Abstract
Stem cell therapy has emerged as a promising tool for the treatment of a variety of diseases. Previously, we have shown that Akt-modified mesenchymal stem cells mediate tissue repair through paracrine mechanisms. Using a comprehensive functional genomic strategy, we show that secreted frizzled related protein 2 (Sfrp2) is the key stem cell paracrine factor that mediates myocardial survival and repair after ischemic injury. Sfrp2 is known to modulate Wnt signaling, and we demonstrate that cardiomyocytes treated with secreted frizzled related protein increase cellular β-catenin and up-regulate expression of antiapoptotic genes. These findings reveal the key role played by Sfrp2 in mediating the paracrine effects of Akt-mesenchymal stem cells on tissue repair and identify modulation of Wnt signaling as a therapeutic target for heart disease.
Despite major advances in our understanding and therapy of coronary artery disease, myocardial infarction remains a major cause of mortality and morbidity in the United States. The limited ability of the damaged heart to regenerate and replace dead myocardium leads to the devastating sequelae of congestive heart failure. The recent interests in the development of cell-based therapeutic strategies are aimed at replenishing the diminished myocyte mass (1). Such cell-based strategies have used a variety of stem and progenitor cells, including skeletal muscle myoblasts, embryonic stem cells, resident cardiac stem cells, mesenchymal stem cells (MSCs), endothelial progenitor cells, and bone marrow-derived mononuclear cells (2). Although the majority of animal and preliminary human studies of cell-based therapy shows an overall improvement in cardiac function when administered to hearts after acute infarction, the effects generally are modest, and the mechanisms underlying such an observed improvement are far from clear (3). Postulated mechanisms include differentiation of transplanted cells or resident cardiac stem cells into cardiomyocytes (4), fusion between donor cells and host cardiomyocytes (5), and/or improved tissue perfusion attributable to enhanced donor cell-derived angiogenesis (6).
We recently have reported that intracardiac implantation of genetically engineered MSCs overexpressing the Akt gene (Akt-MSCs) yielded dramatic diminution of infarct size and restoration of cardiac function in rodent hearts after myocardial injury (7). We observed that these salutary effects occurred as early as 72 h after Akt-MSC implantation (8). Moreover, we demonstrated that Akt-MSC-conditioned medium provided survival signal to adult ventricular myocytes against hypoxia-induced apoptosis in vitro and upon injection into infarcted hearts, dramatically limited infarct size, and prevented ventricular dysfunction in vivo (8). Accordingly, we hypothesized that Akt-MSCs achieve their beneficial effects in part through enhancing early survival of the ischemic myocardium. Furthermore, we postulated that the prosurvival effects of Akt-MSCs on ischemic myocardium are paracrine in nature and mediated by specific Akt-MSC-secreted proteins. To identify the paracrine factors that mediate Akt-MSC effects on myocardial survival and repair, we used a comprehensive functional genomic strategy supported by proteomic analysis. Microarray data confirmed by Western blot analysis demonstrated that the most prominently expressed and secreted protein by Akt-MSCs compared with native MSCs is the secreted frizzled related protein 2 (Sfrp2). Indeed, quantitative PCR (QPCR) showed 100-fold up-regulation of Sfrp2 mRNA in Akt-MSCs compared with control MSCs. Sfrps are secreted glycoprotein molecules that structurally resemble cell surface frizzled receptors but lack the transmembrane domain. They have been increasingly recognized as potent regulators of cellular Wnt signaling (9, 10) and have been implicated in diverse cellular processes such as regulation of cell fate, differentiation, proliferation, and cell death (10).
In this article, we report that Sfrp2 plays a major role in mediating the survival signal of Akt-MSCs on the ischemic myocardium. Our data show that Sfrp2 exerts survival effects on ischemic cardiomyocytes and that the prosurvival effects of Akt-MSCs were markedly attenuated upon knockdown of Sfrp2 with siRNA. We provide evidence that Sfrp2 increases total cellular and nuclear β-catenin in cardiomyocytes in vitro. Stabilization of β-catenin has been demonstrated to protect neonatal rat cardiomyocytes against hypoxia/reoxygenation-induced apoptosis (11). Furthermore, we observed that the canonical Wnt, Wnt3a, is up-regulated in ischemic cardiomyocytes in vitro and that Wnt3a induces apoptosis of cardiomyocytes. Importantly, Sfrp2 blocks the proapoptotic effect of Wnt3a in vitro. Together, our data provide strong evidence supporting the paracrine hypothesis of stem cell action. Importantly, we have identified Sfrp2 as a major paracrine mediator of Akt-MSCs myocardial survival and reparative effects. Furthermore, our results suggest that modulators of Wnt signaling, such as Sfrp2, may hold promise as therapeutic agents in the management of acute myocardial injury.
Results
Profiling of Secreted Factors Expressed in MSCs.
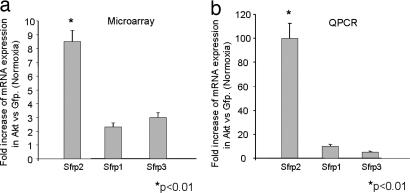
To identify potential Akt-MSC-secreted candidate paracrine factors mediating myocardial cell survival after ischemic injury, we used large-scale microarray expression analysis of mouse Akt-MSCs while using MSCs overexpressing the GFP gene as control (GFP-MSCs). We recognize the limitations of this approach in that it does not examine posttranscriptional events. We used Affymetrix (Santa Clara, CA) GeneChip Mouse Genome 430 2.0 Arrays, which allow analysis of ≈45,000 transcripts. Expression levels and quality analysis were carried out with the Affymetrix MAS 5.0 software. Further analysis was performed with the dChip software (12) based on the following filtering criteria: (i) transcripts expressed (P call) in at least one of the samples compared, and (ii) fold change was at least 1.2 times (90% lower bound confidence). Approximately 650 transcripts were differentially regulated between Akt-MSCs and GFP-MSCs. Included in this list were 169 novel transcripts with unassigned function. Because we were interested specifically in the paracrine effects, the set of 650 transcripts was queried for transcripts coding for secreted proteins. This analysis revealed 62 transcripts encoding for 51 unique genes that potentially could contribute to the paracrine effects of the MSC cells [supporting information (SI) Table 1]. Among these up-regulated genes, Sfrp2 was the most dramatically up-regulated (SI Table 1). Other cytokines such as Vegf, Hgf, and FGF were not differentially expressed between Akt-MSCs and GFP-MSCs under normoxic conditions (data not shown). The expression of Sfrp2 was Akt pathway-dependent. Interestingly, the expression of the other Sfrp family members was altered minimally in Akt-MSCs (Fig. 1a).
Fig. 1.
Sfrps are expressed in MSCs. (a) Levels of Sfrp1, Sfrp2, and Sfrp3 expression as estimated by microarray analysis show a nearly 10-fold up-regulation of Sfrp2 in Akt-MSCs compared with GFP-MSCs. (b) Real-time QPCR validation of mRNA expression levels demonstrates a 100-fold up-regulation of Sfrp2 gene expression in Akt-MSCs compared with GFP-MSCs.
Akt-Regulated Expression of Sfrps in MSCs.
We next confirmed the results of microarray analysis by QPCR. For this purpose, we collected RNA from cultured Akt-MSCs and GFP-MSCs cultured in vitro. As shown in Fig. 1b, the expression patterns of Sfrp1, Sfrp2, and Sfrp3 were consistent with the microarray results. Neither Sfrp1 nor Sfrp3 was significantly up-regulated in Akt-MSCs versus GFP-MSCs, whereas Sfrp2 expression was 100-fold higher in Akt-MSCs than in GFP-MSCs.
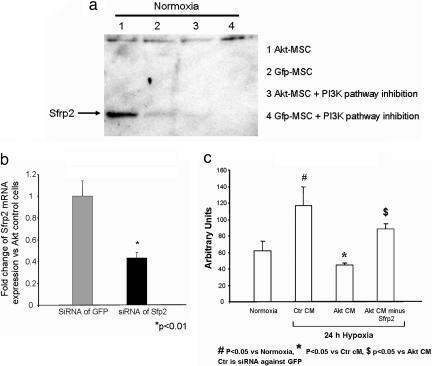
To further validate our observations at the protein level and to evaluate the effect of Akt on Sfrp2 expression, control mouse MSCs and Akt-MSCs were cultured for 6 h with 50 mM phosphatidylinositol 3-kinase (PI3-kinase) inhibitor (LY294002; Cayman Chemical, Ann Arbor, MI) or vehicle. The conditioned medium then was collected and concentrated for Western blot protein analysis. As shown in Fig. 2a, Sfrp2 was secreted at high levels into the conditioned medium from the Akt-MSC cells (lanes 1). The levels of Sfrp2 were low or undetectable in the conditioned medium of GFP-MSCs (lanes 2). Furthermore, the expression/release of Sfrp2 in the Akt-MSC cells depended on the PI3-kinase pathway because inhibition of the PI3-kinase abolished Sfrp2 accumulation in the medium (lanes 3).
Fig. 2.
Paracrine factors from Akt-MSCs mediate the survival signaling on cardiomyocytes. (a) Western blot analysis for Sfrp2 demonstrates presence of Sfrp2 protein in conditioned medium collected from Akt-MSCs or GFP-MSCs and inhibition of its accumulation in the medium in the presence of PI3-kinase inhibitor. (b) Relative reduction in mRNA levels of Sfrp2 in Akt-MSCs after knockdown of Sfrp2 with siRNA. (c) Effect of conditioned medium on apoptosis in ARVCs. Caspase activity of ARVCs after 24 h of hypoxia under different culture conditions (Ctr CM, control conditioned medium; Akt CM, Akt-conditioned medium; and Akt CM minus Sfrp2, Akt-conditioned medium after Sfrp2 knockdown) demonstrates reduction of caspase activity after Akt-conditioned medium treatment (Akt CM) and attenuation of this effect after treatment with Akt-conditioned medium after Sfrp2 knockdown (Akt CM minus Sfrp2).
Akt-MSCs Promote Cardiomyocyte Cell Survival Through Paracrine Mechanisms Mediated by Sfrp2.
To prove whether Sfrp2 is a key paracrine mediator of the survival signaling of Akt-MSCs, we evaluated the apoptotic response (caspase activity) of adult rat ventricular cardiomyocytes (ARVCs) exposed to conditioned medium collected from Akt-MSCs treated with siRNA against Sfrp2 (Akt-MSC CM minus Sfrp2). ARVCs were subjected to hypoxia for 24 h in the presence of Akt-MSC-conditioned medium, Akt-MSC CM minus Sfrp2, or standard growth medium (Fig. 2 b and c). ARVCs maintained in standard growth medium under normoxic conditions for 24 h were viable and exhibited their typical rod-shaped appearance (data not shown), whereas ARVCs grown in the same medium and subjected to 24 h of hypoxia exhibited a 82% increase in caspase activity (Fig. 2c). Compared with hypoxic ARVCs maintained in standard growth medium, hypoxic ARVCs exposed to Akt-MSC-conditioned medium exhibited a 40% reduction in caspase activity (Fig. 2c). Moreover, exposure of hypoxic cardiomyocytes to Akt CM minus Sfrp2 resulted in a significant increase in caspase activity compared with hypoxic ARVCs treated with Akt-conditioned medium. Indeed, we observed a 33% increase in caspase activity in hypoxic ARVCs after knockdown of Sfrp2 expression in Akt-MSCs. These observations demonstrate the key role played by Sfrp2 in mediating survival effects of Akt-MSC-conditioned medium on cardiomyocytes.
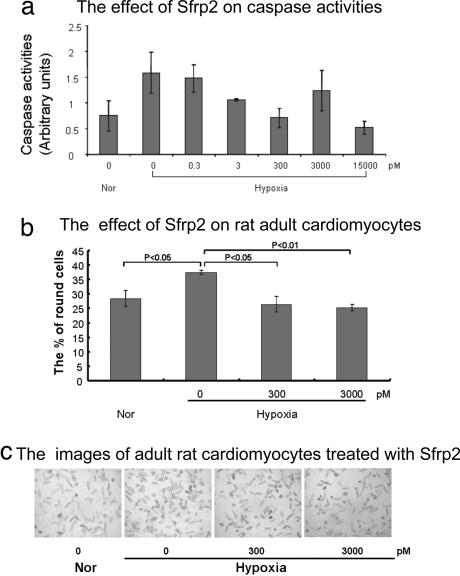
To examine the direct effect of Sfrp2 on ARVCs, we also performed gain-of-function experiments in vitro. ARVCs were maintained in standard growth medium at normoxia or subjected to 24 h of hypoxia. Sfrp2 or vehicle then was added at various concentrations, and apoptosis levels were assessed by measuring caspase activity. As shown in Fig. 3, treatment with Sfrp2 resulted in significant reduction in caspase activity. A dose-dependent cytoprotective response was observed with increasing Sfrp2 concentrations up to 15 nM (Fig. 3a).
Fig. 3.
Sfrp2 decreases caspase 3 activity in vitro. (a) Cleaved caspase 3 activity as measured by a fluorometric assay demonstrates decreased caspase activity in hypoxic cardiomyocytes after Sfrp2 treatment in a dose-dependent manner. The activity was calculated as fold changes with the same control. (b) Number of round-shaped cardiomyocytes was counted in six random high-power fields (×40) after 24 h of hypoxic exposure with/without Sfrp2 treatment and expressed as a percentage of the total number of cells present. Normoxia: 28.4 ± 2.7; hypoxia: 37.3 ± 0.75; hypoxia plus 300 pM Sfrp2: 26.4 ± 2.6; hypoxia plus 3,000 pM Sfrp2: 25.2 ± 1.0. (c) Representative high-power field photographs demonstrating decreased number of round-shaped cardiomyocytes after treatment with Sfrp2.
We further confirmed the cytoprotective effects of Sfrp2 on cardiomyocytes by observing changes in cardiomyocyte cell morphology after exposure to hypoxia in vitro. After 24 h of hypoxia exposure, ARVCs lose their typical rod-shaped morphology, become round in shape, subsequently detach, and die. Hypoxia alone increased the number of round-shaped cardiomyocytes by ≈36% (Fig. 3 b and c). However, when ARVCs were treated with Sfrp2 (3 nM), the number of round-shaped cardiomyocytes was decreased by ≈31% compared with untreated controls (Fig. 3 b and c). Together, our data strongly suggest that Sfrp2 promotes cardiomyocyte survival and protects cardiomyocytes from hypoxic injury in vitro.
Suppression of Sfrp2 Expression in Akt-MSCs Reduces the Paracrine Protection of Myocardial Injury in Vivo.
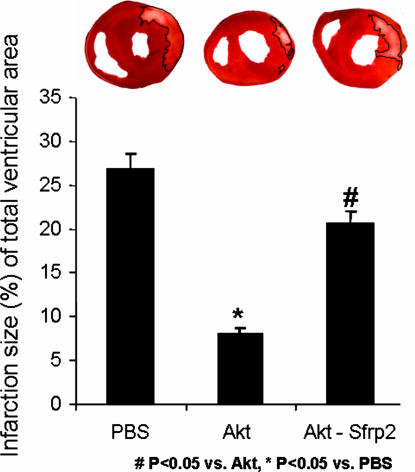
We next studied the physiological significance of Sfrp2 in Akt-MSC-mediated paracrine myocardial protection in vivo. To demonstrate the importance of Sfrp2 as a key paracrine factor mediating prosurvival effects of injected Akt-MSCs, we compared the in vivo effects of conditioned medium collected from Akt-MSCs treated with siRNA against Sfrp2 with those of untreated Akt-MSC-conditioned medium. Akt-MSCs treated with siRNA against Sfrp2 had a 60% decrease in Sfrp2 mRNA expression after 48 h of exposure to siRNA (Fig. 2b). The conditioned medium either from untreated or siRNA-treated cells was collected, concentrated as described in ref. 8, and then injected into five different sites at the infarct border zone 30 min after coronary artery ligation (8). Hearts then were isolated 72 h later, and infarct size was estimated by triphenyl tetrazolium chloride staining. The results were analyzed by an investigator who was blind to the treatment groups. As shown in Fig. 4a and b, injection of Akt-conditioned medium in infarcted hearts resulted in 71% reduction in the infarct size after myocardial infarction within 72 h, whereas injection of conditioned medium from siRNA-treated Akt-MSCs did not show any significant protection. Collectively, these results strongly support the hypothesis that Sfrp2 is an important Akt-MSC-secreted molecule that possesses cell survival signaling properties and mediates myocardial protective effects of implanted Akt-MSCs after myocardial infarction.
Fig. 4.
Akt-MSC-secreted Sfrp2 decreases cardiac infarct size. (Upper) Triphenyl tetrazolium chloride staining in three biventricular sections of similar thickness perpendicular to the long axis of the heart demonstrates decreased infarct size with Akt-conditioned medium and Sfrp2 and attenuation of reduction in infarct size with Akt-MSCs that did express reduced levels of Sfrp2 because of siRNA treatment. (Lower) Infarct size is expressed as a percentage of the total ventricular area. Rat hearts were treated with PBS as control. Akt, Akt-MSC-conditioned medium; Akt-Sfrp2, conditioned medium from Akt-MSCs that did express reduced levels of Sfrp2 because of siRNA treatment.
Sfrp2 Leads to Up-Regulation of β-Catenin in Hypoxic Cardiomyocytes.
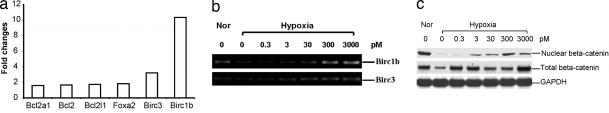
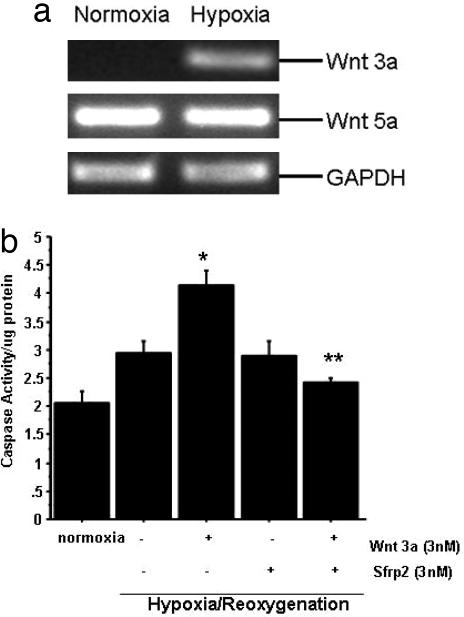
Sfrp2 is a known antagonist of Wnt signaling. Unlike Sfrp1, which can potentiate Wnt signaling under certain conditions, Sfrp2 is not known to activate Wnt signaling (13). However, in this report, we provide evidence that Sfrp2 increases total cellular as well as nuclear β-catenin, mimicking canonical Wnt signaling. Using Western blot analysis, we demonstrate that Sfrp2 induces a dose-dependent increase in nuclear as well as total cellular β-catenin levels in cardiomyocytes exposed to hypoxia (see Fig. 6c). Increased β-catenin within cardiomyocytes has been associated with increased cellular protection against ischemic injury in vitro (11). Together, these data strongly suggest that Sfrp2 promotes the survival of cardiomyocytes against hypoxia-induced apoptosis via potentiation of canonical signaling. We then examined whether Wnts are up-regulated in cardiomyocytes exposed to 24 h of hypoxia and observed that Wnt3a was expressed at very low levels in normoxic cells but increased in hypoxic cells (Fig. 5a). Next, we incubated cardiomyocytes both under normoxia and hypoxia/reoxygenation with Wnt3a alone and in combination with Sfrp2. Our data demonstrated that under normoxic conditions, as compared with control cardiomyocytes, Wnt3a treatment resulted in a modest increase in caspase 3 activity, which was attenuated by Sfrp2 treatment (data not shown). Furthermore, under hypoxia/reoxygenation conditions, Wnt3a treatment resulted in a significant increase in caspase activity, which was inhibited by the addition of Sfrp2 (Fig. 5b)
Fig. 6.
Sfrp2 up-regulates Birc1b and β-catenin in hypoxic cardiomyocytes in vitro. (a) Microarray analysis demonstrates Sfrp2-mediated up-regulation of Birc1b gene expression in hypoxic cardiomyocytes. (b) RT-PCR confirmation of increased Birc1b expression in hypoxic cardiomyocytes after Sfrp2 treatment. (c) Western blot analysis for nuclear and total β-catenin expression in ARVCs demonstrates a reduction of β-catenin after hypoxia and up-regulation after treatment with Sfrp2.
Fig. 5.
Hypoxic cardiomyocytes up-regulate Wnt3a expression, and Sfrp2 blocks proapoptotic effects of Wnt3a. (a) Wnt3a mRNA expression by RT-PCR is increased in hypoxic cardiomyocytes, whereas expression of Wnt5 remains unchanged. (b) Wnt3a (3 nM) increases caspase activity of cardiomyocytes undergoing hypoxia/reoxygenation injury; Sfrp2 at a similar concentration significantly attenuates Wnt3a-induced caspase activity. ∗, P < 0.05 versus normoxia; ∗∗, P < 0.05 versus Wnt plus hypoxia/reoxygenation; n = 6 per group.
Sfrp2 Up-Regulates Expression of Antiapoptotic Gene Birc1b in Hypoxic Cardiomyocytes.
To further investigate the molecular mechanism by which Sfrp2 protects cardiomyocytes from cell death, we collected RNA from hypoxic cardiomyocytes after Sfrp2 treatment (10 ng/ml) and by using a microarray-determined expression of multiple genes involved in cell survival/death pathways. Using an Oligo GEArray (SuperArray Bioscience, Frederick, MD) for rat signal transduction pathways, we analyzed gene expression of 95 marker genes associated with 18 different signaling pathways. In this analysis, 43 genes showed differential expression between the Sfrp2-treated and the control cardiomyocytes. We found that Sfrp2 up-regulated the expression of Birc1b, an antiapoptotic gene belonging to the neuronal apoptosis inhibitory protein family. Our preliminary study showed a decrease in mitochondrial cytochrome C levels (by using Western blot analysis) in response to Sfrp2 (data not shown). Interestingly, expression of other cytoprotective genes such as Bcl2 were increased only minimally in hypoxic cardiomyocytes in the presence of Sfrp2 (Fig. 6a and b).
Discussion
Despite the theoretical potentials of cell-based therapies for cardiac disease, the clinical effects are modest, and the mechanisms underlying their therapeutic effect are still under debate. We previously have hypothesized that one of the principal mechanisms of the stem cell transplantation effect is paracrine in nature (8). Paracrine factors released by modified MSCs potentially can exert effects on various aspects of cardiac pathophysiology such as myocardial cell survival, angiogenesis, remodeling, contractility, and even myocyte regeneration. Indeed, we previously demonstrated that Akt-MSCs exert survival effects and protect the myocardium in vivo by paracrine mechanisms (8). Fazel et al. (14) recently have shown that bone marrow-derived cKit+ cells are mobilized to the heart after myocardial infarction and create a proangiogenic milieu that is critical for cardiac repair. The authors demonstrate that the cells exert these salutary effects on cardiac repair through paracrine mechanisms and not through differentiation into cardiomyocytes or endothelial cells. Consistent with our previous observation (8), Fazel et al. (14) found decreased degrees of apoptosis at the infarct border zones. In this article, we identify the principal paracrine factor, Sfrp2, that mediates, at least in large part, prosurvival signals of Akt-MSCs.
We used a genomic approach to identify genes overexpressed in Akt-MSCs and then restricted our analysis to genes encoding secreted proteins. A filtering strategy focused on secreted proteins therefore would enable identification of paracrine mediators of Akt-MSCs effects. Although a genomic approach, as compared with a proteomic approach, can overlook important posttranscriptional events such as alternative splicing, a genomic approach currently is more comprehensive, is technically less demanding, and enables easier identification of novel genes with previously unknown cell survival effects. Using such an approach, we identified Sfrp2 as an Akt-MSC-secreted protein exerting prosurvival effects on the myocardium. Several lines of evidence support our hypothesis of Sfrp2 as a principal mediator of antiapoptotic effects exerted on the myocardium by Akt-MSCs. First, Sfrp2 expression is dramatically up-regulated (100 times) in Akt-MSCs compared with GFP-MSCs, and its expression/secretion depends on the PI3-kinase/Akt pathway. Secondly, Sfrp2 conferred prosurvival effects on hypoxic cardiomyocytes. Moreover, knockdown of Sfrp2 expression resulted in the attenuation of the prosurvival action of Akt-MSC-conditioned medium both in vitro and in vivo.
Sfrps compete with the frizzled receptor for Wnt ligands by direct binding of Wnts, preventing activation of Wnt signaling in the cell. Wnts are known to regulate cardiac morphogenesis (15–17), but the role of Wnts in the postnatal heart is not known (18). Mice harboring mutant Sfrp2 have no apparent cardiac phenotype. In these mice, Sfrp1 appeared to compensate for the lack of Sfrp2. Double homozygous mutants suffered embryonic lethality. Studies of infarct size in mice lacking Sfrp2 have not been performed yet but would be of considerable interest given our observation. In this study, we provide evidence that Sfrp2 exerts prosurvival action on cardiomyocytes. Indeed, Sfrp2 has been shown to confer cellular resistance against UV- and TNF-induced apoptosis in mammalian cell lines (19). Data in this study demonstrate that Sfrp2 increases total as well as nuclear β-catenin within the hypoxic cardiomyocyte in a dose-dependent manner. The mechanism by which Sfrp2 increases β-catenin within cardiomyocytes is not entirely clear. In this context, Sfrp1 has been shown to potentiate Wnt signaling by directly binding to frizzled receptors under certain circumstances (20). It is possible that cardiomyocytes up-regulate several Wnts after hypoxic injury. Different Wnts acting on the same cells may exert opposing effects (21). In this regard, noncanonical Wnts can antagonize the effects of canonical Wnts within the same cell (21, 22). It thus is possible that Sfrp2 binds locally present Wnts and alters the balance of intracellular Wnt signaling within a cardiomyocyte to favor a canonical pathway. In this study, we observed that Wnt3a is up-regulated in hypoxic cardiomyocytes. It induces cardiomyocyte apoptosis, and Sfrp2 antagonized this effect of Wnt3a. Interestingly, we also found that Sfrp2 up-regulated expression of Birc1b, an antiapoptotic gene belonging to the neuronal apoptosis inhibitory protein family, which confers neurons resistance to hypoxia-induced apoptosis (23) by caspase-dependent and -independent pathways (24, 25). We speculate that Sfrp2-mediated increased β-catenin activates transcription of antiapoptotic genes such as Birc1b in hypoxic cardiomyocytes. Together, these data strongly suggest that, by increasing cellular and nuclear β-catenin, Sfrp2 can enhance the survival response of cardiomyocytes against hypoxia-induced apoptosis.
The role of Sfrps in regulating cardiomyocyte function, homeostasis, and survival currently is unclear. Sfrp3 and Sfrp4 expression was increased in volume-overloaded human hearts (26), whereas Sfrp1 overexpression in transgenic mice was associated with reduced infarct size and improved ventricular function in mice after myocardial infarction (27). Our study sheds light on the potential role of Sfrp2 in regulating cardiomyocyte cell survival and preserving cardiac function after myocardial infarction. Moreover, it points to Sfrp2 as an important paracrine factor mediating beneficial effects of stem cell therapy. Although well recognized antiapoptotic factors such as Vegf, Hgf, and Fgf are expressed by Akt-MSCs, they are not differentially expressed between Akt and GFP-MSCs (data not shown). Furthermore, the critical importance of Sfrp2 is underscored by the fact that the prosurvival effects of conditioned medium were reduced significantly when Sfrp2 expression was knocked down in Akt-MSCs.
The results of this study provide strong evidence for paracrine actions of stem cells. It is well known that the microenvironment of stem cells is important for stem cell survival and proliferation. Our data extend this concept to include the effects of paracrine mediators released by the stem cells on the microenvironment, the possible existence of a transplanted stem cell niche, and their potential role in tissue repair and regeneration. Our data suggest that Sfrp2 alters the local milieu around the infarct zone to favor cardiomyocyte cell survival and raise the possibility that simple administration of Sfrp2 alone can achieve results similar to stem cell-based cardiac therapy. A protein-based therapy has obvious advantages over cell-based cardiac therapy for acute myocardial infarction. It is beyond the scope of this study to examine the therapeutic potential of Sfrp2 injection in vivo. Further studies will be needed to confirm the cardio-protective effects of Sfrp2 and molecules that modulate the Wnt–β-catenin pathway for successful translation of this therapy to the bedside.
Materials and Methods
Purification of MSCs and Retroviral Transduction.
Bone marrow cells from 8- to 10-week-old wild-type male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were collected in a modified MEM supplemented with 17% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 0.25 mg/ml amphotericin B. Mononuclear cells then were isolated from aspirates by Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) gradient centrifugation. For the retroviral transduction, murine Akt1 cDNA tagged with a c-myc epitope by PCR amplification was cloned into pMSCV-IRES-GFP vector. To overexpress Akt/GFP (Akt-MSC) or GFP alone (GFP-MSC), MSCs were infected with high-titer VSV-G pseudotyped retrovirus.
Gene Expression Profiling and RNA Validation.
Eight micrograms of total RNA from mouse GFP-MSCs and Akt-MSCs (n = 3 per group) under normoxia or hypoxia (6 h) was used for microarray analysis. Affymetrix GeneChip Mouse Genome 430 2.0 Arrays, which allow analysis of ≈45,000 transcripts, were performed in triplicate and analyzed with Affymetrix Microarray Suite (MAS 5.0). For further analysis various Dhip was used. All possible comparisons (Akt-MSC normoxia versus GFP-MSC normoxia, Akt-MSC hypoxia versus GFP-MSC hypoxia, GFP-MSC hypoxia versus GFP-MSC normoxia, and Akt-MSC hypoxia versus Akt-MSC normoxia) were tested. The transcripts then were annotated by using various databases to compile a list of potent secreted candidates.
Gene expression profiling was determined by real-time QPCR for selected genes with appropriate primer mixtures (TaqMan Gene Expression Assays; No. 4331182) from Applied Biosystems [Sfrp1, Mm00489161; Sfrp2, Mm00485986; Sfrp3 (Frzb), Mm00441378; and Gapdh, Mm99999915].
Conditioned Media Collection and Concentration.
GFP-MSCs and Akt-MSCs from passages 3–5 reached 90% confluence in 10-cm dishes. The cells then were left in a standard incubator or the hypoxic chamber in the medium [αMEM/0.2% FBS/(insulin transferrin selenite)ITS] for 6 h. Plates with medium only also were left at the same conditions as control conditioned medium. The medium was concentrated up to 50× by using a Millipore Amicon Ultra-15 (Billerica, MA) system with membrane.
Western Blot Analysis.
Proteins from conditioned medium from MSCs were separated by SDS/PAGE gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The blots were incubated with Sfrp2 primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then with the appropriate secondary antibody conjugated with horseradish peroxidase (Amersham Biosciences). Complexes were detected by chemiluminescence (LumiGLO; Cell Signaling Technology, Danvers, MA).
Suppression of Secreted Factor Effect by siRNA.
GFP-MSCs or Akt-MSCs were incubated overnight with OptiMEM medium containing 1 μM siRNA for Sfrps (Sfrp1: sense, 5′-CGGAUUGUAAAGAACUGCATT-3′ and antisense, 5′-UGCAGUUCUUUACAAUCCGTT-3′; Sfrp2: sense, 5′-GGACGACAACGACAUCAUGTT-3′ and antisense, 5′-CAUGAUGUCGUUGUCGUCCTC-3′; and Sfrp3: sense, 5′-CCGUCAAUCUUUAUACCACTT-3′ and antisense, 5′-GUGGUAUAAAGAUUGACGGTG-3′; Ambion, Austin, TX). Rhodamine-labeled GFP siRNA (Qiagen, Valencia, CA) was used to assess transfection efficiency. Cells were incubated in normal medium for 48 h then exposed to a serum-free medium (αMEM/0.2% FBS/ITS) at normoxia or hypoxia as described above. The medium was concentrated for further analysis. The efficiency of the siRNA-mediated reduction of Sfrps was assessed by QPCR using 18S as a control.
Adult Rat Ventricular Myocyte (ARVM) Isolation and Quantification of Apoptotic Cardiomyocytes.
ARVMs were isolated by enzymatic dissociation as described in ref. 8. Cells (1 × 106) were incubated in 10-cm dishes (Becton Dickinson, Franklin Lakes, NJ) overnight with full 199 medium (0.2% albumin, 2 mM carnitine, 5 mM creatine, 5 mM taurine, and 1 μg/ml of recombinant human insulin; Sigma, St. Louis, MO). The next day, the medium was replaced with optimal medium according to different assays. The hypoxic condition was created by incubating the cells at 37°C into a hypoxia chamber with an atmosphere of 5% CO2/95% N2. The oxygen level into the chamber was controlled to 0.5%.
Apoptosis was determined by measuring the activity of cleaved caspase 3 by using a caspase-specific fluorogenic substrate according to the protocol for the Caspase 3 Assay Kit (Sigma). ARVMs were lysed after treatment with Sfrps for 24 h under hypoxia. Then, 5 μl of cell extract was incubated in reaction buffer at room temperature for 1 h. The enzyme-catalyzed release of 7-amino-4-methyl coumarin (AMC) was measured by a fluorescence microplate reader. Fluorescent units were converted to picomoles of AMC per microliter of sample per min per microgram of protein by using a standard curve of AMC.
Quantitation of Morphologic Changes of ARVCs After Hypoxic Exposure.
Isolated cardiomyocytes were seeded in multiwell plates (Becton Dickinson) precoated with laminin (1 μg/cm2) and left overnight in standard growth medium (M199). One day later, the medium was replaced by serum-free medium with different doses of Sfrp2. The ARVCs then were placed in the hypoxia chamber. The viability of the ARVCs was evaluated on the basis of their morphology by using a phase-contrast microscope, and rod-shaped cardiomyocytes were considered viable. The number of round-shaped cardiomyocytes was counted in six random high-power fields and expressed as a percentage of the total number of cells.
Myocardial Infarction Model and Determination of Infarct Size.
Ligation of the left anterior descending coronary artery was performed on 170- to 200-g female Sprague–Dawley rats (Harlan World Headquarters, Indianapolis, IN). Briefly, a left thoracotomy was performed under anesthesia, and the vessel was ligated with a silk suture at midway between the left atrium and the apex of the heart. The infarction then was assessed by the change of color and kinesis of the apex and the anterior-lateral wall. Thirty minutes later, 250 μl of conditioned media was injected in five different sites around the border zone. An equivalent amount of PBS was injected in the control group. Then, the wound was sutured immediately.
Infarct size was analyzed by staining the tissue for 5 min at 37°C with planar morphometry in triphenyl tetrazolium chloride (Sigma Chemicals) followed by fixation of 12 h in 10% phosphate-buffered formalin. Size was expressed as a percentage of the total ventricular area.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (to V.D.).
Abbreviations
- Sfrp
secreted frizzled related protein
- MSC
mesenchymal stem cell
- QPCR
quantitative PCR
- PI3-kinase
phosphatidylinositol 3-kinase
- ARVC
adult rat ventricular cardiomyocyte
- ARVM
adult rat ventricular myocyte.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610024104/DC1.
References
- 1.Caplice NM, Deb A. Nat Clin Pract Cardiovasc Med. 2004;1:90–95. doi: 10.1038/ncpcardio0051. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Zeiher AM, Schneider MD. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Circulation. 2004;109:2386–2393. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 6.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 7.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 8.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 9.Moon RT, Bowerman B, Boutros M, Perrimon N. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 10.Steve E, Jones CJ. BioEssays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann MW, Rechner C, Freund C, Baurand A, El Jamali A, Dietz R. J Mol Cell Cardiol. 2004;37:681–690. doi: 10.1016/j.yjmcc.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Hung Wong W. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-8-research0032. RESEARCH0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano Y, Kypta R. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 14.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li R-K. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Sano M, Songyang Z, Schneider MD. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 17.Schneider VA, Mercola M. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gijn ME, Daemen MJAP, Smits JFM, Blankesteijn WM. Cardiovasc Res. 2002;55:16–24. doi: 10.1016/s0008-6363(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee JL, Lin CT, Chueh LL, Chang CJ. J Biol Chem. 2004;279:14602–14609. doi: 10.1074/jbc.M309008200. [DOI] [PubMed] [Google Scholar]
- 20.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 21.Logan CY, Nusse R. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, MacNaughton WK. Cancer Res. 2005;65:8604–8607. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- 23.Xu DG, Crocker SJ, Doucet JP, St-Jean M, Tamai K, Hakim AM, Ikeda JE, Liston P, Thompson CS, Korneluk RG, et al. Nat Med. 1997;3:997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- 24.Mercer EA, Korhonen L, Skoglosa Y, Olsson PA, Kukkonen JP, Lindholm D. EMBO J. 2000;19:3597–3607. doi: 10.1093/emboj/19.14.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manji HK, Moore GJ, Chen G. J Clin Psychiatry. 2000;61(Suppl 9):82–96. [PubMed] [Google Scholar]
- 26.Schumann H, Holtz J, Zerkowski H-R, Hatzfeld M. Cardiovasc Res. 2000;45:720–728. doi: 10.1016/s0008-6363(99)00376-4. [DOI] [PubMed] [Google Scholar]
- 27.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.