Abstract and Introduction
Abstract
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease characterized by the destruction of interlobular and septal bile ducts that can lead to fibrosis and cirrhosis. Orthotopic liver transplantation (OLT) remains the definitive treatment for decompensated liver disease secondary to PBC. An estimated 10% to 40% of patients develop clinical, biochemical, and histologic changes consistent with recurrent PBC after OLT. However, the presence of recurrent PBC does not appear to affect either graft or patient survival rates. There is conflicting evidence regarding the effect of specific immunosuppressant medications (eg, tacrolimus vs cyclosporine) on the risk of recurrent PBC. Most experts favor the use of ursodeoxycholic acid (UDCA) for recurrent PBC given its beneficial effect in patients with pretransplant PBC and its improvement of biochemical markers in the posttransplant setting. However, despite its potential benefit, there is no evidence that UDCA improves graft or patient survival in recurrent PBC.
Introduction
PBC is a chronic cholestatic liver disease that is characterized by the destruction of interlobular and septal bile ducts, leading to the development of fibrosis and, eventually, cirrhosis. The disease has a female predominance, with a 1:9 male:female ratio. The hallmarks of the disease are fatigue, pruritis, elevated serum alkaline phosphatase, and deficiency in fat-soluble vitamins (A, D, E, and K).
To date, the only US Food and Drug Administration-approved medication for PBC is UDCA. This agent has been shown to improve biochemical markers and transplant-free survival in patients with PBC.[1–4] However, several reports now question the ability of UDCA to affect disease progression and patient survival.[4–8] A recent study showed that liver failure developed in 26% of patients by 10 years after diagnosis.[9] OLT remains the only definitive treatment for PBC, and has been shown to prolong survival.[10] According to one study, survival at 2 and 5 years post OLT was 79% and 68%, respectively, whereas predicted survival without transplant was 55% and 22%, respectively.[10] Following OLT, a small subset of patients exhibit clinical, biochemical, and histologic changes consistent with the diagnosis of recurrent PBC.[11] Initially, several investigators challenged the view that recurrent PBC was indeed a disease entity. It was believed that these changes could be explained by rejection, infection, and various other processes.[11] However, as further data and experience have accumulated, it is now universally accepted that recurrent PBC is a true entity. This article discusses the clinical presentation, epidemiology, diagnosis, and treatment of this condition.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Clinical Presentation of Recurrent PBC
Recurrent PBC is typically suspected when abnormal hepatic biochemical tests are detected on routine laboratory screening. Alternatively, in centers that perform routine liver biopsies for post-OLT surveillance, recurrent PBC may be suspected in the presence of typical histopathologic findings. Similar to pretransplant PBC, symptoms are typically absent, or, when present, are often nonspecific, such as in the case of lethargy, pruritis, and fatigue.[11] Also as in pretransplant PBC, hepatic biochemical tests in recurrent PBC usually exhibit a cholestatic pattern – but histologic evidence of the disease may be present in the setting of normal hepatic biochemical tests.[12–15] In a retrospective review of 400 patients transplanted for PBC, Liermann Garcia and colleagues[13] showed no correlation between abnormal hepatic biochemical tests and histologic diagnosis of recurrent PBC. Other investigators found that only 50% to 57% of patients with recurrent PBC exhibited abnormal hepatic biochemical tests.[14,15] On the basis of these reports, it seems that as many as 50% of patients with recurrent PBC would not be diagnosed if serum alkaline phosphatase was the only test used to screen for recurrent disease.
Similarly, the antimitochondrial antibody (AMA) is not a reliable marker for the presence of recurrent disease. Dubel and colleagues[16] followed 16 patients with PBC for at least 4 years after OLT. AMA titers normalized 1 year after transplantation in 7 patients, declined in 7 others, and remained unchanged in 2. Over the subsequent 4 years, 4 patients demonstrated an increase in AMA titers. By contrast, the antimitochondrial antibodies-2 (anti-M2) never disappeared, as assessed by Western blot analysis. Despite the fluctuations in serum AMA and the persistently detected anti-M2, serum bilirubin and alkaline phosphatase levels normalized in all patients by 1 year after transplantation and remained normal thereafter. Routine liver biopsies performed on a yearly basis did not reveal changes suggestive of recurrent PBC. Thus, the persistence of AMA and anti-M2 is not unexpected following OLT and may only indicate the persistence of the basic abnormality that is associated with development of the disease.[11]
Diagnosis of Recurrent PBC
Given the nonspecific clinical presentation and laboratory findings, the diagnosis of recurrent PBC can be difficult. Numerous entities may present with cholestatic abnormalities in hepatic biochemical tests after OLT. Furthermore, as described previously, recurrent PBC may be present in a completely asymptomatic individual with normal hepatic biochemical tests, in which case a diagnosis will be made only by routine protocol biopsies. At present, the gold standard for the diagnosis of recurrent PBC is a liver biopsy demonstrating the characteristic histologic features, with the exclusion of other entities that can lead to graft dysfunction with similar histologic findings.
Common criteria used for the diagnosis of recurrent PBC include the following:
OLT performed for PBC
Persistence of AMA or anti-M2 antibody
- Absence of other pathology/disorders, including:
- Acute and chronic rejection
- Graft vs host disease
- Biliary obstruction
- Vascular abnormalities
- Cholangitis and other infections
- Viral hepatitis
- Drug toxicity
Figure 1.
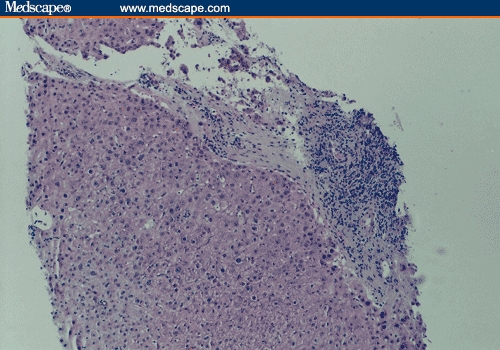
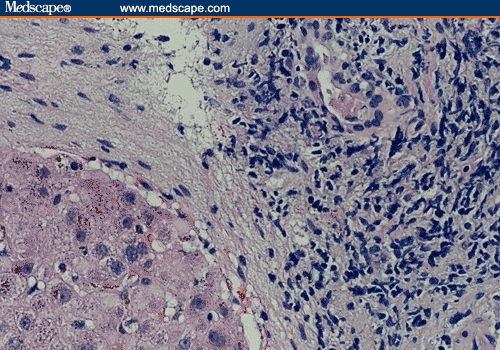
Recurrent PBC post transplant. (a) Mononuclear portal inflammation and bile duct injury (hematoxylin-eosin; original magnification x200). (b) Closer look showing a portal space with a predominantly mononuclear inflammatory infiltrate and bile duct injury (hematoxylin-eosin; original magnification x400).
Figure 2.
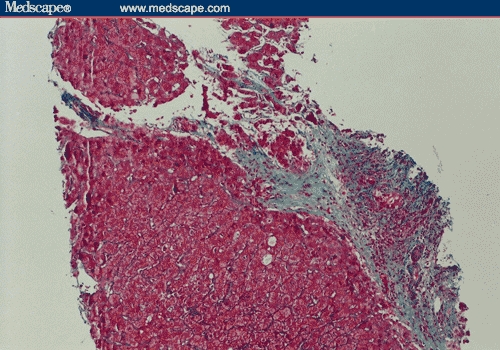
Recurrent PBC post transplant. A portal space showing portal fibrosis and bile duct injury (Masson trichrome; original magnification x200).
A definitive diagnosis of recurrent PBC is made when all 4 of these criteria are present, and in the presence of at least 3 of the 4 histologic features.[17] A likely diagnosis is made when only 2 histologic features are present. One caveat which should be considered in these criteria is that a patient transplanted for AMA-negative PBC can manifest recurrent PBC with a negative AMA or anti-M2 titer. Clearly, this represents a small subgroup of patients, because less than 10% of patients with PBC are AMA negative.
These observations raise the intriguing question of whether routine protocol biopsies should be performed in patients undergoing transplantation for PBC. However, protocol biopsies are not without risk.[18] Additionally, the clinical relevance of recurrent PBC remains unclear, as will be discussed. For this reason, we do not advocate protocol biopsies, but only suggest close clinical follow-up, with standard laboratory investigations.
Incidence and Timing of Recurrence
The published literature reports variable incidence rates of recurrent PBC, ranging from 10% to 40%.[13,15,17,19–21,22,23] <Maria: Ref 22 is cited out of order now. I've renumbered from this point on.> The timing from OLT to recurrent disease is also variable, as shown in Table 1. One large retrospective analysis of 400 patients with PBC demonstrated a recurrence rate of 18% at 5 years.[13] Additional observation of this same patient group revealed a recurrence rate of 30% at 10 years after OLT, suggesting that the risk of recurrent PBC increases with time.[19]
Table 1.
The Timing and Recurrence Rate of PBC in the Post-Transplant Setting
| Study (Reference) | Proportion of Patients With Recurrent PBC (%) | Median Time to Recurrence (Yrs [Range]) | Mean Time to Recurrence (Yrs) |
|---|---|---|---|
| Liermann Garcia[13] | 68/400(17.0*) | NR | 3.0 |
| AbuElmagd[21]†‡ | 54/513(10.5) | 5.58(range NR) | NR |
| Sanchez[28] | 17/156(10.9) | 4.13(1.1-7.1) | NR |
| Sylvestre[15] | 17/100(17) | 3.10(0.3-7.9) | 3.7 |
| Hubscher[17] | 13/83(16) | 1.08(1.0-4.0) | 1.7 |
| Sebagh[49] | 6/69(8.7) | 8.00(1.00-8.00) | 6.3 |
| Balan[50] | 5/60(8.3) | 3.00(2.0-6.0) | 4.0 |
| Guy[22] | 17/48(35.0) | NR | 3.3 |
| Levitsky[27] | 7/46(15.0) | 6.50(2.25-10) | NR |
| Khettry[23] | 8/43(18.6) | 3.50(0.1-14) | 5.6 |
| Jacob[20]† | (14.0) | 5.10(3-10.2) | NR |
After following this cohort for 10 years, Neuberger reports that this rate has increased to 30%
Published in abstract form
Kaplan-Meier analysis shows the estimated cumulative risk of recurrent PBC is 7.9% at 5 years and 21.6% at 10 years
NR = not reported
Risk Factors for Recurrent PBC
Studies attempting to identify risk factors for the development of recurrent PBC have yielded conflicting results. Although some identified donor age,[13,20] recipient age,[13,21] warm ischemia time,[13] and cold ischemia time[21] as significant risk factors, others failed to show significant differences for these criteria,[22,24] and thus, their clinical relevance remains unclear. Race, ethnicity, sex mismatch, HLA mismatch, serum bilirubin, albumin, international normalized ratio, and creatinine level were not statistically different in most studies.[13,21,23,24] Guy and colleagues[22] failed to show any significant influence of donor/recipient cytomegalovirus (CMV) status, or of the dose of immunosuppression on the risk of PBC recurrence – although they did find a trend toward more episodes of acute cellular rejection in patients with recurrent PBC (P < .07).
An area of much deliberation is whether the specific immunosuppression regimen used in the post OLT setting can be a risk factor for recurrent PBC. Several reports indicate that the use of tacrolimus is associated with early, more aggressive recurrence.[21,25,26] Liermann Garcia and colleagues[13] found that the rate of PBC recurrence was 29.5%, with a median onset of 22 months in patients receiving tacrolimus, vs 14.5%, with a median onset of 37 months in those receiving cyclosporine (P = .04). In a multivariate analysis, Neuberger and colleagues[24] showed that tacrolimus was associated with an increased risk (hazard ratio of 2.73) and earlier recurrence (123 months vs 62 months) of PBC compared with cyclosporine. Although similar findings were also reported by other groups,[20,22] some studies dispute the deleterious effects of tacrolimus. Levitsky and colleagues[27] as well as others[23,28] found no significant differences in the incidence and timing of recurrent PBC between patients treated with tacrolimus and those treated with cyclosporine. Given these conflicting data and the fact that the relevance of recurrent PBC remains debatable, as discussed in the next section, it seems that at present, there are no established risk factors that identify a subgroup of patients who should undergo aggressive surveillance for recurrent PBC. Furthermore, on the basis of current knowledge, we would advocate that cyclosporine- and tacrolimus-based regimens are equally acceptable for immunosuppression of patients with PBC, as dependent on the individual transplant center's preference.
Natural History of Recurrent PBC
Controversy exists regarding the natural history of recurrent PBC and whether recurrent disease is clinically relevant. Several reports suggest that recurrent PBC does not significantly affect graft function and has very little effect on morbidity and mortality.[29] According to one study involving 68 patients who developed recurrent PBC, only 1 progressed to cirrhosis 3 years after the diagnosis of recurrent disease, and 1 required retransplantation 8 years after OLT.[13] There was no statistical difference in either graft or patient survival between those patients with recurrent PBC and those without. In another study,[27] 6 of 7 (86%) patients with recurrent PBC were alive 10 years after OLT; the 1 death occurred 10 years after transplantation, and it was unclear whether this death was related to the disease recurrence. Jacob and colleagues[20] reported that out of 14 patients who developed recurrent PBC, only 2 (14%) developed graft dysfunction, and that, overall, recurrent PBC had no impact on patient survival. The only study that reported increased mortality in patients with recurrent PBC included both patients with definite recurrence of PBC and those with autoimmune features that were not specified.[23] Thus, the increased mortality may not reflect the true consequence of recurrent PBC. The results of another study (a retrospective analysis of 17 patients with recurrent PBC) demonstrated more advanced hepatic fibrosis in PBC patients when compared with a control group of 35 patients transplanted for other liver diseases.[15] However, it is unclear whether this increased stage of fibrosis was clinically relevant.
Treatment
Once the diagnosis of recurrent PBC is made, controversy exists as to the most appropriate initial intervention. Although data are lacking, most recommend the initiation of UDCA therapy.[30] The exact mechanism of action of UDCA remains unknown, but its ability to stabilize cell membranes, decrease the prevalence of toxic hydrophobic bile acids, and alter histocompatibility expression have been advanced as hypotheses.[1,2,31–33] In the nontransplanted patient, UDCA is the only drug that has been shown to delay and possibly prevent progression of the disease.[1–4,10] It is assumed that these same effects of UDCA would also benefit recurrent disease[11,30]; however, the precise benefit of this agent in this setting remains unknown.[19,34] Anecdotal evidence suggests that hepatic biochemical tests show improvement in patients with recurrent PBC who are treated with UDCA.[19]
One study reported on 7 patients who were treated with UDCA for recurrent PBC (dose not specified).[27] Three (43%) had improvement in hepatic biochemical tests, and 3 other patients had elevated but stable hepatic biochemical tests. One patient died due to multiorgan failure 10 years after transplantation. It is important to note that all 7 patients were alive 5 years post OLT, and that UDCA had an excellent safety profile in the posttransplant setting. Despite the limited number of patients and the brief follow-up period, the study authors recommend the use of UDCA once recurrent disease is diagnosed.[27] As discussed previously, in a recent analysis of 17 patients with recurrent PBC, 75% of patients treated with UDCA showed a marked decrease in serum alkaline phosphatase (mean decrease, 111 ± 157).[22] One patient could not tolerate the treatment due to worsening pruritis, and 2 did not exhibit any improvement in their alkaline phosphatase levels. No patient underwent repeat liver biopsy after the initiation of UDCA. Thus, it is unknown whether the biochemical improvement in serum alkaline phosphatase level correlated with a histologic improvement.[22] Several investigators have suggested that a prospective, randomized trial comparing UDCA with placebo is required to prove the benefit of UDCA in the setting of recurrent PBC. However, given the variable and slow natural history of recurrent PBC, it is unlikely that such a study is practical.[11]
Another potential benefit of UDCA in the post-transplant setting may be its effect on the risk of colon cancer. Liver transplantation increases the risk of colon cancer in allograft recipients. The relative risk for the development of colon cancer may be as high as 12.5.[35–39] This risk is especially pronounced in those patients with underlying primary sclerosing cholangitis (PSC) and/or inflammatory bowel disease,[40,41] although it is increased in all post-transplant patients. UDCA has been shown to reduce the risk of colon cancer in a subgroup of patients with ulcerative colitis and PSC.[42] A randomized clinical trial confirmed this observation, with 10% of UDCA-treated patients developing dysplasia vs 35% of patients who received placebo.[43] This relative risk reduction may also apply to the PBC population and is another argument in favor of initiating the drug in this setting.[30] Although existing data are limited, UDCA appears to be a safe drug, with multiple potential benefits. We favor initiating therapy with UDCA in all patients with recurrent PBC.
To date, there is no evidence to support the use of altered corticosteroid taper protocols post OLT for prevention of recurrent PBC; however, this remains an area for further investigation. The combination of corticosteroids with UDCA was shown to be superior to UDCA alone in the nontransplanted patient with PBC,[44,45] but unfortunately, corticosteroids carry a significant risk of adverse effects. Recent literature suggests a potential role for budesonide, an oral glucocorticoid with over 90% first-pass metabolism by the liver, resulting in fewer systemic side effects.[46–48] Nevertheless, there is no information regarding the effect of budesonide on PBC post OLT.
Conclusion
PBC is a chronic cholestatic liver disease that can progress to cirrhosis, necessitating OLT. It is now well accepted that PBC can recur in the post-transplant setting in 10% to 40% of patients. Because the disease is often clinically silent, the true rate of recurrence may currently be underestimated. On the basis of the largest analyses, the presence of recurrent disease does not appear to affect either graft or patient survival rates. There may be several potential risk factors for the development of recurrent disease, including age, ischemia time, and the use of tacrolimus. However, reports in the current literature are conflicting, and no definitive risk factors can be positively identified. Most experts favor the use of UDCA for the treatment of recurrent disease because of its multiple potential benefits, but at present, there is no evidence indicating that UDCA is indeed beneficial in this setting.
Contributor Information
Ian Schreibman, Center for Liver Diseases, Division of Hepatology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida.
Arie Regev, Center for Liver Diseases, Division of Hepatology, University of Miami Leonard M. Miller School of Medicine, Miami, Florida. Email: aregev@med.miami.edu.
References
- 1.Poupon RE, Poupon R, Balkau B, UDC-PBC Study Group Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 2.Combes B, Carithers RL, Maddrey WC, et al. A randomized double blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–766. [PubMed] [Google Scholar]
- 3.Lindor KD, Poupon R, Heathcote EJ, et al. Ursodeoxycholic acid for primary biliary cirrhosis. Lancet. 2000;355:657–658. doi: 10.1016/S0140-6736(05)72401-6. [DOI] [PubMed] [Google Scholar]
- 4.Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 5.Goulis J, Leandro G, Burroughs A. Randomized controlled trials of ursodeoxycholic acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354:1053–1060. doi: 10.1016/S0140-6736(98)11293-X. [DOI] [PubMed] [Google Scholar]
- 6.Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD000551. [DOI] [PubMed] [Google Scholar]
- 7.Poupon RE, Lindor KD, Pares A, et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12–16. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 8.Papatheodoridis GV, Hadziyannis ES, Deusch M, et al. Ursodeoxycholic acid for primary biliary cirrhosis: final results of a 12-year prospective randomized, controlled trial. Am J Gastroenterol. 2002;97:2063–2070. doi: 10.1111/j.1572-0241.2002.05923.x. [DOI] [PubMed] [Google Scholar]
- 9.Prince M, Chetwynd A, Newman W, et al. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044–1051. doi: 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- 10.Tinmouth J, Tomlinson G, Heathcote EJ, et al. Benefit of transplantation in primary biliary cirrhosis between 1985-1997. Transplantation. 2002;73:224–227. doi: 10.1097/00007890-200201270-00012. [DOI] [PubMed] [Google Scholar]
- 11.Neuberger J. Recurrent primary biliary cirrhosis. Liver Transpl. 2003;9:539–546. doi: 10.1053/jlts.2003.50096. [DOI] [PubMed] [Google Scholar]
- 12.Mitchison HC, Bassendine MF, Hendrick A, et al. Positive antimitochondrial antibody but normal alkaline phosphatase: is this primary biliary cirrhosis. Hepatology. 1986;6:1279–1284. doi: 10.1002/hep.1840060609. [DOI] [PubMed] [Google Scholar]
- 13.Liermann Garcia RF, Evangelista Garcia CE, McMaster P, et al. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology. 2001;33:22–27. doi: 10.1053/jhep.2001.20894. [DOI] [PubMed] [Google Scholar]
- 14.Slapak GI, Saxena R, Portmann BC, et al. Graft and systemic disease in long-term survivors of liver transplantation. Hepatology. 1997;25:195–202. doi: 10.1002/hep.510250136. [DOI] [PubMed] [Google Scholar]
- 15.Sylvestre PB, Batts KP, Burgart LJ, et al. Recurrence of primary biliary cirrhosis after liver transplantation: histologic estimate of incidence and natural history. Liver Transpl. 2003;9:1086–1093. doi: 10.1053/jlts.2003.50213. [DOI] [PubMed] [Google Scholar]
- 16.Dubel L, Farges O, Bismuth H, et al. Kinetics of anti-M2 antibodies after liver transplantation for primary biliary cirrhosis. J Hepatol. 1995;23:674–680. doi: 10.1016/0168-8278(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 17.Hubscher S, Elias E, Buckels J, et al. Primary biliary cirrhosis: histological evidence of disease recurrence after liver transplantation. J Hepatology. 1993;18:173–184. doi: 10.1016/s0168-8278(05)80244-2. [DOI] [PubMed] [Google Scholar]
- 18.Neuberger J, Wilson P, Adams D. Protocol liver biopsies: the case in favour. Transplant Proc. 1998;30:1497–1499. doi: 10.1016/s0041-1345(98)00332-7. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger J. Liver transplantation for primary biliary cirrhosis: indications and risk of recurrence. J Hepatology. 2003;39:142–148. doi: 10.1016/s0168-8278(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 20.Jacob DA, Neumann UP, Bahra M, et al. Influence of primary biliary cirrhosis recurrence after liver transplantation in a long term follow-up. Hepatology. 2005;42:321A–322A. [Google Scholar]
- 21.Abu-Elamgd K, Demetris J, Rakela J, et al. Transplantation for primary biliary cirrhosis: recurrence and outcome in 421 patients. Hepatology. 1997;26:176A. [Google Scholar]
- 22.Guy JE, Qian P, Lowell JA, et al. Recurrent primary biliary cirrhosis: peritransplant factors and ursodeoxycholic acid treatment post-liver transplant. Liver Transpl. 2005;11:1252–1257. doi: 10.1002/lt.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khettry U, Anand N, Faul PN, et al. Liver transplantation for primary biliary cirrhosis: a long-term pathologic study. Liver Transpl. 2003;9:87–86. doi: 10.1053/jlts.2003.36392. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger J, Gunson B, Hubscher S, et al. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2004;10:488–491. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 25.Wong PY, Portmann B, O'Grady JG, et al. Recurrence of primary biliary cirrhosis after liver transplantation following FK506-based immunosuppression. J Hepatology. 1993;17:284–287. doi: 10.1016/s0168-8278(05)80206-5. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida EM, Singh RA, Vartanian RK, et al. Late recurrent post-transplant primary biliary cirrhosis in British Columbia. Can J Gastroenterol. 1997;11:229–233. doi: 10.1155/1997/790906. [DOI] [PubMed] [Google Scholar]
- 27.Levitsky J, Hart J, Cohen SM, et al. The effect of immunosuppressive regimens on the recurrence of primary biliary cirrhosis after liver transplantation. Liver Transpl. 2003;9:733–736. doi: 10.1053/jlts.2003.50132. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez EQ, Levy MF, Goldstein RM, et al. The changing clinical presentation of recurrent primary biliary cirrhosis after liver transplantation. Transplantation. 2003;76:1583–1588. doi: 10.1097/01.TP.0000090867.83666.F7. [DOI] [PubMed] [Google Scholar]
- 29.Balan V, Abu-Elmagd K, Demetris A. Autoimmune liver diseases: recurrence after liver transplantation. Surg Clin North Am. 1999;79:147–152. doi: 10.1016/s0039-6109(05)70011-6. [DOI] [PubMed] [Google Scholar]
- 30.Neuberger, James Chronic allograft dysfunction: diagnosis and management. Is it always progressive? Liver Transpl. 2005;11:s63–s68. doi: 10.1002/lt.20603. [DOI] [PubMed] [Google Scholar]
- 31.Guldutuna S, Zimmer G, Imhof M, et al. Molecular aspects of membrane stabilization by ursodeoxycholate. Gastroenterology. 1993;104:1736–1744. doi: 10.1016/0016-5085(93)90653-t. [DOI] [PubMed] [Google Scholar]
- 32.Calmus Y, Weill B, Ozier Y, et al. Immunosuppressive properties of chenodeoxycholate and ursodeoxycholic acids in the mouse. Gastroenterology. 1992;103:617–621. doi: 10.1016/0016-5085(92)90855-s. [DOI] [PubMed] [Google Scholar]
- 33.Paumgartner G, Beuers U. Mechanism of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis. 2004;8:67–81. doi: 10.1016/S1089-3261(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 34.Faust, Thomas W. Recurrent primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis after transplantation. Liver Transpl. 2001;7:s99–s108. doi: 10.1053/jlts.2001.28514. [DOI] [PubMed] [Google Scholar]
- 35.Penn I. Cancers in renal transplant recipients. Adv Ren Replace Ther. 2000;7:147–156. doi: 10.1053/rr.2000.5269. [DOI] [PubMed] [Google Scholar]
- 36.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23:101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 37.Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998:147–148. [PubMed] [Google Scholar]
- 38. Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1991-2002. Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs. Division of Transplantation, Rockville, Maryland; 2001 United Network for Organ Sharing, Richmond, Virginia; University Renal Research and Education Association, Ann Arbor, Michigan.
- 39.Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 40.Vera A, Gunson BK, Ussatoff V, et al. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983–1988. doi: 10.1097/01.TP.0000058744.34965.38. [DOI] [PubMed] [Google Scholar]
- 41.Fabia R, Levy MF, Testa G, et al. Colon carcinoma in patients undergoing liver transplantation. Am J Surg. 1998;176:265–269. doi: 10.1016/s0002-9610(98)00141-x. [DOI] [PubMed] [Google Scholar]
- 42.Tung BY, Emond MJ, Haggitt RC, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 43.Pardi DS, Loftus EV, Jr, Kremers WK, et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 44.Leuschner M, Güldütüna S, You T, et al. Ursodeoxycholic acid and prednisolone versus ursodeoxycholic acid and placebo in the treatment of early stages of primary biliary cirrhosis. J Hepatol. 1996;25:49–57. doi: 10.1016/s0168-8278(96)80327-8. [DOI] [PubMed] [Google Scholar]
- 45.Wolfhagen FH, van Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. Neth J Med. 1994;44:84–90. [PubMed] [Google Scholar]
- 46.Rautiainen H, Karkkainen P, Karvonen A-L, et al. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747–752. doi: 10.1002/hep.20646. [DOI] [PubMed] [Google Scholar]
- 47.Leuschner M, Maier KP, Schlichting J, et al. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918–925. doi: 10.1016/s0016-5085(99)70351-3. [DOI] [PubMed] [Google Scholar]
- 48.Angulo P, Jorgensen RA, Keach JC, et al. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology. 2000;31:318–323. doi: 10.1002/hep.510310209. [DOI] [PubMed] [Google Scholar]
- 49.Sebagh M, Farges O, Dubel L, et al. Histologic features predictive of recurrence of primary biliary cirrhosis after transplantation. Transplantation. 1998;65:1328–1333. doi: 10.1097/00007890-199805270-00008. [DOI] [PubMed] [Google Scholar]
- 50.Balan V, Batts BM, Porayko M, et al. Histologic evidence for recurrence of primary biliary cirrhosis after liver transplantation. Hepatology. 1993;18:1392–1398. [PubMed] [Google Scholar]


