Abstract and Background
Abstract
Migraine is a chronic neurologic disorder with heterogeneous characteristics resulting in a range of symptom profiles, burden, and disability. Migraine affects nearly 12% of the adult population in occidental countries, imposing considerable economic and social losses. The pharmacologic treatment of migraine includes preventive and acute strategies. A better understanding of the migraine pathophysiology along with the discovery of novel molecular targets has lead to a growing number of upcoming therapeutic proposals. This review focuses on new and emerging agents for the treatment of migraine.
Background
Migraine is a highly prevalent, disabling, undiagnosed, and undertreated disease.[1] The phenomenon is a primary neurologic disorder with a clear genetic basis.[2,3] For some uncommon forms of migraine, such as familial hemiplegic migraine, specific pathogenic genes have been identified. The most common mutation affects a gene on chromosome 19 that encodes for a neuronal calcium channel.[4] This observation suggests that other forms of migraine may also be ion channelopathies. During the migraine attack, neural events result in the dilatation of meningeal blood vessels that, in turn, causes pain, further nerve activation, and inflammation.[5] Because neural events are linked to vascular events, migraine is considered a neurovascular headache disorder.
Migraine probably results from dysfunction of brainstem areas involved in the modulation of craniovascular afferent fibers.[2–5] Brainstem activation may also lead to activation of ascending and descending pathways, with initiation of a perimeningeal vasodilatation and neurogenic inflammation. The pain is understood as a combination of altered perception (related to peripheral or central sensitization) of stimuli that are usually not painful, and the activation of a feed-forward neurovascular dilator mechanism in the first (ophthalmic) division of the trigeminal nerve. Cortical spreading depression is the presumed substrate of migraine aura; spreading depression also occurs in migraine without aura.
The past 15 years has witnessed the development of an arsenal of drugs that act on excitatory glutamate-mediated activity or inhibitory gamma-aminobutyric acid (GABA)-mediated activity, actions that theoretically provide cortical stabilization, therefore counteracting the imbalance supposedly existent in the migraineur's brain.[4,5] In addition, the progressive knowledge about the sequence of phenomena occurring during a migraine attack has stimulated interest in agents that may block the cortical spreading depression, a presumed substrate of migraine. Other targets include the blockage of proinflammatory substances released at the level of the trigeminal end, including neuropeptides involved in initiating the pain of migraine, and substances that may block the sensitization of peripheral and central trigeminal nociceptive pathways.[1,2,5–9]
In this review, we discuss new and emerging agents for the treatment of migraine. For both preventive and acute therapies, we first discuss medications that have been recently proposed for migraine, and then medications in development. None of the drugs discussed, with the exception of topiramate (TPM), have received an indication for the treatment of migraine, according to regulatory agencies.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Need for New Treatments for Migraine
As soon as a clinical diagnosis of migraine is made and disability and comorbidities have been assessed, the next task is to develop an individualized treatment plan. This plan usually has a number of goals that vary in priority with the patient's headache characteristics and treatment preferences. The plan usually includes educating patients about their illness and its management (eg, mechanisms, recognizing and avoiding triggers, and lifestyle changes), acute treatment, and preventive treatment.
The objective of acute migraine therapy is to restore the patient's ability to function by rapidly and consistently alleviating the head pain and the associated symptoms.[8,10] The objective of prevention is to reduce the frequency and impacts of attacks.
Despite the tremendous advances in the pharmacologic management of migraine, available options are still far from the optimum. Nearly 31% of the patients taking a triptan for acute migraine treatment discontinue its use because of lack of efficacy, headache recurrence, cost, and/or side effects.[11] In most trials, the therapeutic gain (efficacy of the drug subtracted by the efficacy of placebo) for the triptans is roughly 25% to 35% at 2 hours after treatment, and the absolute response usually does not exceed 70%.[12] In most trials of migraine prophylaxis, only 50% of the subjects experience more than 50% reduction of their headache frequency after 3 months of treatment.[8,10] Therefore, despite the advances in the past decade, new medicines for the management of migraine are needed.
Brief Review of Existing Treatments
Pharmacologic treatment of migraine is divided into acute and prophylactic modalities. Acute treatment can be subdivided into nonspecific agents (such as aspirin, acetaminophen, nonsteroidal anti-inflammatory drugs, opiates, and combination analgesics) and migraine-specific treatments (ergotamine, dihydroergotamine, and the triptans). The US Headache Consortium Guidelines recommend stratified care that is based on the level of disability to help physicians target patients who require careful assessment and treatment.[13] Thus, substantial clinical evidence exists for using disability to guide the assessment and treatment strategy (Figure 1). For migraine sufferers with attack-related disability and no contraindications, triptans (Table 1) should be the class of choice.[14]
Figure 1.
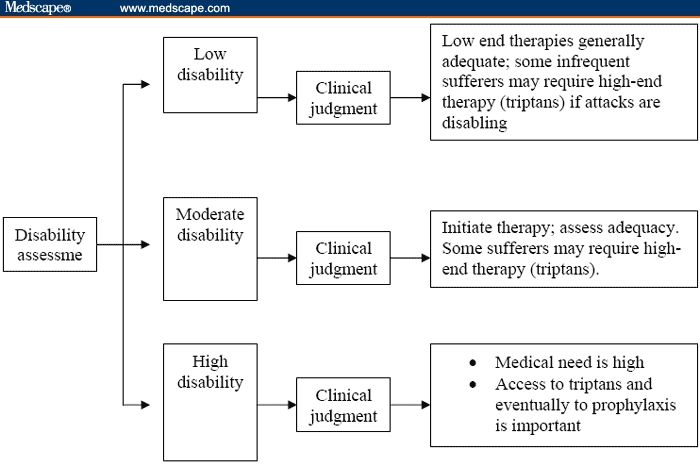
The stratified care for migraine acute treatment.
Table 1.
The Triptan Formulations
| Generic | Formulations | Doses (mg) | Maximum Daily Dose (mg) |
|---|---|---|---|
| Sumatriptan | Oral tablet | 25, 50, 100 | 200 |
| Nasal spray | 5, 20 | 40 | |
| Subcutaneous injection | 6 | 12 | |
| Zolmitriptan | Oral tablet | 2.5, 5 | 10 |
| Orally disintegrating tablet | 2.5, 5 | 10 | |
| Rizatriptan | Oral tablet | 5, 10 | 30 |
| Orally disintegrating tablet | 5, 10 | 30 | |
| Naratriptan | Tablet | 1, 2.5 | 5 |
| Almotriptan | Tablet | 12.5 | 25 |
| Frovatriptan | Tablet | 2.5 | 7.5 |
| Eletriptan | Tablet | 20, 40 | 80 |
According to the US Headache Consortium Guidelines, preventive treatment should be considered when (1) the migraine significantly interferes with the patient's daily routine despite acute treatment (eg, 2 or more attacks a month that produce disability that lasts ≥ 3 days or headache attacks that are infrequent but produce profound disability); (2) failure, contraindication to, or troublesome side effects occur from acute medications; (3) patients overuse acute medications; or (4) very frequent headaches (more than 2 a week) occur, or the pattern of attacks increases over time, with the risk of developing rebound headache from the repeated use of medicines taken for the acute attack.[9] Selected current preventive medications are displayed in Table 2.
Table 2.
Choices of Preventive Treatment in Migraine
| Comorbid Condition | ||||
|---|---|---|---|---|
| Drug | Efficacy | Adverse Events | Relative Contraindication | Relative Indication |
| Beta blockers | 4+ | 2+ | Asthma, depression, congestive heart failure, Raynaud's disease, diabetes | Hypertension, angina |
| Antiserotonin | ||||
| Pizotifen | 4+ | 2+ | Obesity | Orthostatic hypotension |
| Methysergide | 4+ | 4+ | Angina, vascular disease | |
| Calcium channel | ||||
| blockers | 2+ | 1+ | Constipation, hypotension | Aura, hypertension, angina |
| Verapamil | 4+ | 2+ | Parkinson's disease, depression | asthma |
| Flunarizine | Dizziness, vertigo | |||
| Antidepressants | ||||
| TCAs | 4+ | 2+ | Mania, urinary retention, heart block | Depression, anxiety, insomnia |
| SSRIs | 4+ | 1+ | Mania | pain |
| MAOIs | 4+ | 4+ | Unreliable patient | Depression, OCD |
| Refractory depression | ||||
| Anticonvulsants | ||||
| Divalproex/valproate | 4+ | 2+ | Liver disease, bleeding disorders | Mania, epilepsy, anxiety |
| Gabapentin | 2+ | 2+ | Liver disease, bleeding disorders | Mania, epilepsy, anxiety |
| Topiramate | 4+ | 2+ | Kidney stones | Mania, epilepsy, anxiety |
| NSAIDs | 2+ | 2+ | Ulcer disease, gastritis | Arthritis, other pain disorders |
NSAIDs = nonsteroidal anti-inflammatory drugs; TCAs = tricyclic antidepressants; SSRIs = selective serotonin reuptake inhibitors; MAOIs = monoamine oxidase inhibitors; OCD = obsessive-compulsive disorder
Ratings are on a scale from 1+ (lowest) to 4+ (highest) on the basis of the strength of the evidence.
Recent Advances in Migraine Prophylaxis
In this section we highlight drugs that recently received approval for migraine treatment (TPM) or are available and sometimes used off-label. All drugs discussed in this section are for the preventive treatment of migraine.
TPM
TPM was recently approved by the US Food and Drug Administration (FDA) for migraine prophylaxis. It is a neuromodulator with a structurally unique formula that provides multiple mechanisms of action and can influence the activity of some types of voltage-activated Na+ and Ca++ channels, the GABAA receptor, and the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/kainate subtype of glutamate receptors. TPM also has inhibition properties on specific carbonic anhydrase (CA) II and CA IV isozymes of CA.[15,16] TPM exerts its effects on voltage-activated Na+ and Ca++ channels, GABAA receptors, and AMPA/kainate receptors through protein phosphorylation. Because some of its effects are influenced by the phosphorylation state of these receptors, it has been postulated that TPM may bind to the membrane channel complexes and modulate the ionic conductance through the channels.[15]
TPM is rapidly and almost completely absorbed after oral administration. Its plasma concentration increases linearly with the increasing dose. The metabolism of TPM depends on hepatic P450 microsomal enzymes; therefore, its clearance is increased in the presence of enzyme-inducing antiepileptic drugs (AEDs) resulting in reduced TPM concentrations. It readily penetrates the central nervous system and nearly 70% to 80% is eliminated unchanged in the urine. The half-life is 21 hours with normal renal function and the time to steady state is 4-5 days.[17]
After initial evidence from open-label studies demonstrated efficacy,[18] the largest multicenter, randomized, double-blind, placebo-controlled trials ever conducted in migraine prevention confirmed the usefulness of this drug.[19,20] In both trials, participants were given varying doses of TPM or placebo. The best results were achieved at a dose of 100 mg or 200 mg, with no difference in efficacy observed between the 2 doses. Participants experienced a significant reduction in the frequency of migraine headaches, number of migraine days, and use of acute medications. On the basis of information about efficacy and tolerability, 100 mg/day of TPM should be the initial target dose for most patients, a lower dose than that used to treat epilepsy. The most common adverse events were paresthesias, fatigue, loss of appetite, nausea, diarrhea, weight loss, and taste perversion. TPM is currently considered a first-line migraine preventive drug and should especially be considered a preferred treatment for all patients who are concerned about gaining weight, who are currently overweight, or who have coexisting epilepsy.
TPM may be useful for pediatric migraineurs as well. Campistol and colleagues[21] evaluated 24 patients aged 6-14 years in a 4-month trial, observing efficacy and safety of TPM with a mean dose of 3.5 mg/kg/day after titration. A significant decline in severity and duration of attacks was shown with good/excellent impressions of efficiency. Two patients withdrew because of paresthesias or lack of efficacy.
Tiagabine
Tiagabine (TGB) is an effective add-on therapy for partial seizures. TGB inhibits the neuronal and glial reuptake of GABA and therefore enhances GABA-mediated inhibition. TGB does not induce or inhibit the function of hepatic enzymes and does not displace tightly protein-bound drugs, such as carbamazepine, theophylline, warfarin, and digoxin. Total and unbound TGB concentrations are increased in patients with hepatic dysfunction but are unaffected in patients with renal failure.[22]
TGB was initially studied in patients with refractory migraine,[23] with doses titrated to 4 mg, 4 times daily. In an open-label study of 41 migraine patients who had been previously treated with divalproex and discontinued therapy because of adverse events or relative lack of efficacy, Freitag and colleagues[24] used a mean dose of 10 mg/day. In this study, 5 patients experienced a remission of their migraine attacks, and 33 of the 41 studied had at least a 50% reduction in their attacks. The reported side effects, usually mild to moderate in severity and almost always resolving without medical intervention, included dizziness, asthenia (fatigue or generalized muscle weakness), nervousness, tremor, trouble concentrating, mental lethargy, slowness of thought, depression, aphasia, and abdominal pain.[24] The drug is not a standard treatment for migraine and does not carry an FDA indication for migraine.
Levetiracetam
Levetiracetam (LCT) is a new AED of unknown mechanism of action, although it has proved to be a broad-spectrum anticonvulsant in animal models.[25] It is rapidly and nearly completely absorbed after oral administration; peak serum concentrations are achieved within 2 hours, and daily doses are linearly related with plasma concentrations. LCT is metabolized primarily by hydrolysis of the acetamide group to the inactive carboxylic derivative and it is poorly protein-bound (< 10%).[26] The metabolic degradation of LCT is independent of the hepatic system of cytochrome P450, and therefore is not affected by the concomitant use of other AEDs. In children as well as in adults, steady state is achieved after 2 days of twice-daily dosing.
Anecdotal evidence suggests the usefulness of LCT in the prevention of migraine.[27,28] A recent study assessed LCT as prophylaxis of transformed migraine. Mean headache frequency per month at baseline was 24.9 and a significant reduction of headache frequency was obtained in 1 month (19.4, P < .001), 2 months (18.4, P < .001), and 3 months (18.0, P < .001).[29] The most common side effects reported in these initial clinical trials included fatigue or tiredness, somnolence, dizziness, and infection (common cold or upper respiratory tract infection).
Recently, the efficacy and safety of LCT for pediatric migraine was evaluated in a population of 30 children or adolescents aged 6 to 19 years (mean 12.9 years). This was a 10-week, open-label study. Among the 19 patients who completed the study, 6 patients experienced at least a 50% reduction in headache frequency and severity; 8 had at least 75% improvement; 3 became headache-free; and 2 developed worsening headaches. In 16 of the participants, disability decreased and quality of life improved, evaluated by PedMIDAS. One patient reported delusions and violent behavior; 1 patient developed a seizure disorder; 5 patients did not comply; and 4 withdrew because of lack of efficacy.[30]
Zonisamide
Zonisamide (ZNS) is a sulfonamide derivative that is structurally and chemically unrelated to other AEDs. It has been used for adjunctive therapy of partial seizures, and it is rapidly and nearly completely absorbed after oral administration with negligible first-pass metabolism.[31] ZNS is unaffected by tightly protein-bound drugs, is binding to plasmatic proteins between 40% and 60%, and does not affect the protein binding of other drugs. The plasma half-life of ZNS in healthy volunteers after a single oral dose ranges from 50 hours to 68 hours, but in the presence of enzyme-inducing AEDs, it decreases by approximately 50%.[31–33]
ZNS presents a unique combination of pharmacologic actions: It blocks voltage-dependent sodium and T-type (but not L-type) calcium channels; reduces glutamate-mediated excitatory neurotransmission; inhibits excessive nitric oxide (NO) production, scavenging hydroxyl and NO radicals; and inhibits carbonic anhydrase. All of these mechanisms may play a role in headache and pain modulation, possibly via neuronal stabilization.[31–34]
ZNS was studied for migraine prevention in 2 open-label trials presented. The first evaluated 33 patients with mixed headache disorders and refractory migraines.[35] Most had not responded to at least 2 previous preventive agents. ZNS was started at a dosage of 100 mg at bedtime every third day for 4-5 doses. The dosage frequency was then increased to every other day for another 4-5 doses, followed by the same dosage on a daily regimen. Dosage was adjusted upward every 2-3 weeks and in some cases reached as high as 600 mg/day. A total of 18% of the participants reported a 65% or better reduction in the frequency of migraine attacks and other headaches; 24.2% reported a 25% to 50% decrease in the same parameter; and 27% did not respond or were noncompliant with the protocol.
In the second study,[36] 34 patients with migraine with and without aura who were refractory to other preventive therapies received an initial dosage of 100 mg of ZNS daily, which was titrated as tolerated to 400 mg daily. Headache severity was significantly reduced as well as the other headache measures. The side effects reported included paresthesia, fatigue, anxiety, and weight loss. Agitated dysphoria and difficulty concentrating were also observed.
The tolerability of ZNS is favorable if titrated slowly. The most common side effects reported are somnolence, ataxia, anorexia, confusion, abnormal thinking, nervousness, fatigue, and dizziness. ZNS is weight-neutral and some patients report weight loss. Reports on development of kidney stones, leukopenia, and abnormal liver enzyme levels have been described.[32,34]
Petasites
Petasites is an extract from the plant Petasites hypridus (butterbur), which is a perennial shrub found throughout Europe and parts of Asia and North America. It has been used medicinally for centuries and during the Middle Ages was used to treat plague and fever. In the 17th century, butterbur was used frequently in treating cough, asthma, and skin wounds. In addition, petasites has been reported to inhibit peptide-leukotriene biosynthesis, possibly through calcium channel regulation.[37,38]
The efficacy of petasites in migraine prevention was studied in 2 trials. A small randomized, double-blind, placebo-controlled trial reported that a low dose of petasites, 50 mg twice daily, significantly reduced the number of migraine attacks per month and the number of migraine days per month.[39] In a larger double-blind, 5-month trial, Lipton and colleagues[40] randomized patients to receive either petasites 50 mg or 75 mg twice daily, or placebo. Compared with placebo-treated patients, the 4-month mean attack count was reduced by 48% in patients treated with petasites 75 mg twice daily, 34% with petasites 50 mg twice daily, and 26% with placebo (P < .01). All the other parameters showed similar results. The investigators concluded that petasites 75 mg twice daily may be an effective alternative preventive treatment for migraine.[40]
The tolerability of this herbal product seems to be good because side effects from petasites extracts have not been reported. However, the plant's pyrrolizidine alkaloids are thought to cause liver damage and to be carcinogenic in animals.[38] Therefore, extracts are commercially available in which the pyrrolizidine alkaloids have been removed. There are no known interactions with either pharmaceutical or over-the-counter anti-inflammatory agents; however, use of petasites extracts during pregnancy and lactation is contraindicated.[40]
Carvedilol
The use of beta blockers for migraine prevention is not new. The evidence for the use of this pharmacologic class was well established with propranolol, timolol, atenolol, and nadolol. The use of novel beta blockers, such as carvedilol, for the prophylactic treatment of migraine is a new concept because it offers additional alpha-1 blocking and antioxidant properties. This nonselective alpha-1 and beta-1 antagonist reduces blood pressure by reducing peripheral vascular resistance with no alteration of heart frequency or cardiac debit.[41] The results are a very favorable adverse event profile, which may represent an appeal in migraine prevention because traditional beta blockers have limiting side effects.
Carvedilol was initially studied for migraine in a prospective, open-label trial involving 76 patients. The dosages were titrated from 3.125 mg/day to 6.25 mg twice daily over 2 weeks. After 6 weeks of maintaining a stable dosage, patients were required to choose between keeping the 6.25 mg twice-daily dosage, increasing it to 12.5 mg twice daily, or decreasing it to 3.125 mg twice daily. Of the 68 patients who completed the study, 40 (59%) experienced a 50% reduction in monthly migraine attack frequency at the third month of treatment. Ten (15%) didn't present any significant response, and 18 (26%) withdrew because of lack of efficacy, or as a result of adverse events, including diarrhea, insomnia, nausea, dizziness, and myalgias.[42]
Tizanidine
Tizanidine hydrochloride is an alpha2-adrenergic presynaptic agonist that inhibits the release of norepinephrine in the brainstem and spinal cord. The antinociceptive effect does not involve the opioid system but is expressed on the alpha-adrenergic system at the alpha receptors located at the substantia nigra pars compacta.[43] Tizanidine is not an antihypertensive medication but contains several pharmacologic similarities to clonidine, another alpha2-adrenergic presynaptic agonist that has been advocated for migraine prophylaxis. Studies with cats demonstrated an inhibitory effect on vasoconstrictor and vasodilator responses to noradrenaline, adrenaline, isoprenaline, and angiotensin.[44]
The efficacy of tizanidine in headache was shown in a controlled study involving the treatment of chronic daily headache, especially in chronic migraineurs.[43] In addition, an open study of 220 patients demonstrated efficacy in both migraine and chronic tension-type headache,[46] which makes this drug attractive as a possible prophylactic treatment of episodic migraine and tension-type headache.
Quetiapine
Quetiapine (QTP) is a dibenzothiazepine derivative classified as an atypical antipsychotic drug with a low affinity for dopaminergic D1 and D2 receptors but high affinity for D4 receptors. QTP has an interesting characteristic of presenting antagonistic properties at many neurotransmitter receptors. In addition, it has interesting, more pronounced effects on mesolimbic than on nigrostriatal dopaminergic pathways, which results in better tolerability with regard to extrapyramidal symptoms.[47] QTP represents a new hope for migraineurs because it also possesses high affinity for 5-HT2 receptors, partial agonistic activity at 5-HT1A receptors, and a blocking activity at alpha1-adrenergic receptors with a consequent potential for migraine prevention.[47,48] QTP has a short half-life of 2-3 hours, requiring a twice-daily regimen for schizophrenic patients. It presents a favorable side-effect profile if titrated gradually, but somnolence and dizziness may be common. In 7% of the patients, orthostatic hypotension may occur. Its pharmacokinetics is not affected by sex, ethnic group, cigarette smoking, or body weight.[49]
The role of QTP in migraine was studied in 24 migraineurs who had a history of not responding at least to 2 agents. QTP was initiated as an add-on therapy at a dose of 25 mg daily with a progressive titration to a maximum of 150 mg daily. At an average dose of 75 mg daily, 21 of the 24 patients showed significant improvement in either frequency or severity, or both, of migraine. Disability, evaluated by the Migraine Disability Assessment (MIDAS) score, improved by at least 1 grade in 18 patients, with none presenting serious side effects or extrapyramidal symptoms. One patient discontinued the drug because of sedation.[49] The clinical impression is that QTP may represent a very important resource for patients with refractory migraine or patients with psychological disturbances.[48,49]
Botulinum Toxin
Botulinum toxin (BTX) is a bacterial neurotoxin approved for the treatment of strabismus, blepharospasm, and hemifacial spasm; BTX also has been safely used for spasticity, tremor, dystonia, and other neuromuscular disorders of inappropriate muscular contraction. BTX has also been used to reduce wrinkles and hyperfunctional lines of the face. BTX causes long-term cholinergic blockade at the neuromuscular junction, which is thought to be responsible for its chemodenervating action and the therapeutic effect causing muscle paresis or paralysis. In addition, it has antinociceptive properties, an effect unrelated to its inhibition of the muscle contraction.[50] BTX may work in migraine through the suggested inhibition of substance P, calcitonin gene-related peptide (CGRP), and glutamate.[50,51]
BTX has been evaluated in open-label trials and large multicenter studies.[52,53] Silberstein and associates[53] examined the safety and efficacy of BTX in migraine prevention with a double-blind, vehicle-controlled design. A total of 123 patients from 12 headache centers were recruited in this double-blind, randomized, placebo-controlled (vehicle-controlled), parallel-group prospective study. The requirements of the protocol included a 1-month baseline, an injection visit, 3 monthly postinjection visits, and completion of a daily headache diary. Patients with migraine (with or without aura) who had experienced an average of 2-8 moderate-to-severe migraines per month during the 3 previous months were eligible. Patients were randomized to 1 of 3 groups: BTX 25 U or 75 U, or vehicle. The sites of the injections were symmetric into the glabellar, frontalis, and temporalis muscles. Participants kept diaries for 3 months post injection.
The group of patients that received 25 U of BTX received a significantly better performance than the vehicle in all endpoints. The 75-U BTX treatment group was significantly more improved than the vehicle group on patient global assessment for days 31-60 but not other parameters. BTX was well tolerated, but the group that was injected with 75 U showed significantly more treatment-related adverse events than vehicle. The conclusion of the study was that pericranial injection of BTX, 25 U, is effective for the treatment of migraine. The adverse effects, usually transient and mild, included blepharoptosis, diplopia, and injection-site weakness, which is an expected drug effect.
In other study, BTX reduced the number of headache days 60 patients with either chronic tension-type headache or chronic migraine compared with placebo, but this difference was not statistically significant.[54] Results from another large multicenter trial of BTX in the preventive treatment of chronic daily headaches, already conducted, are anxiously awaited.
Compounds in Development
The ideal migraine drug should be effective both for prophylaxis and for acute treatment. The need for a well-tolerated preventive drug that can significantly reduce or eliminate migraine attacks, preferentially acting on different biological systems, is obvious. Cost is an issue as well. The probability that such a drug will be developed in the near future is low, however, because the nature of migraine is multifactorial and still emerging, and because patients' central nervous system responses are so variable. Although recent additions to the migraine pharmacologic arsenal demonstrate multiple effects on pathophysiologic mechanisms of migraine, a definitive drug is still far from reality.
Progress in finding new effective treatments for acute migraine has been more promising. Several drugs and potential targets are under development and will be highlighted in the next section.
Drugs Acting on 5-HT (Serotonin) Receptors
The 5-HT1B/1D agonists, or triptans, target the trigeminovascular system. They are vasoconstrictive medications, an action mediated through the 5-HT1B postsynaptic receptor subtype.[55] Because of the presence of 5HT1B receptors on peripheral and coronary vascular beds, the potential for serious adverse vascular events precludes the unlimited use of triptans because of safety concerns in subjects at a higher risk for cardiovascular events.[56] Theoretically, drugs targeting 5-HT1D, but not 5-HT1b, receptors could be effective without vasoconstrictive effects. In addition, 5-HT1F receptors appear to play an important role in migraine attacks. Several emerging migraine medications target these receptors subtypes without activity at the 5-HT1B receptor.[57] Two agents acting primarily on 5-HT1D and/or 5-HT1F receptors are PNU-142633 and the LY334370.
PNU-142633 is a highly selective 5-HT1D agonist with at least 1000-fold selectivity for the 5-HT1D receptor compared with the 5-HT1B receptor.[58] Animal model studies suggest that PNU-142633 blocks the neurogenic inflammation and the rise in trigeminal nucleus blood flow normally elicited by stimulation of trigeminal afferent fibers, with no evidence of vasoconstriction on carotid, meningeal, or coronary arteries.[58] Its safety and tolerability were demonstrated in a phase 1, single-dose, double-blind study. Thirty-nine patients received doses from 1 mg to 100 mg, with no serious adverse events reported; the most common side effects were headache and dizziness.[58] However, another study using a 50-mg oral dose of PNU-142633 failed to show a significant treatment effect compared with placebo.[59] With regard to safety issues, 3 of the 34 patients receiving PNU-142633 experienced QTc interval prolongation on electrocardiography and 2 of these reported chest pain. Definite conclusions about this study are of questionable validity because PNU-142633 was developed with gorilla 5HT1D receptors, and differences in relative potency on the 5-HT1D receptors must be considered. Another 5-HT1D agonist, PNU-109291, did not demonstrate efficacy in preclinical models of migraine. Further studies are still under way with this compound.[60]
Most of the triptans currently in use were shown to exert an effect at cloned human 5-HT1F receptors; in addition, in animal models, selective 5-HT1F agonism inhibited neurogenic inflammation.[61,62] Recently, a selective 5-HT1F receptor agonist, LY334370, has been developed. In phase 1 studies, LY334370 was not associated with evidence of cardiac ischemia on electrocardiogram. However, intravenous doses up to 20 mg and oral doses up to 200 mg caused somnolence, mild-to-moderate asthenia, dizziness, and paresthesias.[63] In a double-blind, randomized, placebo-controlled study, migraine sufferers were randomized to receive placebo or 20 mg, 60 mg, or 200 mg of LY334370.[64] Headache response, pain-free, and sustained pain-free rates after 2 hours were significantly better in the 60-mg and 200-mg treatment groups, compared with those receiving placebo. The patients receiving 200 mg experienced the best results; 71% of these patients had headache response at 2 hours and 38% achieved pain-free status. In all, 33% demonstrated sustained pain-free response at 24 hours. The tolerability was troublesome; however, 80% of patients receiving the 200-mg dose reported at least 1 adverse event. In addition, a high toxicity in animal studies was observed and the development of the drug was interrupted. Further studies with other agents of this pharmacologic class are in process.[64]
Adenosine Receptors
Adenosine has an established antinociceptive effect in humans. Recent findings in rats suggest that the both chronic and acute analgesic effects may be mediated by adenosine A1 receptors within the spinal cord.[65] However, despite the existence of the A1 receptor protein in human trigeminal ganglia, the relevance of these findings for migraine is still unknown.[66] Currently, 2 selective A1 receptor agonists, GR79236 and GR190178, are under development. In studies with cats, these compounds inhibited trigeminal nociceptive transmission and the peripheral release of CGRP in the cranial circulation and at the central trigeminal synapse. In addition, in the rat model, GR79236 inhibited the firing of second-order neurons in response to electrical stimulation of nociceptive afferents of the trigeminal nucleus caudalis.[67,68] This process, if operative, may prevent activation of central trigeminal neurons.[69] In fact, measured by suppression of nociceptive blink reflex, GR79236 has been demonstrated to effectively inhibit trigeminal nociception in humans, which supports its potential as an acute migraine treatment.[70]
Vanilloid Receptors
The vanilloid type 1 (VR1) receptors are located on small and medium-sized neurons, which are either unmyelinated C-fibers or thinly myelinated A-delta fibers. VR1 receptors also are present on neurons in the human trigeminal ganglia.[71] Capsaicin is the best known vanilloid and activates afferent fibers involved in nociceptive transmission as well as in neurogenic inflammation. This activation leads to rapid desensitization, and loss of sensitivity to heat and chemical stimulation with consequent inability to release the neurochemicals involved in neuronal transmission and inflammation, such as substance P and CGRP. In addition, intravenous capsaicin promotes the release of the proinflammatory neuropeptides from trigeminal neurons and has been shown to cause dilatation of the vasculature of rat dura.[72] The possibility that VR1 receptor activation results in CGRP-induced vasodilatation at the trigeminovascular junction have lead to the study of a capsaicin derivative as a potential antimigraine drug. Civamide is a cis-monomer of capsaicin with vanilloid agonistic properties as well as a neuronal calcium channel effect. Its antimigraine action may be through the release of neurotransmitters to meningeal and dural blood vessels, reducing neurogenic inflammation, peripheral sensitization, and headache pain.[73]
The possible efficacy of intranasal civamide in migraine was studied in a non-placebo-controlled study of 34 patients with migraine. In all, 55% of the patients in whom nasal burning and lacrimation occurred frequently achieved headache relief at 2 hours. No systemic side effects were reported with the intranasal use of civamide.[73]
CGRP
CGRP is a neuropeptide found within cell bodies of the sensory terminals in the trigeminal nerve. Along with other neuropeptides, such as substance P and neurokinin A, it innervates the cerebral vasculature and may exert a counterbalance effect on cerebrovascular contraction. This action, which is induced by dilatation of cerebral vessels, increasing cerebral blood flow and mediating the trigeminal reflex, relates to CGRP receptors existing in various cerebral and cranial arteries.[74] In addition, in animal models, stimulation of trigeminal ganglion fibers results in release of CGRP leading to neurogenic vasodilation.[75]
CGRP has an important role in migraine. CGRP levels are increased in the external jugular venous blood during spontaneous migraine attacks or following electrical or chemical stimulation, with normalization of levels after treatment with sumatriptan.[76,77] Furthermore, infusion of human CGRP in migraineurs induces migrainelike headache.[76] The investigation of the CGRP role in migraine has led to the development of CGRP antagonists, which are still in preclinical phase trials.
BIBN4096BS is a small molecule with a high affinity and specificity for the human CGRP receptor. It is a nonpeptide CGRP-receptor antagonist.[78] Healthy volunteers were tested to evaluate the safety and tolerability of BIBN4096BS. They were randomized to receive intravenous placebo or drug in doses ranging from 0.1 mg to 10 mg. There were no clinically relevant changes in pulse rate, blood pressure, respiratory rate, electrocardiography, laboratory tests, or forearm blood flow. Fatigue and paresthesias were the most reported adverse events.[79] The first study in migraine was carried out with a multicenter, randomized, double-blind design.[80] The primary endpoint was headache efficacy (severe or moderate headache at baseline to mild or no headache at 2 hours). The 2.5-mg dose resulted in a response rate of 66%, compared with 27% for the placebo. Other endpoints, such as pain-free rate at 2 hours; sustained response over 24 hours; recurrence of headache; improvement of nausea, photophobia, phonophobia, andfunctional capacity, were also evaluated and demonstrated the superiority of BIBN4096BS over placebo. A total of 25% of the patients receiving the 2.5-mg dose reported adverse events, consisting mostly of paresthesias and no serious adverse events.[80]
Drugs Modulating Glutamate
Glutamate is the major excitatory neurotransmitter in central nociceptive pathways and has been implicated in the pathophysiology of migraine. In animal models, glutamate promotes excitation of neurons at the trigeminal nucleus caudalis. In addition, noxious stimuli trigger rising levels of glutamate in the trigeminal nucleus caudalis.[81] The glutamate receptors may be a G-protein-coupled receptor (metabotropic) (such as mGlu receptors groups I, II, or III) or ion-forming channel (ionotropic) receptors (such as NMDA, non-NMDA [AMPA], and kainite [KA] types).[82] The path to develop ionotropic NMDA receptor antagonists has been frustrating because of its bad tolerability profile.[83] However, non-NMDA AMPA/KA antagonists, such as LY293558, have shown promising preclinical performance.[84] The efficacy of LY293558 in migraine was evaluated recently in a pilot study in which 45 patients were enrolled and 44 completed the trial with response rates of 69% vs 25% of the placebo (P = .017). LY293558 has an acceptable tolerability profile, with visual distortions as the most prominent adverse event. Minor effects, such as dizziness and sedation, were also reported, and no serious side effects were described.[85]
Drugs Acting at the NO Synthase
NO is a small molecule gas synthesized from L-arginine with potent vasodilator properties. NO is found in endothelial cells, granulocytes, platelets, and the brain. In the normal brain, endothelial and neuronal NO are expressed, but a third isozyme that synthesizes NO appears to be inducible in response to tissue damage or following nitroglycerine administration.[86] The relationship of nitric oxide synthase (NOS) inhibition on neuronal activity in the trigeminal nucleus has been demonstrated in animal models. The infusion of a NOS inhibitor significantly reduced neuronal activity, suggesting that NO may play an important role in sensitized neurons in the trigeminal nucleus. In addition, the administration of the exogenous NO donor nitroglycerin in rats induces delayed plasma protein extravasation in dura mater. Furthermore, the cortical spreading depression, which releases CGRP from nerve terminals triggering the migraine cascade, also releases NO.[87] These observations along with the knowledge that intravenous nitroglycerine infusion causes a migraine attack in migraineurs but not in nonmigraine controls suggests the important role of NO in migraine.[88]
Thus far, LNMAH 546C88 is the only NOS inhibitor being studied. This agent was administered intravenously to 15 patients with migraine, and compared with 14 subjects who received placebo. Ten of the 15 patients experienced relief 2 hours after the infusion compared with 2 of 14 in the placebo group.[89]
Although well tolerated, LNMAH 546C88 did not alleviate histamine-induced migraine, but in a randomized, double-blind, crossover trial of 16 patients with chronic tension-type headache (patients were assigned intravenous infusion of 6 mg/kg L-NMMA or placebo for 2 days separated by at least 1 week in a randomized order), L-NMMA reduced pain intensity on the visual analog scale significantly more than placebo. These preliminary results suggest that NOS inhibition may represent a safe and effective way of treating acutely primary headache in which NO mechanisms may be involved.[90,91]
Potential Development Issues
Although many of the drugs presented in this review provide effective migraine relief, they do not approach having the prerequisites of an ideal antimigraine medication. With the exception of sodium divalproex and TPM, most of the current prophylactic agents have not been studied in randomized controlled trials with an acceptable number of subjects. We look forward to a drug, or at least a strategy of treatment, that can act on the glutamatergic-GABAergic transmission imbalance in addition to exerting a modulating effect on the serotonergic system.
Although effective, most of the current options for acute therapy do not offer consistent and fast pain-free endpoints in all patients. Acting simultaneously in different pathophysiologic mechanisms of a migraine attack, such as inflammation and “low serotonin,” is an attractive goal. In addition, developing a serotonergic agonist devoid of vasoconstrictor effects (ie, no 5-HT1B action) would represent a significant advance, as would an agent that acts on different targets as the CGRP receptor.
The importance of rational polytherapy is indisputable. Combining pharmacologic agents with actions on specific, different neurotransmitter systems and targets is a necessary goal. As a multifactorial disease, migraine should be managed through multimodal pharmacotherapy, which currently cannot be provided by a single agent.
Conclusion
The possibility of better modulating the imbalance between central neurotransmitters that occurs with migraine has created an exciting search for new pharmacologic sites. The use of animal models to predict human effects has not been as successful as expected but more clinically predictive pharmacologic models are being developed. Drugs acting on the early stages of migraine and nonvasoactive therapies are in the late stages of development. Neuromodulators for the prevention of multiple mechanisms related to migraine are already available. Obtaining synergies by combining agents with different sites of action is a valuable approach. The better use of available drugs, beyond conservative monotherapy, may represent an important strategy for helping migraine patients until better drugs are developed.
Contributor Information
Marcelo E. Bigal, The New England Center for Headache, P.C., Stamford, Connecticut; Department of Neurology, Albert Einstein College of Medicine, Bronx, New York.
Abouch V. Krymchantowski, Outpatient Headache Unit, Instituto de Neurologia Deolindo Couto, Rio de Janeiro, Brazil; Headache Center of Rio de Janeiro, Rio de Janeiro, Brazil.
References
- 1.Stewart WF, Shechter A, Lipton RB. Migraine heterogeneity, disability, pain intensity, and attack frequency and duration. Neurology. 1994;44:S24–S39. [PubMed] [Google Scholar]
- 2.Goadsby PJ. Pathophysiology of migraine. In: Silberstein SD, Lipton RB, Dalessaio DJ, editors. Wolff's Headache and Other Head Pain. 7th ed. Oxford: Oxford University Press; 2001. pp. 57–72. [Google Scholar]
- 3.May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiology implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Lipton RB, Ferrari MD. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 5.Welch KMA, Barkley GL, Tepley N, et al. Central neurogenic mechanisms of migraine. Neurology. 1993;43:S21–S25. [PubMed] [Google Scholar]
- 6.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 7.Tfelt-Hansen P, Block G, Dahlof C, et al. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20:765–786. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review) Neurology. 2000;55:754–763. doi: 10.1212/wnl.55.6.754. [DOI] [PubMed] [Google Scholar]
- 9.Dodick DW. Acute and prophylactic management of migraine. Clin Cornerstone. 2001;4:36–52. doi: 10.1016/s1098-3597(01)90038-9. [DOI] [PubMed] [Google Scholar]
- 10.Ramadan MN, Silberstein SD, Freitag FG, Gilbert TT, Frishberg GM. Pharmacological management for prevention of migraine. Available at: http://www.aan.com/public/practiceguidelines/ Accessed July 2005.
- 11.Sheftell FD, Feleppa M, Tepper SJ, Volcy M, Rapoport AM, Bigal ME. Patterns of use of triptans and reasons for switching them in a tertiary care migraine population. Headache. 2004;44:661–668. doi: 10.1111/j.1526-4610.2004.04124.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 13.Matchar DB, Young WB, Rosenerg J, et al. Multispecialty consensus on diagnosis and treatment of headache: pharmacological management of acute attacks. Neurology. 2000;54 doi: 10.1212/wnl.54.8.1553. Available at: http://www.aan.com/public/practiceguidelines/ Accessed July 2005. [DOI] [PubMed]
- 14.Bigal ME, Lipton RB, Krymchantowski AV. The medical management of migraine. Am J Ther. 2004;11:130–140. doi: 10.1097/00045391-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41:35–39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 16.Sigel E. Functional modulation of ligand-gated GABAA and NMDA receptor channels by phosphorilation. J Receptor Signal Transduct Res. 1995;15:325–332. doi: 10.3109/10799899509045224. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs JW, Sombati S, Delorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainite-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41:S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 18.Shuaib A, Ahmed F, Muratoglu M, Kochanski P. Topiramate in migraine prophylaxis: a pilot study. Cephalalgia. 1999;19:379–380. [Google Scholar]
- 19.Silberstein SD, Neto W, Schmitt J, Jacobs D, Migr-001 Study Group Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61:490–495. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- 20.Brandes JL, Saper JR, Diamond M, et al. Migr-002 Study Group Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291:965–973. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- 21.Campistol J, Campos J, Casas C, Herranz JL. Topiramate in the prophylactic treatment of migraine in children. J Child Neurol. 2005;20:251–253. [PubMed] [Google Scholar]
- 22.Pelloch JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving anti-epileptic drugs. Neurology. 1991;41:961–964. doi: 10.1212/wnl.41.7.961. [DOI] [PubMed] [Google Scholar]
- 23.Drake ME, Jr, Kay AM, Knapp MS, et al. An open-label trial of tiagabine for migraine prophylaxis. Headache. 1999;39:352. [Google Scholar]
- 24.Freitag FG, Diamond S, Diamond ML, et al. The prophylaxis of migraine with the GABA-agonist, tiagabine: a clinical report. Headache. 1999;39:354. [Google Scholar]
- 25.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent model of seizures and epilepsy. Eur J Pharmacol. 1998;353:191. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas J-M, Collart P, Gerin B, et al. In vitro evaluation of potential drug interactions with levetiracetam, a new antiepileptic agent. Drug Metab Disp. 1999;27:250–254. [PubMed] [Google Scholar]
- 27.Drake ME, Greathouse NI, Armentbright AD, Renner JB. Levetiracetam for preventive treatment of migraine. Cephalalgia. 2001;21:373. [Google Scholar]
- 28.Krusz JC. Levetiracetam as prophylaxis for resistant headaches. Cephalalgia. 2001;21:373. [Google Scholar]
- 29.Rapoport AM, Sheftell FD, Tepper SJ, Bigal ME. Levetiracetam in the preventive treatment of transformed migraine. Curr Ther Res. 2005;66:212–221. doi: 10.1016/j.curtheres.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaisleb I, Neft R, Schor N. Role of Levetiracetam in prophylaxis of migraine headaches in childhood. Neurology. 2005;64:A343. [Google Scholar]
- 31.Schmidt D, Jacob R, Loiseau P, et al. Zonisamide for add-on treatment of refractory partial epilepsy: A European double-blind trial. Epilepsy Res. 1993;15:67–73. doi: 10.1016/0920-1211(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Hori M, Kadokawa T. Effects of zonizamide (AD-810) on tungistic acid gel-induce thalamic generalized seizures and conjugated estrogen-induced cortical spike-wave discharge in cats. Epilepsia. 1986;27:367–374. doi: 10.1111/j.1528-1157.1986.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 33.Yagi K, Seino M. Methodological requirements for clinical trials in refractory epilepsies: our experience with zonisamide. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:79–85. doi: 10.1016/0278-5846(92)90010-c. [DOI] [PubMed] [Google Scholar]
- 34.Mimaki T. Clinical pharmacology and therapeutic drug monitoring of zonisamide. Ther Drug Monit. 1998;20:593–597. doi: 10.1097/00007691-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Krusz JC. Zonisamide in the treatment of headache disorders. Cephalalgia. 2001;21:374–375. [Google Scholar]
- 36.Drake ME, Greathouse NI, Armentbright AD, Renner JB. Preventive treatment of migraine with zonisamide. Cephalalgia. 2001;21:374. [Google Scholar]
- 37.Eaton J. Butterbur, herbal help for migraine. Nat Pharm. 1998;2:23–24. [Google Scholar]
- 38.Lin H, Chien CH, Lin YL, et al. Inhibition of testosterone secretion by S-petasin in rat testicular interstitial cells. Chin J Physiol. 2000;43:99–103. [PubMed] [Google Scholar]
- 39.Grossmann M, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Int J Clin Pharmacol Ther. 2000;38:430–435. doi: 10.5414/cpp38430. [DOI] [PubMed] [Google Scholar]
- 40.Lipton RB, Gobel H, Wilks K, Mauskop A. Efficacy of petasites (an extract from Petasites rhizone) 50 and 75 mg for prophylaxis of migraine: results of a randomized, double-blind, placebo controlled study (abstract) Neurology. 2002;58:A472. [Google Scholar]
- 41.Cleophus TJ, Zwinderman AH. Beta-blockers and heart failure: meta-analysis of mortality trials. Int J Clin Pharm Ther. 2001;39:383–387. doi: 10.5414/cpp39383. [DOI] [PubMed] [Google Scholar]
- 42.Kaniecki RG. Migraine prevention with Carvedilol: a prospective, open-label trial. Headache. 2003;43:589. [Google Scholar]
- 43.Zaimis E, Hanington E. A possible pharmacological approach to migraine. Lancet. 1969;2:298–300. doi: 10.1016/s0140-6736(69)90058-0. [DOI] [PubMed] [Google Scholar]
- 44.D'Andrea G, Perini F, Granella F, Cananzi A, Sergi A. Efficacy of transdermal clonidine in the treatment of cluster headache. Headache Treatment. In: Olesen J, Tfelt-Hansen P, editors. Trial Methodology and New Drugs. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 335–340. [Google Scholar]
- 45.Saper JR, Lake AE, Cantrell DT, Winner PK, White JR. Chronic daily headache prophylaxis with tizanidine: a double blind, placebo controlled, multicenter outcome study. Headache. 2002;42:470–482. doi: 10.1046/j.1526-4610.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 46.Krusz JC, Belanger J, Mills C. Tizanidine: a novel effective agent for the treatment of chronic headaches. Headache. 2000;11:41–45. [Google Scholar]
- 47.Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine and quetiapine. Am J Psychiatry. 1999;156:876–884. doi: 10.1176/ajp.156.6.876. [DOI] [PubMed] [Google Scholar]
- 48.Schatzberg AF, Cole JO, Debattista C. Manual of Clinical Psychopharmacology. 4th ed. Washington, DC: American Psychiatric Publishing; 2002. pp. 199–200. [Google Scholar]
- 49.Brandes JL, Roberson SC, Pearlamn SH. Quetiapine for migraine prophylaxis. Headache. 2002;42:450–451. [Google Scholar]
- 50.Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 51.Volknandt W. Commentary: the synaptic vesicle and its targets. Neuroscience. 1995;64:277–300. doi: 10.1016/0306-4522(94)00408-w. [DOI] [PubMed] [Google Scholar]
- 52.Evers S, Rahmann A, Vollmer-Haase J, Husstedt IW. Treatment of headache with botulinum toxin A – a review according to evidence-based medicine criteria. Cephalalgia. 2002;22:699–710. doi: 10.1046/j.1468-2982.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 53.Silberstein SD, Mathew NT, Saper J, Jenkins S, Botox Migraine Clinical Research Group Botulinum toxin type A as a migraine preventive treatment. Headache. 2000;40:445–450. doi: 10.1046/j.1526-4610.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 54.Ondo W, Vuong K, Derman H. Botulinum toxin A for chronic daily headache: a randomized, placebo-controlled, parallel-design study. Cephalalgia. 2004;24:6–15. doi: 10.1111/j.1468-2982.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 55.Humphrey PPA, Goadsby PJ. Controversies in headache. The mode of action of sumatriptan is vascular? A debate Cephalalgia. 1994;14:401–410. doi: 10.1046/j.1468-2982.1994.1406401.x. [DOI] [PubMed] [Google Scholar]
- 56.Maassen Vandenbrink A, Reekers M, Bax WA, et al. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation. 1998;98:25–30. doi: 10.1161/01.cir.98.1.25. [DOI] [PubMed] [Google Scholar]
- 57.Buchanan TM, Ramadan NM, Aurora S. Future pharmacologic targets for acute and preventive treatments of migraine. Expert Rev. Neurother. 2004;4:391–430. doi: 10.1586/14737175.4.3.391. [DOI] [PubMed] [Google Scholar]
- 58.Mccall RB, Huff R, Chio CL, et al. Preclinical studies characterizing the antimigraine and cardiovascular effects of the selective 5-HT1D receptor agonist PNU-142633. Cephalalgia. 2002;22:799–806. doi: 10.1046/j.1468-2982.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 59.Fleishaker JC, Pearson LK, Knuth DW, et al. Pharmacokinetics and tolerability of a novel 5-HT1D agonist, PNU-142633. Int J Clin Pharmacol Ther. 1999;37:487–492. [PubMed] [Google Scholar]
- 60.Gomez-Mancilla B, Cutker NR, Leibowitz MT, et al. Safety and tolerability of PNU-142633, a selective 5-HT1D agonist, in patients with acute migraine. Cephalalgia. 2001;21:727–732. doi: 10.1046/j.1468-2982.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- 61.Adham N, Kao HT, Schecter LE, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci U S A. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shepheard S, Edvinsson L, Cumberbatch M, et al. Possible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370. Cephalalgia. 1999;19:851–858. doi: 10.1046/j.1468-2982.1999.1910851.x. [DOI] [PubMed] [Google Scholar]
- 63.Phebus LA, Johnson KW, Zgombick JM, et al. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 1997;61:2117–2126. doi: 10.1016/s0024-3205(97)00885-0. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein DJ, Roon KI, Offen WW, et al. Selective serotonin 1F (5-HT(1F) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet. 2001;358:1230–1234. doi: 10.1016/s0140-6736(01)06347-4. [DOI] [PubMed] [Google Scholar]
- 65.Reeve AJ, Dickenson AH. The roles of spine adenosine receptors in the control of acute and more persistent nociceptive responses of dorsal horn neurons in the anesthetized rats. Br J Pharmacol. 1995;116:2221–2228. doi: 10.1111/j.1476-5381.1995.tb15057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schindler M, Harris CA, Hayes B, Papotti M, Humphrey PP. Immunohistochemical localization of adenosine A1 receptors in human brain regions. Neurosci Lett. 2001;297:211–215. doi: 10.1016/s0304-3940(00)01643-8. [DOI] [PubMed] [Google Scholar]
- 67.Gurden MF, Coates J, Ellis F, et al. Functional characterization of three adenosine receptor subtypes. Br J Pharmacol. 1993;109:693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bland-Ward PA, Fentiuk W, Humphrey PPA. The adenosine A1 receptor agonist GR79236 inhibits evoked firing of trigeminal nucleus caudalis (TNC) neurons in the rat. Cephalalgia. 2000;20:271–274. [Google Scholar]
- 69.Goadsby PJ, Hoskin KL, Storer RJ, Edvinsson L, Connor HE. Adenosine (A1) receptor agonists inhibit trigeminovascular nociceptive transmission. Brain. 2002;125:1392–1401. doi: 10.1093/brain/awf141. [DOI] [PubMed] [Google Scholar]
- 70.Giffin NJ, Kowacs F, Libri V, et al. Effect of the adenosine A1 receptor agonist GR79236 on trigeminal nociception with blink reflex recordings in healthy humans subjects. Cephalalgia. 2003;23:287–292. doi: 10.1046/j.1468-2982.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 71.Hou M, Uddman R, Tajti J, Kanje M, Edvinsson L. Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci Lett. 2002;330:223–226. doi: 10.1016/s0304-3940(02)00741-3. [DOI] [PubMed] [Google Scholar]
- 72.Markowitz S, Saito K, Moskowitz M. Neurogenically mediated leakage of plasma proteins occurs from blood vessels in dura mater but not brain. Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diamond S, Freitag F, Phillips S, Bernstein J, Saper J. Intranasal civamide for the acute treatment of migraine headache. Cephalalgia. 2000;20:597–602. doi: 10.1046/j.1468-2982.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 74.Jansen-Olesen I, Jorgensen L, Engel U, Edvinsson L. In-depth characterization of CGRP receptors in human intracranial arteries. Eur J Pharmacol. 2003;481:207–216. doi: 10.1016/j.ejphar.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 76.Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43:S16–S20. [PubMed] [Google Scholar]
- 77.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 78.Doods H, Hallermayer G, Wu D, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iovino M, Feifel U, Young CL, Wolters JM, Wallestein G. Safety, tolerability and pharmacokinetics of BIBN4096BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia. 2004;24:645–656. doi: 10.1111/j.1468-2982.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 80.Olesen J, Diener HC, Husstedt IW, et al. BIBN4096BS Clinical Proof of Concept Study. Calcitonin gene-related peptide receptor antagonist BIBN4096BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 81.Bereiter DA, Benetti AP. Excitatory amino acid release within spinal trigeminal nucleus after mustard oil injection into the temporomandibular joint region of the rat. Pain. 1996;67:451–459. doi: 10.1016/0304-3959(96)03156-9. [DOI] [PubMed] [Google Scholar]
- 82.Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- 83.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 84.Sang CN, Hostetter MP, Gracely RH, et al. AMPA/kainite antagonist LY-293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology. 1998;89:1060–1067. doi: 10.1097/00000542-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Sang CN, Ramadan NM, Wallihan RG, et al. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and web-tolerated in acute migraine. Cephalalgia. 2004;24:596–602. doi: 10.1111/j.1468-2982.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 86.Tassorelli C, Joseph SA, Buzzi MG, Nappi G. The effects on central nervous system of nitroglycerine – putative mechanism and mediators. Prog Neurobiol. 1999;57:607–624. doi: 10.1016/s0301-0082(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 87.Read SJ, Smith MI, Hunter AJ, Parsons AA. Enhanced nitric oxide release during cortical spreading depression following infusion of glyceryl trinitrate in the anesthetized cat. Cephalalgia. 1997;17:159–165. doi: 10.1046/j.1468-2982.1997.1703159.x. [DOI] [PubMed] [Google Scholar]
- 88.Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity. A possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Lassen LH, Ashina M, Christiansen I, et al. Nitric oxide synthase inhibition: a new principle in the treatment of migraine attacks. Cephalalgia. 1998;18:27–32. doi: 10.1046/j.1468-2982.1998.1801027.x. [DOI] [PubMed] [Google Scholar]
- 90.Lassen LH, Christiansen I, Iversen HK, Jansen-Olesen I, Olesen J. The effect of nitric oxide synthase inhibition on histamine induced headache and arterial dilatation in migraineurs. Cephalalgia. 2003;23:877–886. doi: 10.1046/j.1468-2982.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 91.Ashina A, Lassen LH, Bendtsen L, Janssen R, Olesen J. Effect of inhibition of nitric oxide synthase on chronic tension-type headache: a randomised crossover trial. Lancet. 1999;353:287–289. doi: 10.1016/s0140-6736(98)01079-4. [DOI] [PubMed] [Google Scholar]


