Abstract
We investigated finger strength and the ability to control digit force/torque production in children with Developmental Coordination Disorder (DCD) using manipulative tasks with different kinetic redundancies (KNR). Age-related changes in finger strength and finger force/torque control in typically developing (TD) children were also examined to provide a developmental landscape that allows a comparison with children with DCD. Forty-eight TD children (7-, 9-, and 11- year-olds) and sixteen 9-year-old children with DCD participated in the study. Three isometric tasks with different KNR were tested: constant index finger pressing force production (KNR=0), constant thumb-index finger pinching force production (KNR=1), and constant thumb-index finger torque production (KNR=5). Each subject performed two conditions for each isometric task: maximum voluntary force/torque production and constant force/torque control (40% of maximum force/torque). The results showed that the maximum force/torque production increased and the variability of constant force/torque control decreased with age in all tasks in TD children. Children with DCD showed larger variability than TD children in the constant thumb-index finger pinching torque production. These results suggest that children with DCD, as compared to TD children, are capable of producing the same level of maximum finger force, but have poor control in manipulation tasks with a large number of kinetic redundancies.
Keywords: motor redundancy, finger force control, development, children, DCD
For successful achievement of everyday manipulative tasks, the central nervous system (CNS) is required to control digit-tip forces and moments. During a simple multi-digit prehension task, the hand provides an infinite number of digit-tip contact force and torque solutions available to achieve the motor task. This problem is commonly referred to as motor redundancy [5;28], which requires the CNS to find a solution from the many available kinetic variables. Previous studies have addressed the problem of motor redundancy in manipulative tasks of adults, specifically during prehension of hand-held objects [24;25;29]. However, far less attention has been drawn to how children solve the problem of motor redundancy and no studies have examined how children with motor difficulties such as those with Developmental Coordination Disorder (DCD) [2] handle this problem.
Children with DCD often show impairments in manipulative tasks which are required in daily and school activities such as writing and drawing [13;23]. Previous studies have shown that children with DCD, compared to typically developing (TD) children, have higher variability in controlling finger force pulses [19;21], more excessive grip force generation [16;17], and larger variability in finger forces during pinching grip tasks [20]. However, the observed poor motor performance in children with DCD may result from impairment in controlling the large number of kinetic redundancies commonly found in these manipulative tasks. Our knowledge about how children with DCD and TD children perform motor tasks with different numbers of kinetic redundancy (KNR) is limited. This study systematically investigated finger strength and the ability to control digit force/torque production in children with DCD using manipulative tasks with different numbers of KNR. To further understand the development of children with DCD in relation to TD children, age-related changes in finger strengths and finger force/torque control were also examined.
Forty-eight TD children aged 7, 9, and 11 years (8 male and 8 female children in each age group; mean ages: 7.6±0.5, 9.6±0.3, 11.4±0.6 years) and sixteen children with DCD (mean age: 9.6±0.2 years; age- and gender-matched with the nine-year-old TD children group), participated in the study. Potential children were screened in a two step process: a) children were reported by their teachers as experiencing difficulties in school motor activities, and b) assessment by the Movement Assessment Battery for Children (MABC) [15]. In this study, the children with DCD were defined as those with the MABC scores at or below the 5th percentile (a standard cut-off point for this disorder [14]). The TD children were defined as those whose scores on the MABC test were at or above the 35th percentile. All subjects were right-handed, according to their preferential hand use during writing and eating, and had no history of neuropathy or trauma to the upper limbs. A parent or legal guardian of each child gave his/her informed consent prior to participation in the study. All procedures were approved by the Committee Board of Federal University of Rio Grande do Sul, Brazil. [22]
Three isometric tasks with different numbers of KNR were tested: index finger pressing force production (PR) (KNR = 0), thumb-index finger pinching force production (PN) (KNR= 1), and thumb-index finger torque production (TQ) (KNR = 5). At the kinetic level, the PR task has one variable; index normal force , with one constraint ( , where is the target constant force for the PR task), thus, there is no kinetic redundancy in the system. The PN task has two variables; index finger and thumb normal forces ( and , respectively), and one constraint (, where is the target constant force for the PN task), which creates one KNR. Although both PN and TQ tasks have the same thumb and index finger joint configurations, the TQ task has increased redundancy due to the larger number of kinetic degrees-of-freedom (DoF). There are six variables (, and , where and are index and thumb tangential forces and , and are moment arms for normal forces) with one constraint (, where r is grip width/2 and Tconst is the target constant torque). Therefore, there exist five redundant DoF in the torque production task.
For the PR and PN tasks, customized strain gauge force sensors were used to measure the index finger pressing force and thumb-index pinching force, respectively. For the TQ task, we used a strain gauge force sensor with a moment arm (2.5 cm) and the torque produced by the thumb and index finger was calculated as torque = force * moment arm. All sensors were mechanically fixed and all tasks were isometric. The signals from the strain gauge sensors were amplified (MSC6, ENTRAN, Canada) and routed to the analog-digital converter then saved in a microcomputer (Satellite 110CS, Toshiba, USA). The sampling frequency was 500 Hz.
During the experiment, the subject sat on a chair facing an oscilloscope that showed the force/torque he/she applied to the strain gauge sensors. The subjects performed two conditions in each isometric task (maximum voluntary force/torque (MAX) and constant force/torque (CONST) production) and one trial for each condition. In the MAX condition, the subject produced maximum force/torque for 5 s in each task [maximum index finger pressing force (MAXPR), maximum thumb-index finger pinching force (MAXPN), and maximum thumb-index finger pinching torque (MAXTQ]. For the CONST condition, the subject maintained an isometric, constant, and continuous force/torque at 40% of his/her maximum in the given task for 20 s [constant index finger pressing force (CONSTPR), constant thumb-index finger pinching force (CONSTPN), and constant thumb-index finger pinching torque (CONSTTQ)]. The oscilloscope screen displayed a fixed horizontal line to indicate the target force/torque (40% of MAX) and a moving horizontal line to show the force/torque produced by the subject as online feedback. Each trial started with a “get ready, go” signal, and the subject was instructed to match the moving line to the fixed target line. The first trial of each task served as a practice trial and thus was discarded in the analyses. All signals were low-pass filtered (2nd-order Butterworth, zero-lag, 25-Hz cutoff).
For the MAX condition, the instant peak force/torque was selected as the maximum. Since the torque task has a different unit, the maximum values were normalized by the mean value of the 11-year-old group for between-task comparisons. For the CONST tasks, the coefficient of variation (CV = standard deviation/mean) was computed over the last 15 s of each trial as an index of variability. The data from the initial 5 s of each trial were removed to exclude the initial period of force/torque adjustment to the visual feedback.
Regression analyses were used to examine developmental changes in each task in TD children. Statistical significance was set at p=.05. With a sample size of 48, the critical value of significance for the empirical coefficients of correlation is 0.288 at p=.05. For the comparison between children with DCD and the TD children, standard descriptive statistics and mixed-effects ANOVAs with the factors of GROUP (2 levels: TD and DCD) and TASK (3 levels: pressing, pinching, and torque tasks) were used.
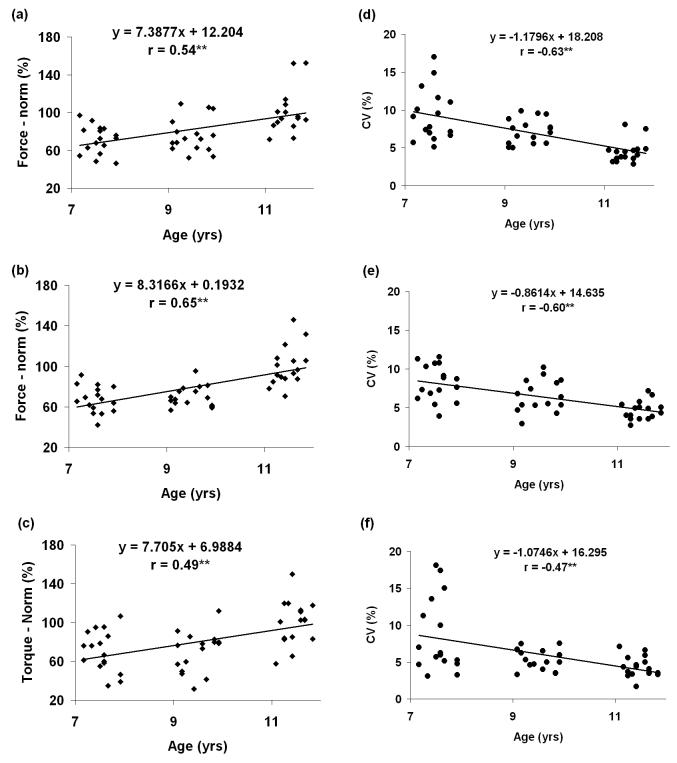
The maximum force and torque production showed an age-related increase in TD children [Figures 1a, 1b, and 1c] in all three tasks. This result was supported by the regression analysis between the MAX values and ages (all p < .01). These results complement the findings of previous studies that showed age-related increases of children's maximum force production abilities in other motor tasks such as hand grasping tasks and index finger pressing [4;27]. CVs of the children's performance during CONSTPR, CONSTPN, and CONSTTQ tasks all decreased with age in TD children (Figure 1d, 1e, and 1f) (all p < .01). This indicates consistent age-related improvements of TD children's isometric control of finger force/torque in all tasks. Similar findings have been reported in previous studies with finger pressing tasks [7;18;27] and pinching tasks [8-10].
Figure 1.
–The maximum voluntary force/torque values (normalized by the mean values of 11-year-old group and expressed as a %) as a function of age in TD children in: (a) index finger pressing force task, (b) thumb-index finger pinching force task, and (c) thumb-index finger pinching torque task. Coefficient of variations (CV), as a function of age in TD children in: (d) constant index finger pressing force task, (e) thumb-index finger pinching force task, and (f) thumb-index finger pinching torque task are also shown. ** p<.01.
For the comparison between children with DCD and age-matched TD children in their maximum force or torque production, no significant factor effect or interaction (all p > .05) was found. The mean MAX values for the TD children were MAXPR=6.12 ±1.45 N, MAXPN=6.31 ±0.91 N, and MAXTQ=0.16±0.04 Nm, and for children with DCD were MAXPR=5.30±1.10 N, MAXPN=6.29±1.21 N, and MAXTQ=0.15±0.02 Nm. While the variability during constant force production tasks (CONSTPR, CONSTPN) in children with DCD did not differ from the TD control group, children with DCD showed a significantly larger variability during the constant torque production task (CONSTTQ), as compared to TD children (Figure 2). This finding was supported by two-way ANOVA which showed significant effects of GROUP [F(1,30)=7.2, p<.01] and GROUP × TASK interaction [F(2,60)=4.1, p<.05].
Figure 2.
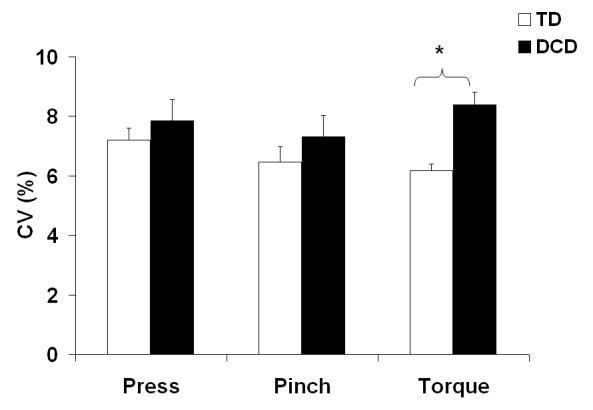
–Coefficient of variation (CV) (mean ± S.E.) for children with DCD and age-matched TD children during constant index finger pressing force task, thumb-index finger pinching force task, and thumb-index finger pinching torque task. * p<.05.
While larger variability of finger force production in children with DCD has been reported in other studies [16;17;19;20], our current study showed no statistically significant group differences in the CONSTPR and CONSTPN tasks. However, a unique finding of this study is the larger variability difference between the children with DCD and TD children found in a constant torque production task. We intentionally used similar tasks with different number of KNR (PR: KNR = 0, PN: KNR= 1, and TQ: KNR = 5).
Compared to the age-matched control group, children with DCD tend to show higher variability across the tasks [pressing (9%) pinching (13%) and torque (46%), (Figure 3)]. The larger variability in the DCD group during the CONTTQ as compared with the CONTPR and CONTPN may suggest that children with DCD have deficits in controlling tasks with larger numbers of DoF not only at the kinetic level but also at the muscular level. The CONTTQ task involves a larger number of muscles (i.e., muscles involved in hand supination, index finger adduction, and thumb abduction in addition to the muscles associated with thumb-index pinching) as compared to CONTPR and CONTPN.
Using the regression equations obtained from TD children, developmental differences of children with DCD were examined. The results showed that in the CONTTQ task, the average performance of the 9-year-old children with DCD (mean age = 9.6 years) was similar to the 7-year-old TD children (mean age = 7.9 years), while the pressing and pinching force tasks showed relatively smaller differences (mean ages = 8.6 and 8.7 years, respectively) between children with DCD and the TD children. This comparison complements our suggestion of the poor control by children with DCD in a manipulative task with a large number of kinetic and motor redundancies. Grasping force control during a simple static task [25;26] such as holding a glass of water allows a relatively large margin of force without manipulative errors. The force should not exceed the limit to break the glass nor fail to reach the slipping threshold. As compared to controlling the grasping force, controlling torque does not allow a margin of force: the resultant torque should be equal to zero for the static grasping. If the fingers produced a torque that offsets the target torque (i.e., zero resultant torque), there will be an immediate rotation of the glass causing water to spill. Thus, the impairments of torque control in children with DCD may result in a greater challenge in daily manipulative tasks.
In any control system, the computational load will increase with the number of independent variables to be controlled. In our study, for example, the constant torque production task involved a larger number of independent kinetic variables to be controlled by the central nervous system (i.e., larger kinetic redundancy) than the constant force production tasks. Thus, the greater problem for the children with DCD in controlling a constant torque may be viewed as their deficits in governing numerous independent variables. A similar phenomenon has been previously observed in Parkinson's disease patients who showed a problem in controlling a large number of degrees of freedom or independent variables [1]. Although the underlying mechanisms of DCD are to date unknown, it has been suggested that the increased variability in finger force control in children with DCD could be related to a compromised balance among the basal ganglia nuclei [19]. Also a recent study of children on and off methylphenidate (a stimulant that amplifies the release of dopamine) found that children with DCD improved manual dexterity when they were on the drug [12]. However, in addition to the basal ganglia areas, other areas such as primary motor cortex (M1) and sensory motor cortex (S1) may also contribute to the motor behavior deficits as their circuitries are interconnected [3]. Adult brain images have shown a bilateral circuit of sensory and motor areas involved in the control of the fingertip forces during precision grip tasks [11]. In view of these findings, it appears rather unlikely that the increased variability in finger force control can be specifically attributed to basal ganglia dysfunction. Other motor function-related cortical fields, including primary motor, sensory, supplementary motor area, premotor, prefrontal, parietal and cingulated cortices, and cerebellum, may be necessary for controlling precise static force of finger muscles [6]. Thus, deficits in several areas of the brain may affect the finger force or torque control in children with DCD.
In summary, our results showed that children with DCD can produce the same level of maximum finger strength as TD children but have poor control in a manipulation task with a large number of kinetic redundancies. Thus, we interpret the difficulties in everyday manipulation tasks, often observed in children with DCD during handwriting, tying shoe laces or grasping/holding objects, are not related to the magnitude of force production itself, but with the torques adjustments among the fingers involved in these tasks. Our findings suggest that our neuromechanical paradigm may offer a novel and intriguing approach to future research on manipulative difficulties in children with DCD. Finally, we recognize that the employed tasks were functionally different (i.e., pressing and rotation) and suggest, for the next studies, investigating the effects of kinetic redundancy (e.g., different number of fingers employed for the same pressing force matching task) on the same motor task (e.g., constant pressing force control). Furthermore, neuroimaging techniques would be valuable in matching neural sources to behavioral performances for a clearer understanding of the underlying mechanisms involved in the force control of children with DCD.
Acknowledgements
This research was supported by the National Institute of Health grant R01HD42527 to the last author.
Reference List
- 1.Alberts JL, Tresilian JR, Stelmach GE. The co-ordination and phasing of a bilateral prehension task. The influence of Parkinson's disease, Brain. 1998;121(Pt 4):725–742. doi: 10.1093/brain/121.4.725. [DOI] [PubMed] [Google Scholar]
- 2.APA . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 3.Baufreton J, Zhu ZT, Garret M, Bioulac B, Johnson SW, Taupignon AI. Dopamine receptors set the pattern of activity generated in subthalamic neurons. FASEB J. 2005;19:1771–1777. doi: 10.1096/fj.04-3401hyp. [DOI] [PubMed] [Google Scholar]
- 4.Beenakker EA, van der Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4-16 years by hand-held dynamometry. Neuromuscul. Disord. 2001;11:441–446. doi: 10.1016/s0960-8966(01)00193-6. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein N,A. The co-ordination and regulation of movements. Pergamon Pres; Oxford: 1967. [Google Scholar]
- 6.Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Experimental Brain Research. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch KM, Newell KM. Age differences in noise and variability of isometric force production. J. Exp. Child Psychol. 2001;80:392–408. doi: 10.1006/jecp.2001.2642. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch KM, Newell KM. Children's coordination of force output in a pinch grip task. Dev. Psychobiol. 2002;41:253–264. doi: 10.1002/dev.10051. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch KM, Newell KM. Deterministic and stochastic processes in children's isometric force variability. Dev. Psychobiol. 2003;43:335–345. doi: 10.1002/dev.10140. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch KM, Newell KM. Changes in the structure of children's isometric force variability with practice. J. Exp. Child Psychol. 2004;88:319–333. doi: 10.1016/j.jecp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: An fMRI study. Journal of Neurophysiology. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- 12.Flapper BCT, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention-deficit-hyperactivity disorder and developmental coordination disorder. Developmental Medicine and Child Neurology. 2006;48:165–169. doi: 10.1017/S0012162206000375. [DOI] [PubMed] [Google Scholar]
- 13.Geuze RH. Motor impairment in DCD and activities of daily living. In: Sugden D, Chambers M, editors. Children with Developmental Coordination Disorder. Whurr Publishers; London: 2005. pp. 19–46. [Google Scholar]
- 14.Geuze RH, Jongmans MJ, Schoemaker MM, Smits-Engelsman BCM. Clinical and research diagnostic criteria for developmental coordination disorder: a review and discussion. Human Movement Science. 2001;20:7–47. doi: 10.1016/s0167-9457(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 15.Henderson SE, Sugden D. Movement Assessment Battery for Children. The Psychological Corporation; London: 1992. [Google Scholar]
- 16.Hill EL, Wing AL. Developmental disorders and the use of grip force to compensate for inertial forces during voluntary movement. In: Connolly KJ, editor. The Psychobiology of the Hand. Mac Keith Press; London: 1998. pp. 199–212. [Google Scholar]
- 17.Hill EL, Wing AL. Coordination of grip force and load force in Developmental Coordination Disorder: a case study. Neurocase. 1999;5:537–544. [Google Scholar]
- 18.Lazarus JC, Whitall J, Franks C. Isometric force regulation in children. Journal of Experimental Child Psychology. 1995;60:245–260. [Google Scholar]
- 19.Lundy-Ekman L, Ivry R, Keele S, Woollacott M. Timing and force control deficits in clumsy children. Journal of Cognitive Neuroscience. 1991;3:367–376. doi: 10.1162/jocn.1991.3.4.367. [DOI] [PubMed] [Google Scholar]
- 20.Pereira HS, Landgren M, Gillberg C, Forssberg H. Parametric control of fingertip forces during precision grip lifts in children with DCD (developmental coordination disorder) and DAMP (deficits in attention motor control and perception) Neuropsychologia. 2001;39:478–488. doi: 10.1016/s0028-3932(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 21.Piek JP, Skinner RA. Timing and force control during a sequential tapping task in children with and without motor coordination problems. J. Int. Neuropsychol. Soc. 1999;5:320–329. doi: 10.1017/s1355617799544032. [DOI] [PubMed] [Google Scholar]
- 22.Piek JP, Skinner RA. Timing and force control during a sequential tapping task in children with and without motor coordination problems. J. Int. Neuropsychol. Soc. 1999;5:320–329. doi: 10.1017/s1355617799544032. [DOI] [PubMed] [Google Scholar]
- 23.Polatajko HJ, Macnab JJ, Anstett B, Malloy-Miller T, Murphy K, Noh S. A clinical trial of the process-oriented treatment approach for children with developmental co-ordination disorder. Developmental Medicine and Child Neurology. 1995;37:310–319. doi: 10.1111/j.1469-8749.1995.tb12009.x. [DOI] [PubMed] [Google Scholar]
- 24.Santello M, Soechting JF. Force synergies for multifingered grasping. Exp. Brain Res. 2000;133:457–467. doi: 10.1007/s002210000420. [DOI] [PubMed] [Google Scholar]
- 25.Shim JK, Latash ML, Zatsiorsky VM. Prehension synergies in three dimensions. J. Neurophysiol. 2005;93:766–776. doi: 10.1152/jn.00764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim JK, Latash ML, Zatsiorsky VM. Prehension synergies: trial-to-trial variability and principle of superposition during static prehension in three dimensions. J. Neurophysiol. 2005;93:3649–3658. doi: 10.1152/jn.01262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smits-Engelsman BC, Westenberg Y, Duysens J. Development of isometric force and force control in children. Brain Res. Cogn Brain Res. 2003;17:68–74. doi: 10.1016/s0926-6410(03)00081-8. [DOI] [PubMed] [Google Scholar]
- 28.Turvey MT. Coordination. Am. Psychol. 1990;45:938–953. doi: 10.1037//0003-066x.45.8.938. [DOI] [PubMed] [Google Scholar]
- 29.Zatsiorsky VM, Gregory RW, Latash ML. Force and torque production in static multifinger prehension: biomechanics and control. I. Biomechanics. Biol. Cybern. 2002;87:50–57. doi: 10.1007/s00422-002-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]