esophagus.
Dysplasia, the precancerous neoplastic alteration of the gastrointestinal epithelium which has the potential to progress to invasive malignancy, remains the best predictor for the development of invasive malignancy (Figure 3).[5] The classification of neoplastic change in the gastrointestinal mucosa includes 5 diagnostic categories: negative for dysplasia; indefinite for dysplasia; low-grade dysplasia, high-grade dysplasia; and invasive carcinoma.[27–30] Inter- and intra-observer studies have demonstrated that pathologists show acceptable levels of diagnostic agreement when there are 2 major comparative categories used: high-grade dysplasia combined with carcinoma, vs negative for dysplasia combined with indefinite and low-grade dysplasia (kappa values of 0.8). However, when statistical analysis was performed using diagnostic division into 4 clinical categories (negative for dysplasia; combined indefinite for dysplasia and low-grade dysplasia; high-grade dysplasia; and carcinoma), there were poorer levels of agreement (intra-observer kappa values of 0.64; inter-observer kappa values of 0.43).[31] The vital separation of high-grade dysplasia from intramucosal cancer is dependent on the penetration of neoplastic cells through the basement membrane. This diagnostic classification is also difficult, with interobserver agreement between all pathologists and specific gastrointestinal pathologists for high-grade dysplasia and intramucosal carcinoma having a kappa value of 0.42 and 0.56, respectively, even based on resection specimens. These data have serious consequences for patients undergoing endoscopic surveillance when the number of collected biopsies are small. Difficulties in orientation of biopsy specimens also make it more challenging to differentiate high-grade dysplasia from invasive cancer.[32,33]
Figure 3.
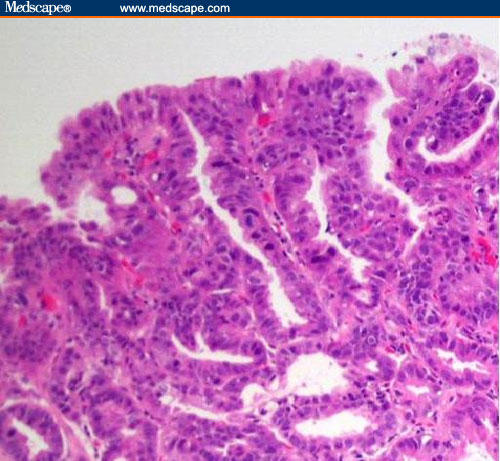
High-grade dysplasia in Barrett's esophagus.
Current Trials
Once detected, the question of when to intervene becomes important. Medical and, indeed, endoscopic intervention in Barrett's esophagus may become costly, is associated with adverse events, is inconvenient to patients, and, in rare instances, can result in premature death.[34,35] Proponents of Barrett's surveillance suggest that randomized controlled trials evaluating the cost-effectiveness of surveillance strategies are not possible because they will be too expensive. At present, our group is conducting a trial of endoscopic surveillance, and we have started a second trial evaluating the efficacy of aspirin and standard and high-dose acid suppression in preventing esophageal adenocarcinoma in patients with Barrett's esophagus (Aspirin Esomeprazole Chemoprevention Trial [AspECT]. This study, funded by Cancer Research UK, involves randomizing 5000 patients with Barrett's esophagus to high- vs standard-dose proton-pump inhibitor (PPI) therapy and aspirin (300 mg once a day) vs no aspirin in a 2 × 2 factorial design. In this trial, patients undergo surveillance endoscopy every 2 years. Recruitment is progressing very well, but results will not be reported for several years. Very good experimental data are available suggesting that this strategy may be effective for the prevention of progression in patients with Barrett's esophagus.[36]
The Future: New Endoscopic Methods of Surveillance
The current gold standard of visual white light endoscopy with protocol biopsy is not sufficient to detect early cancer and dysplasia. Magnification endoscopy, chromoendoscopy, and spectroscopy may allow for earlier detection of malignant and dysplastic changes in Barrett's esophagus. These techniques can be stratified into those that provide better morphologic information and those that have the potential for providing molecular information. Morphologic information provides in vivo histology, and the relevant techniques include optical coherence tomography, elastic/light scattering spectroscopy, and confocal microscopy. Fluorescence imaging and spectroscopy provide both morphologic and biochemical data. Raman spectroscopy provides the most powerful tool for obtaining molecular data.
Laser-induced fluorescence spectroscopy has the potential to detect high-grade dysplasia using the technique of differential normalized fluorescence, with excitation at 410 nm.[33] There are significant spectral differences at 480 and 600 nm between normal and neoplastic tissue. There is difficulty in the detection of focal high-grade dysplasia and areas of low-grade dysplasia, and in differentiating these from inflammation. The use of delayed laser-induced fluorescence in combination with the administration of topical 5-amino-levulinic acid sprayed onto the Barrett's mucosa to generate the endogenous photosensitizer (protoporphyrin IX) improves the detection of high-grade dysplasia by a factor of 2.8.[34] It is important to note that this method successfully differentiated low-grade dysplasia from nondysplastic tissue and identified specialized intestinal metaplasia in short-segment disease.[37]
Although the combination of white light reflectance with fluorescence excitation-emission with inelastic (Raman) scattering appears highly promising,[38] widespread application remains some time away. An investigation of the novel methods of fluorescence imaging and chromoendoscopy were directly compared with the standard conventional protocol biopsy using video endoscopy, and the researchers concluded that the newer techniques had a low sensitivity for the detection of foci of high-grade dysplasia and cancer. Thus, they could not replace careful endoscopy and protocol biopsy.[39] The sensitivity and specificity for fluorescence detection was 21% and 91%, respectively, and for chromoendoscopy with methylene blue dye spray, 37% and 91%, respectively. There may be some improvement using magnification chromoendoscopy in the detection of high-grade dysplasia, but not in the detection of low-grade dysplasia.[40] Very detailed morphologic changes, as well as imaging of cells and nuclei, can be obtained using fluorescence confocal endomicroscopy with exogenously applied fluorophores (topical acriflavine and intravenous fluorescein).[41] These new techniques have yet to be investigated in a routine clinical setting.
Optical coherence tomography[42,43] offers the prospect of in vivo histologic imaging of the mucosa, submucosa, and muscularis. The penetration depth of standard optical coherence tomography is limited to about 2 mm, with a resolution of 5–15 micrometers. Ultra-high resolution and 3-dimensional imaging can resolve < 5 microns, 3 times finer than the standard techniques. Whether this is of benefit practically remains to be evaluated.[44] The imaging of dysplasia has proven difficult, and in our experience it is detectable by being more homogeneous than nondysplastic tissue (Figure 4). This confirms data from other groups, who have attempted to characterize Barrett's esophagus with optical coherence tomography.[45,46]
Figure 4.
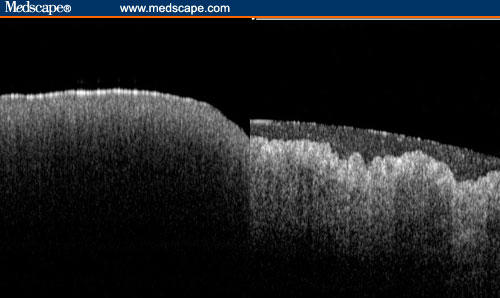
Optical coherence tomography of high-grade dysplasia in Barrett's esophagus (left image) and nondysplastic Barrett's esophagus (right image), which has a layer of mucus on the surface.
Optical Biopsy for Surveillance in Barrett's Esophagus
Photons usually interact with tissue by wavelength-dependent elastic scattering or absorption. The signal depends on the sizes and shapes of the cell nuclei, cell crowding, and cell shape. With 76 sites in 13 patients examined, the application of this technology in patients with Barrett's esophagus has been promising. The sensitivity and specificity of light-scattering spectroscopy for detecting low-grade dysplasia and high-grade dysplasia was 90% and 90%, respectively, with all high-grade dysplasia detected and 87% of low-grade dysplasia sites detected.[47] Some of the photons will set up molecular vibrations and can provide a molecular fingerprint for the detection and identification of dysplasia and cancer. This is the basis of Raman spectroscopy for the detection and classification of the changes associated with molecular degeneration in Barrett's esophagus.[48,49] It is a promising technique for histopathology sections and is being developed as an in vivo endoscopic probe (Figure 5).
Figure 5.
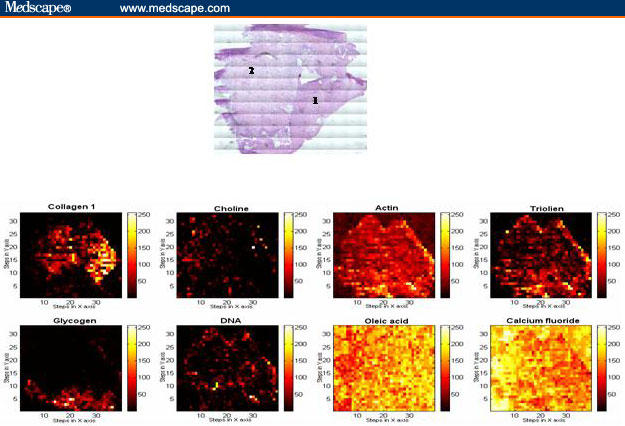
Raman basis map showing the distribution of different biochemical constituents across the sample, generated using mathematical least square fit method and contiguous H&E-stained normal squamous (1) and low-grade dysplasia (2) section.
Other endoscopic techniques are becoming available for the early detection of neoplastic transformation in patients undergoing endoscopic surveillance. Narrow band imaging limits the bandwidth of spectral transmission of the RGB (red/green/blue) optical filters and can define the mucosal capillary and pit pattern. Preliminary data suggest that it has a sensitivity of 56% with a specificity of 95% for the detection of intestinal metaplasia in Barrett's esophagus.[50] Similarly, confocal endomicroscopy provides very high-resolution images (1–2 microns) to a depth of 300 microns. It has been evaluated more extensively in the colon but clearly has a role for the surveillance of patients with a Barrett's segment. The most widely evaluated endoscopic technique is fluorescence imaging, either following an administered fluorophore or using autofluorescence. It can detect high-grade dysplasia with a specificity of 75%–100% and a sensitivity of 70%–90%, although problems with the identification of focal dysplasia and inflammation remain.[46,51]
Screening
The establishment of a screening program for the detection of Barrett's esophagus and esophageal cancer has been suggested for patients with chronic heartburn. The basis for this proposal derives from the Swedish data showing that patients with severe heartburn have a 44-fold increased rate of esophageal adenocarcinoma, although 40% of patients who developed cancer denied problematic heartburn.[52] It is estimated that there is an incidence of 1 cancer per 1000 patients with chronic heartburn.[53] Thus, screening to detect esophageal cancer is difficult to support, although it would identify Barrett's esophagus, which is present in up to 15% of patients.[54] Therefore, there is consideration given to screening patients over the age of 50 years to detect Barrett's esophagus, and to then enter those patients into a surveillance program.[55] Other practice guidelines do not recommend this approach.[12] It is important to respond to patients with heartburn who develop dysphagia, vomiting, weight loss, or anemia.
Conclusions and Current Clinical Guidelines
There is intense debate and controversy regarding how to survey and, indeed, whether to survey patients with Barrett's esophagus.[56–59] These patients are at risk of progression to cancer. In addition, most patients with Barrett's esophagus are asymptomatic,[1] possibly because they have decreased sensation in the lower esophagus.[60]
Current research recognizes that chronic heartburn is a major risk factor for esophageal adenocarcinoma, and that the risk increases with severity. However, there is no evidence to suggest that an endoscopic screening program of patients with heartburn to detect Barrett's, dysplasia, and cancer is worthwhile. Screening for Barrett's esophagus has been suggested for those individuals over the age of 50 but, again, is not recommended.[12] For patients diagnosed as having columnar-lined esophagus, surveillance performed every 2 years with quadrant biopsy every 2 cm is suggested.[12,61] In addition, all visible lesions should be biopsied. A Markov model based on United Kingdom National Health Service Costings estimates that this strategy costs £19,000 per life saved.[12,62] We eagerly await the results of ongoing randomized trials.
Figure 1.
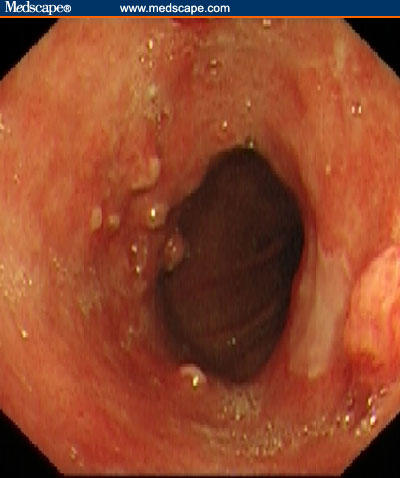
A segment of Barrett's esophagus with nodular area of high-grade dysplasia and early cancer in the most proximal part.
Contributor Information
Hugh Barr, Cranfield Postgraduate Medical School in Gloucestershire, Gloucestershire Royal Hospital, Gloucester, United Kingdom. Email: hugh.barr@glos.nhs.uk.
Catherine Kendall, Cranfield Postgraduate Medical School in Gloucestershire, Gloucestershire Royal Hospital, Gloucester, United Kingdom.
Florian Bazant-Hegemark, Cranfield Postgraduate Medical School in Gloucestershire, Gloucestershire Royal Hospital, Gloucester, United Kingdom.
Paul Moayyedi, Department of Gastroenterology, McMaster University Medical Centre, Hamilton, Ontario, Canada.
G. Shetty, Cranfield Postgraduate Medical School in Gloucestershire, Gloucestershire Royal Hospital, Gloucester, United Kingdom.
Nicholas Stone, Cranfield Postgraduate Medical School in Gloucestershire, Gloucestershire Royal Hospital, Gloucester, United Kingdom.
References
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Wei JT, Shaheen NJ. The changing epidemiology of esophageal adenocarcinoma. Semin Gastrointest Dis. 2003;14:112–127. [PubMed] [Google Scholar]
- 3.El Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Endoscopy Clin N Am. 2002;31:421–440. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 4.Mason AC, Eloubeidi MA, el Serag HB. Temporal trends in survival of patients with esophageal adenocarcinoma.1973–1997. Gastroenterology. 2001;122:A30. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 5.Jankowski J, Harrison RF, Perry I, Balkwill F, Tselepis C. Seminar: Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski J, Provenzale D, Moayyedi P. Oesophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the West. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 7.Fennerty MB. Endoscopic diagnosis and surveillance of Barrett's esophagus. Tech Gastrointest Endosc. 2005;7:89–94. doi: 10.1016/s1052-5157(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett's oesophagus: results from a population based study. Gut. 2003;52:1081–1084. doi: 10.1136/gut.52.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jankowski J, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the West. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 10.Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;183:274–279. doi: 10.1016/s0002-9610(02)00795-x. [DOI] [PubMed] [Google Scholar]
- 11.Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 12. Report of Working Party of the British Society of Gastroenterology. Guidelines for the diagnosis and management of Barrett's columnar-lined oesophagus, 2005.
- 13.Fountoulakis A, Zafirellis KD, Dolan K. Effect of surveillance for Barrett's oesophagus on oesophageal cancer. Br J Surg. 2004;91:997–1003. doi: 10.1002/bjs.4591. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 15.Dulai GS. Surveying the case for surveillance. Gastroenterology. 2002;122:820–825. doi: 10.1053/gast.2002.32093. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, McQuaid K, Dent J, et al. AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett's esophagus. Gastroenterology. 2004;127:310–330. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Moayyedi P. Should patients with GERD be screened once at least for Barrett's epithelium? Con: Patients with GERD should be screened once at least for Barrett's esophagus–medicine or magic? Am J Gastroenterol. 2004;99:2293–2295. doi: 10.1111/j.1572-0241.2004.41295_2.x. [DOI] [PubMed] [Google Scholar]
- 18.Sampliner RE. Should patients with GERD be screened once at least for Barrett's epithelium? Pro: The need to screen GERD patients for Barrett's esophagus–a greater yield than surveillance. Am J Gastroenterol. 2004;99:2291–2293. doi: 10.1111/j.1572-0241.2004.41295_1.x. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ. 2000;321:1252–1255. doi: 10.1136/bmj.321.7271.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaheen NJ, Provenzale D, Sandler RS. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: a review of the evidence. Am J Gastroenterol. 2002;97:1319–1327. doi: 10.1111/j.1572-0241.2002.05767.x. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg A, Soni A, Sampliner RE. Medical decision analysis of endoscopic surveillance of Barrett's oesophagus to prevent oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2002;16:41–50. doi: 10.1046/j.1365-2036.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- 22.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick M, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 23.Provenzale D, Schmitt C, Wong JB. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 24.Barr H. The pathological implications of surveillance, treatment and surgery for Barrett's oesophagus. Current Diagnostic Pathology. 2003;9:242–251. [Google Scholar]
- 25.Couvelard A, Cauvin J-M, Goldfain D, Rotenberg A, Robasziewicz, Flejou J-F. Cytokeratin immunoreactivity of intestinal metaplasia at normal oesophagogastric junction indicates its aetiology. Gut. 2001;49:761–766. doi: 10.1136/gut.49.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger K, Meining A, Werner M, Hofler H, Classen M, Rosch T. Endoscopic measurement of Barrett's esophagus length is unreliable: a prospective comparative biopsy study. Z Gastroenterol. 2004;42:499–502. doi: 10.1055/s-2004-813061. [DOI] [PubMed] [Google Scholar]
- 27.Riddell RH, Goldman H, Ransohoff D. Dysplasia in inflammatory bowel disease. Standardised classification with provisional clinical application. Hum Pathol. 1983;14:931–966. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 28.Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 29.Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982–993. doi: 10.1016/0046-8177(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 30.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett's oesophagus: A reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 32.Ormsby AH, Petras RE, Henricks WH, et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51:671–676. doi: 10.1136/gut.51.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderson D. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma: another spanner in the works. Gut. 2002;51:620–621. doi: 10.1136/gut.51.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr H. Photodynamic therapy for high-grade dysplasia in Barrett's esophagus. Tech Gastrointest Endosc. 2005;7:60–65. doi: 10.1016/j.gie.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenberg A, Fennerty MB. Medical decision analysis of chemoprevention against adenocarcinoma. Gastroenterology. 2003;124:1758–1766. doi: 10.1016/s0016-5085(03)00393-7. [DOI] [PubMed] [Google Scholar]
- 36.Triadafilopoulos G, Kaur B, Sood S, Traxler B, Levine D, Westons A. The effects of esomeprazole combined with aspirin or rofecoxib on prostaglandin E2 production in patients with Barrett's oesophagus. Aliment Pharmacol Ther. 2006;23:997–1005. doi: 10.1111/j.1365-2036.2006.02847.x. [DOI] [PubMed] [Google Scholar]
- 37.Panjepour M, Overholt BF, Vo-Dinh T, Haggitt RC, Edwards DH, Buckley FP. Endoscopic fluorescence detection of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 1996;111:93–111. doi: 10.1053/gast.1996.v111.pm8698231. [DOI] [PubMed] [Google Scholar]
- 38.Ortner M-A, Ebert B, Hein E, et al. Time gated fluorescence spectroscopy in Barrett's oesophagus. Gut. 2003;52:28–33. doi: 10.1136/gut.52.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tunnell JW, Desjardins AE, Galindo L, et al. Instrumentation for multi-modal spectroscopic diagnosis of epithelial dysplasia. Technol Cancer Res Treat. 2003;2:505–514. doi: 10.1177/153303460300200603. [DOI] [PubMed] [Google Scholar]
- 40.Egger K, Werner M, Meining A, et al. Biopsy surveillance is still necessary in patients with Barrett's oesophagus despite new endoscopic imaging techniques. Gut. 2003;52:18–23. doi: 10.1136/gut.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma P, Weston AP, Topalovski M, et al. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut. 2003;52:24–27. doi: 10.1136/gut.52.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper and lower-GI tract. Gastrointest Endoscopy. 2005;62:686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Tearney GJ, Brezinski ME, Bouma BE. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276:2037–2039. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 44.Hsiung P-L, Pantanowitz L, Aaron D, et al. Ultrahigh-resolution and 3-dimensional optical coherence tomography ex vivo imaging of the large and small intestines. Gastrointest Endosc. 2005;62:561–574. doi: 10.1016/j.gie.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Poneros JM, Brand S, Bouma BE, et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7–12. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 46.Wong Kee Song L-M, Wilson BC. Optical detection of high-grade dysplasia in Barrett's esophagus. Tech Gastrointest Endosc. 2005;7:78–88. [Google Scholar]
- 47.Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett's esophagus using light-scattering spectroscopy. Gastroenterology. 2000;119:677–682. doi: 10.1053/gast.2000.16511. [DOI] [PubMed] [Google Scholar]
- 48.Stone N, Kendall C, Shepherd N, Crow P, Barr H. Near-infrared spectroscopy for classification of epithelial pre-cancers and cancers. J Raman Spectrosc. 2002;33:564–573. [Google Scholar]
- 49.Kendall C, Stone N, Shepherd N, et al. Raman spectroscopy a potential tool for the objective identification and classification of neoplasia in Barrett's oesophagus. J Pathol. 2003;200:602–609. doi: 10.1002/path.1376. [DOI] [PubMed] [Google Scholar]
- 50.Hamamoto Y, Endo T, Nosho K, et al. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett's esophagus. J Gastroenterol. 2004;39:14–20. doi: 10.1007/s00535-003-1239-z. [DOI] [PubMed] [Google Scholar]
- 51.Panjepour M, Overholt BF, Vo-Dinh T, et al. Endoscopic fluorescence detection of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 1996;111:93–101. doi: 10.1053/gast.1996.v111.pm8698231. [DOI] [PubMed] [Google Scholar]
- 52.Loft DE, Alderson D, Heading RC. Screening and surveillance in columnar-lined oesophagus. British Society of Gastroenterology. 2005;28:28–31. Working Party Report. [Google Scholar]
- 53.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett's esophagus and esophageal cancer. JAMA. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 54.Csendes A, Smok G, Burdiles P, et al. Prevalence of Barrett's esophagus by endoscopy and histologic studies: a prospective evaluation of 306 control subjects and 376 patients with symptoms of gastroesophageal reflux. Dis Esophagus. 2000;13:5–11. doi: 10.1046/j.1442-2050.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 55.Sampliner RE. Updated guidelines for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 56.Dulai GS. Surveying the case for surveillance. Gastroenterology. 2002;122:820–825. doi: 10.1053/gast.2002.32093. [DOI] [PubMed] [Google Scholar]
- 57.Barr H. Endoscopic surveillance of patients with Barrett's oesophagus. Gut. 2002;51:313–314. doi: 10.1136/gut.51.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Playford RJ. Endoscopic surveillance of patients with Barrett's oesophagus. Gut. 2002:314–315. doi: 10.1136/gut.51.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corley DA, Levin TR, Weiss NS, Buffer PA. Surveillance and survival in Barrett's adenocarcinoma: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 60.Tremble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excessive gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GERD. Gut. 1995;37:7–12. doi: 10.1136/gut.37.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's oesophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 62.Povenzale D, Shmitt C, Wong J. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]


