Abstract
Type 1 diabetes in both humans and nonobese diabetic (NOD) mice results from autoreactive T cell destruction of insulin-producing β cells. Cure of type 1 diabetes may require both reversal of autoimmunity and regeneration of β cells. Induction of chimerism via allogeneic hematopoietic cell transplantation has been shown to reestablish tolerance in both prediabetic and diabetic NOD mice. However, it is unclear whether this therapy augments β cell regeneration. Furthermore, this procedure usually requires total body irradiation conditioning of recipients. The toxicity of total body irradiation conditioning and potential for graft-versus-host disease (GVHD) limit the application of allogeneic hematopoietic cell transplantation for treating type 1 diabetes. Here we report that injection of donor bone marrow and CD4+ T cell-depleted spleen cells induced chimerism without causing GVHD in overtly diabetic NOD mice conditioned with anti-CD3/CD8 and that induction of chimerism in new-onset diabetic NOD mice led to elimination of insulitis, regeneration of host β cells, and reversal of hyperglycemia. Therefore, this radiation-free GVHD preventive approach for induction of chimerism may represent a viable means for reversing type 1 diabetes.
Keywords: hematopoietic cell transplantation, type 1 diabetes, anti-CD3-conditioning, reversal of diabetes, reversal of autoimmunity
Type 1 diabetes is an autoimmune disease resulting from destruction of insulin-secreting β cells by pathogenic T cells (1, 2). The nonobese diabetic (NOD) mouse is currently the most widely used animal model of human type 1 diabetes (3). Studies have revealed that NOD mice, as well as type 1 diabetic patients, have defects in negative selection of autoreactive T cells in the thymus (4, 5) as well as in peripheral regulatory T (Treg) cells such as CD25+CD4+ T and natural killer T cells that control autoreactive T cell activation and expansion in the periphery (6, 7).
Although an extensive number of therapies have been reported to prevent diabetes in NOD mice, only a few (i.e., antibodies specific for CD3) have proven efficacious when administered at the time of symptomatic onset (8). These therapies tend to reset the peripheral tolerance. For example, multiple injections of anti-CD3 were shown to reverse autoimmunity and hyperglycemia in the majority of new-onset diabetic NOD mice (9), which induced TGF-β-secreting CD25+CD4+ Treg cells that suppress autoimmunity (10). Interestingly, those treated mice still had autoreactive T cells and showed insulitis (11). Nondepleting anti-CD3 treatment of recent-onset type 1 diabetes patients has also been reported to ameliorate diabetes and to induce CD25+CD8+FoxP3+ and IL-10-secreting CD4+CD25+ Treg cells (12–15). However, the beneficial effects of anti-CD3 treatment in type 1 diabetic patients appeared to decline after 1 year (16). It remains unclear why reversal of autoimmunity by anti-CD3 treatment is unstable in type 1 diabetic patients. It might be because of an accumulation of autoreactive T cells from the defective thymus that overwhelm the peripheral Treg cells induced by anti-CD3 treatment. Therefore, a treatment that could correct both central and peripheral tolerance defects may be required for a permanent reversal of autoimmunity in type 1 diabetes.
Another problem with type 1 diabetes is the destruction of insulin-producing β cells. Investigators have been searching for therapies that would support regeneration of islet β cells in type 1 diabetic individuals. Recent studies in animal models as well as patients indicate three potential mechanisms for islet regeneration in type 1 diabetes (17–20): replication of existing β cells, neogenesis of β cells from pancreatic ductal epithelium stem cells, and transdifferentiation of exocrine into endocrine β cells.
It has recently been proposed that induction of chimerism via allogeneic hematopoietic cell transplantation (HCT) can be an effective therapy for refractory autoimmune diseases (21, 22). Induction of chimerism via transplantation of bone marrow (BM) cells from nonautoimmune donors into prediabetic and diabetic NOD mice was shown to restore self-tolerance and induce tolerance to donor islet grafts (23–26). However, it was unclear whether induction of chimerism could lead to β cell regeneration. In addition, the HCT procedures used in those reports require total body irradiation conditioning of the recipients, and the toxicity of radiation and potential for graft-versus-host disease (GVHD) limits its application in treating type 1 diabetic patients. We recently reported that transplantation of allogeneic donor BM and CD8+ T cells into prediabetic NOD mice conditioned with anti-CD3 mAb induced chimerism without causing GVHD (27). In the current study, we showed that injection of donor BM and CD4+ T cell-depleted spleen (SPL) cells induced chimerism in diabetic NOD mice conditioned with anti-CD3 and anti-CD8 mAb (anti-CD3/CD8). We also demonstrated that induction of chimerism in new-onset diabetic NOD mice led to elimination of insulitis, regeneration of host islet β cells, and a reversal of diabetes. This radiation-free regimen provides for induction of chimerism without GVHD in overtly diabetic NOD mice, and we demonstrate that this chimerism augments the regeneration of host islet β cells.
Results
Induction of Chimerism with Donor BM and CD4+ T Cell-Depleted SPL Cells in Diabetic NOD Mice Conditioned with Anti-CD3/CD8.
We recently reported that anti-CD3 conditioning of prediabetic NOD mice induced stable long-term chimerism without causing GVHD after a single injection of 100 × 106 donor BM cells and two injections of 20 × 106 donor CD8+ T cells (27). However, we also observed that the same dose of donor BM and CD8+ T cells did not induce chimerism in diabetic NOD mice, whose blood glucose had been controlled with implantation of insulin pellets (data not shown).
To induce chimerism in diabetic NOD mice, we first tested whether replacing the positively selected donor CD8+ T cells with CD4+ T cell-depleted SPL (CD4− SPL) cells induced chimerism. The use of CD4− SPL cells could avoid the negative impact of anti-CD8 mAb on donor CD8+ T cells and could also take the advantage of allogeneic donor natural killer cells that were reported to augment donor cell engraftment without causing GVHD (28). Accordingly, prediabetic and diabetic NOD mice were conditioned with anti-CD3 on day −5, and were injected with FVB/N donor BM (100 × 106) along with CD4− SPL cells (100 × 106), which contained ≈10–15 × 106 CD8+ T cells and 1–2 × 106 natural killer cells, on day 0. The CD4− SPL cells were injected again on day 5 after the first injection. Whereas 100% (8/8) of prediabetic NOD mice developed stable long-term chimerism, only 25% (4/16) of the diabetic NOD mice developed chimerism (Table 1). Therefore, anti-CD3 conditioning is not sufficient for induction of chimerism in diabetic NOD mice.
Table 1.
Donor CD4+T cell-depleted SPL and BM cells induced chimerism in overtly diabetic NOD mice conditioned with anti-CD3 and anti-CD8
| Conditioning | NOD mice | % chimeric recipients |
|---|---|---|
| Anti-CD3 | Prediabetic | 100 (8/8) |
| Diabetic | 25 (4/16) | |
| Anti-CD3/anti-CD8 | Prediabetic | 100 (8/8) |
| Diabetic | 96 (30/31) |
The percentage of donor-type cells among blood mononuclear cells from the chimeric recipients 8 weeks after HCT was usually >30%, and donor-type cells were not detectable in nonchimeric recipients.
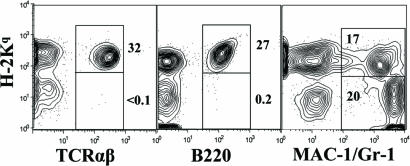
As it has been reported that anti-CD8 treatment facilitated donor cell engraftment in NOD recipients conditioned with total body irradiation (29), we tested whether combined anti-CD3 and anti-CD8 conditioning would allow for induction of chimerism in nonirradiated diabetic NOD mice. Accordingly, diabetic NOD mice were conditioned with anti-CD3/CD8 at a dose of 20 μg/g of body weight each on day −5. The mice were injected with donor BM and CD4− SPL cells (100 × 106 each) on day 0, and the CD4− SPL cells were injected again on day 5. We found that 100% (8/8) of the prediabetic NOD recipients as well as 96% (30/31) of the diabetic NOD recipients developed stable chimerism (Table 1 and Fig. 1). The chimeric recipients showed no signs of GVHD (data not shown).
Fig. 1.
Induction of chimerism in anti-CD3/CD8-conditioned diabetic NOD recipients given donor BM and CD4+ T cell-depleted SPL cells. Twelve weeks after HCT, blood mononuclear cells of the recipients were stained with anti-H-2Kq (donor marker), anti-TCRαβ, anti-B220, and anti-Mac-1/Gr-1. One representative is shown of four examined recipients.
Induction of Chimerism in New-Onset Diabetic NOD Mice Reversed Diabetes.
It has been reported that new-onset diabetic NOD mice treated with multiple injections of anti-CD3 or anti-CD3 and insulin vaccination or anti-lymphocyte globulin (ATG) and exendin-4 showed reversal of diabetes (9, 30, 31), and that BM cells could augment endogenous islet cell regeneration in nonautoimmune mice (32). We tested whether induction of chimerism in new-onset and late-stage of NOD mice could reverse diabetes. Thus, NOD mice older than 14 weeks were routinely checked twice a week for urine and/or blood glucose. Mice having blood glucose levels higher than 300 mg/dl for 3 consecutive days were diagnosed as “diabetic.” The “new-onset” diabetic NOD mice were conditioned with anti-CD3/CD8 within 3 days after diagnosis, and the “late-stage” diabetic NOD mice were conditioned 14 days after diagnosis. All diabetic NOD mice were implanted with insulin pellets right after diagnosis to temporarily normalize blood glucose.
The new-onset and late-stage diabetic NOD mice were conditioned and given one injection of BM and two injections of CD4− SPL cells from FVB/N donors as described above. As shown in Fig. 2A, 83% (20/24) of the new-onset NOD mice given conditioning only became hyperglycemic again ≈20–40 days after diagnosis possibly because of the expiration of insulin pellets. In contrast, none (0/20) of the chimeric recipients showed hyperglycemia 200 days after HCT (P < 0.01) (Fig. 2B). However, all (12/12) of the chimeric late-stage NOD mice also showed hyperglycemia 20–40 days after HCT (Fig. 2C). The new-onset diabetic NOD mice given anti-CD3/CD8 conditioning and one injection of donor CD4− SPL and BM cells showed no chimerism, and 75% (9/12) of them showed hyperglycemia again, which was not significantly different from those given conditioning only. These results indicate that induction of chimerism markedly augments the reversal of diabetes in new-onset diabetic NOD mice.
Fig. 2.
Induction of chimerism reversed diabetes in new-onset diabetic NOD mice. NOD mice with blood glucose levels higher than 300 mg/dl for 3 consecutive days were diagnosed as diabetic. The new-onset diabetic NOD mice were conditioned with anti-CD3 and anti-CD8 within 3 days after diagnosis, and the late-stage diabetic NOD mice were conditioned 14 days after diagnosis. The diabetic NOD mice were implanted with insulin pellets immediately after diagnosis to temporarily normalize blood glucose. Kinetic changes of blood glucose levels of 24 new-onset diabetic NOD mice given anti-CD3/CD8 conditioning only (A), 20 new-onset diabetic NOD mice given conditioning and HCT (B), and 12 late-stage diabetic NOD mice given conditioning and HCT (C) are shown. Mice with recurrence of hyperglycemia were usually killed by day 70.
Marked Increase of β Cell Function and Quantity in Chimeric New-Onset Diabetic NOD Mice with Reversal of Diabetes.
Because all (20/20) chimeric and just a few (7/36) nonchimeric new-onset diabetic NOD mice showed reversal of hyperglycemia, we first determined the degree of glycemic control in the chimeric recipients and the nonchimeric NOD mice by i.p. glucose tolerance test (GTT) 120 days after conditioning and/or HCT. Accordingly, after overnight fasting, 3-week-old NOD, untreated new-onset diabetic NOD, nonchimeric NOD, as well as chimeric recipients with reversal of hyperglycemia were injected with glucose at a dose of 2 mg/g of body weight. As shown in Fig. 3A, after glucose injection, the blood glucose of the untreated new-onset diabetic NOD mice increased to more than 400 mg/dl within 5 min and sustained at this high level for more than 3 h. It is of interest that the kinetics of changes of the blood glucose levels of the nonchimeric NOD with reversal of hyperglycemia were similar to that of the untreated new-onset diabetic NOD mice, although the former had normal glycemia for 120 days already, and the latter had high blood glucose before fasting. In contrast, the blood glucose levels of the chimeric recipients recovered to nearly normal after ≈30 min, although their blood glucose levels also reached 400 mg/dl within 5 min after glucose injection. The kinetics of changes of blood glucose levels of the chimeric recipients were similar to that of 3-week-old NOD mice.
Fig. 3.
Increased β cell insulin-secreting capacity and quantity in chimeric NOD recipients with reversal of diabetes. One hundred twenty days after anti-CD3/CD8 conditioning and/or HCT, chimeric NOD recipients and nonchimeric NOD mice with reversal of diabetes, new-onset diabetic NOD mice before treatment, and 3-week-old NOD mice were fasted overnight and then injected i.p. with glucose at a dose of 2 mg/g of body weight. Blood glucose levels were measured before injection and at serial time points after injection (A). Serum insulin levels were measured before injection and 5 min after injection (B). Total insulin-secreting β cell surface and total pancreatic tissue surface of five sections with 75-μm distance of new-onset diabetic NOD mice before (day −5) and after conditioning and/or HCT were measured longitudinally. The percentage of total β cell surface among total pancreatic tissue surface of each mouse was calculated. Mean ± SE of six mice at each time point are shown (C). The nonchimeric diabetic NOD mice had too few residual islets to be detected on days 45 and 60 after conditioning, so their β cell surface at those time points are not shown. Only 7 of 36 nonchimeric new-onset diabetic NOD mice showed reversal of hyperglycemia, and they were used for the day-120 time point; thus, no nonchimeric mice are shown for day 90 or day 200.
In addition, the serum insulin concentration of the above NOD mice before and after glucose injection was compared. As shown in Fig. 3B, after glucose injection, the serum insulin concentration of the untreated new-onset NOD mice and the nonchimeric NOD with reversal of hyperglycemia was similar and only a little higher than before injection. In contrast, the serum insulin concentration of the chimeric recipients after glucose injection increased ≈2-fold, as compared with before injection (P < 0.01), and was ≈2-fold higher than that of the untreated new-onset diabetic NOD mice and the nonchimeric NOD mice after glucose injection (P < 0.01), although there was no significant difference among them before glucose injection. These results indicate that the chimeric recipients have markedly increased insulin-secreting capacity over the untreated new-onset diabetic NOD as well as the nonchimeric NOD mice with reversal of hyperglycemia. We should point out that the serum insulin levels in the chimeric recipients was ≈35% lower than that of 3-week-old NOD before and after glucose injection (P < 0.01).
Furthermore, we evaluated the change of β cell quantity by measuring the percentage of β cell surface among the total pancreatic tissue surface, as described by Bonner-Weir and colleagues (33). As shown in Fig. 3C, the percentage of β cell surface in new-onset diabetic NOD mice before treatment was 0.0068 ± 0.002% of total pancreatic tissue surface. There was no significant change 5 and 15 days after anti-CD3 conditioning and/or HCT as compared with before treatment (day −5). However, β cell surface in chimeric recipients began to increase 30 days after HCT and reached a plateau of ≈0.125% by 60 days after HCT, which was more than 10-fold higher than before treatment (P < 0.01), although it was still ≈5-fold lower than that (0.61 ± 0.11%) of 3-week-old NOD mice (P < 0.01). There was no significant difference observed in the chimeric recipients between 60 and 200 days after HCT. In contrast, the β cell surface (0.007 ± 0.003%) of the nonchimeric NOD mice with reversal of hyperglycemia 120 days after conditioning and/or donor cell injection was no different from before treatment (Fig. 3C). The β cell surface of nonchimeric NOD mice on days 45, 60, 90, and 200 after conditioning was not shown because of lack of detectable residual β cells or unavailability of mice. These results indicate that the increased insulin-secreting capacity in the chimeric recipients is due to the increase of β cell quantity. These results also indicate that induction of chimerism augments β cell regeneration in new-onset diabetic NOD mice.
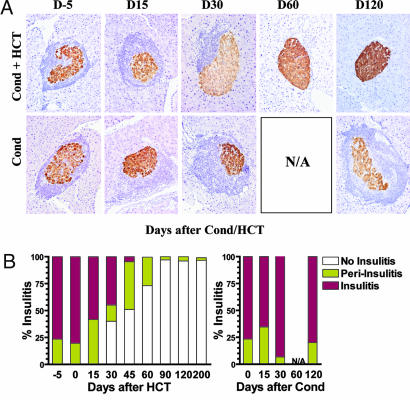
Induction of Chimerism in New-Onset Diabetic NOD Mice Eliminated Insulitis.
Insulitis is the primary cause of β cell destruction (1, 2). Because chimeric, but not nonchimeric, NOD mice with reversal of hyperglycemia showed increased β cell quantity and insulin-secreting capacity, we next longitudinally evaluated the change of insulitis in the mice. Residual islets were divided into insulitis, periinsulitis, and no insulitis based on the severity of infiltration. As shown in Fig. 4B, before treatment (day −5), the majority (76 ± 14%) of residual islets in the new-onset diabetic NOD mice showed insulitis and the rest showed periinsulitis (24 ± 7%), and 15 days after HCT, there was no significant change in the severity of insulitis. Representative residual islets with insulitis before treatment (day −5) and 15 days after treatment are shown in Fig. 4A. However, 30 days after HCT, the severity of insulitis was markedly reduced in chimeric recipients as compared with before treatment. The percentage of residual islets with insulitis in the chimeric recipients was reduced to 45 ± 8% (P < 0.01), and the islets showing no insulitis was increased to 40 ± 9% (P < 0.01), although the percentage of islets with periinsulitis was similar to before treatment. A representative of periinsulitis is shown in Fig. 4A. As time progressed, insulitis in the chimeric recipients was further reduced. By 60 days after HCT, no residual islets were with insulitis, and islets with periinsulitis were at only 27 ± 8%. The rest of islets were of no insulitis, and a representative of them is shown in Fig. 4A. From day 90 onward, almost all (>97%) of the islets in the chimeric recipients showed no insulitis and the rest only minimum periinsulitis (a few infiltrating cells next to the islet) (Fig. 4).
Fig. 4.
Induction of chimerism gradually eliminated insulitis in diabetic NOD mice. Severity of insulitis in chimeric recipients given anti-CD3/CD8 conditioning and HCT, or nonchimeric NOD mice given conditioning only, were evaluated longitudinally. (A) Representative histopathology patterns of islets at different time points. (B) Percentage of residual islets with different level of insulitis at different time points. The mean of six examined mice is shown.
In contrast, there was no reduction in the severity of insulitis in the nonchimeric new-onset diabetic NOD mice with or without reversal of hyperglycemia at different time points after anti-CD3 conditioning and/or injection of donor cells (Fig. 4). By day 45 after conditioning, most of the mice showed hyperglycemia (>500 mg/dl), and it was too difficult to find insulin-secreting islets in those mice for evaluation. As mentioned above, a few (7/36) of the nonchimeric mice showed reversal of hyperglycemia, but even 120 days after conditioning, the mice still showed no reduction of insulitis (Fig. 4). Taken together, these results indicate that induction of chimerism is required for elimination of insulitis in diabetic NOD mice.
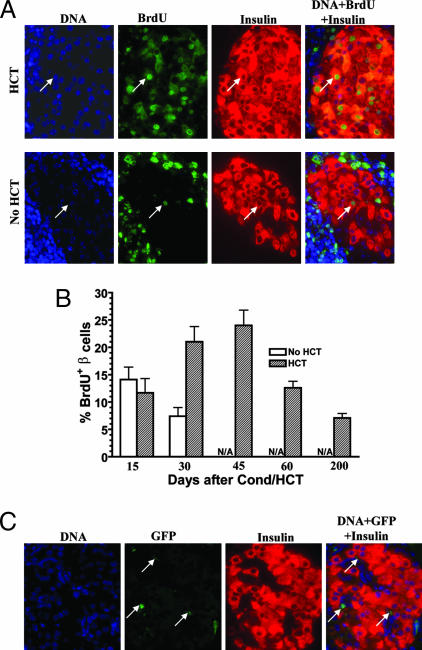
Residual Host β Cell Proliferation in Chimeric New-Onset Diabetic NOD Mice.
Because we observed a significant increase in β cell quantity in the chimeric but not in the nonchimeric new-onset diabetic NOD mice with reversal of hyperglycemia, we tested whether there was a proliferation of insulin-secreting β cells in those recipients, using a 2-week in vivo BrdU labeling assay described by Melton and colleagues (18). Formalin-fixed tissues were stained for DNA, BrdU, and insulin. A cell that was positive for all three stainings was identified as BrdU+insulin+ proliferating β cells. Fig. 5A shows representative patterns of BrdU labeling from chimeric recipients and nonchimeric NOD 30 days after conditioning and/or HCT. We found that, in the chimeric recipients, there were 11.7 ± 2.6% BrdU+insulin+ proliferating β cells among the residual islet β cells 15 days after HCT, and those islets were with insulitis or periinsulitis (Figs. 4B and 5B). Thirty and 45 days after HCT, the percentage of BrdU+insulin+ β cells increased to 21 ± 2.8% and 23.4 ± 2.8%, respectively. This increase was significant when compared with day 15 (P < 0.05). The islets with the most BrdU+insulin+ cells were with no insulitis or only minimum periinsulitis (Figs. 4B and 5A). However, 60 and 200 days after HCT, the percentage of BrdU+insulin+ cells declined to 12.6 ± 1.2% and 7.1 ± 0.8%, respectively, although most islets showed no insulitis. The percentage of proliferating β cells in the long-term chimeric recipients with normal glycemia was similar to that of 20-week-old nondiabetic NOD mice (6 ± 1.4%) and nonautoimmune BALB/c mice (5.2 ± 0.2%). Thus, the decline of proliferating β cells in the chimeric recipients may be due to a feedback of normal glycemia. It has been reported that hyperglycemia triggered β cell regeneration (34).
Fig. 5.
Proliferation of host residual β cells in chimeric NOD recipients with reversal of diabetes. Chimeric new-onset diabetic NOD mice given anti-CD3/CD8 conditioning and HCT, or nonchimeric new-onset diabetic NOD mice given anti-CD3/CD8 conditioning alone, were daily injected i.p. with BrdU (50 μg/g of body weight) for 2 weeks, and the BrdU+insulin+ proliferating β cells were measured longitudinally. (A) Representative patterns of BrdU, DNA, and insulin staining 30 days after conditioning and/or HCT. Arrows point to a representative BrdU+insulin+ β cell. (B) Percentage of BrdU+insulin+ β cells at various time points. Mean ± SE of six mice in each group at each time point are shown. The residual islets in nonchimeric NOD mice on days 45 and 60 after conditioning were too few to be found, and no nonchimeric NOD mice were available on day 200. (C) Representative patterns of DNA, GFP, and insulin staining of islets from chimeric recipients given BM and CD4+ T cell-depleted SPL cells 90–120 days after HCT. Arrows point to GFP+insulin− cells. One of six examined recipients is shown.
In contrast, in the nonchimeric recipients, there were 14.1 ± 2.3% BrdU+insulin+ cells 15 days after anti-CD3/CD8 conditioning, and the percentage of BrdU+insulin+ cells declined to 7.4 ± 1.6%, 30 days after conditioning. This decline was significant as compared with day 15 (P < 0.05). Most of the residual islets in those mice had insulitis, and only a few had periinsulitis, and insulitis was even worse on day 30 than on day 15 after conditioning (Figs. 4B and 5B). Later time points were not measured because there were too few insulin-secreting islets left, and they could not be readily identified. The percentage of BrdU+insulin+ cells in the nonchimeric diabetic NOD mice 30 days after conditioning was at least 3 fold less than that of chimeric recipients 30 days after HCT (P < 0.01) (Fig. 5B). These results indicate that induction of chimerism augments the proliferation of insulin-secreting β cells in new-onset diabetic NOD mice.
Furthermore, we determined whether the proliferating insulin-secreting β cells were of donor- or host-type, using donor BM and CD4− SPL cells from GFP transgenic mice in which all of the islet β cells and hematopoietic cells express GFP. We found that all insulin-secreting β cells in the chimeric recipients 90–120 days after HCT were GFP− (Fig. 5C), indicating that they are host-type but not donor-type cells.
Discussion
Autoimmune insulitis led to destruction of insulin-producing β cells (1, 2). We observed that insulitis in the chimeric NOD recipients with reversal of diabetes was gradually reduced and eventually eliminated after HCT. In contrast, insulitis in the nonchimeric diabetic NOD mice was not reduced, even in those mice with reversal of hyperglycemia. Therefore, induction of chimerism with allogeneic HCT is required for elimination of insulitis in diabetic NOD mice. Induction of chimerism was proposed to eliminate the mature autoreactive T cells in the host peripheral lymphoid tissues via graft versus autoimmunity mediated by donor T cells in the graft, and to eliminate the de novo developed autoreactive T cells by negative selection mediated by donor antigen-presenting cells in the host thymus (21, 22), although this hypothesis needs to be tested. Our preliminary studies showed that induction of mixed chimerism in autoreactive BDC2.5 TCR transgenic NOD mice deleted 99% of the BDC2.5 T cells among host CD4+CD8+ thymocytes, as indicated by I-Ag7/mimotope tetramer that specifically stains the BDC2.5 T cells (C.Z. and D.Z., unpublished data).
We observed that induction of chimerism in new-onset diabetic NOD mice with anti-CD3/CD8 led to reversal of diabetes and a >10-fold increase of β cell quantity, which was due to a vigorous proliferation/regeneration of host islet β cells. This was not demonstrated in previous studies. For example, although multiple injections of low-dose anti-CD3 or in combination with insulin vaccination reversed new-onset diabetes in NOD mice as well as patients (10–15, 30), it was unknown whether anti-CD3 treatment led to regeneration of islet β cells. Although a therapy with combination of ATG and exendin-4 was reported to reverse overt diabetes in NOD mice, the evidence for β cell regeneration was not found (31). Although injection of semiallogeneic donor splenocytes and complete Freund's adjuvant were shown to reverse diabetes via regeneration of β cells from the injected donor SPL cells (35), the results were not confirmed by others, and the source of β cell regeneration was still unclear (36–38). A previous report showed that β cell regeneration in nonautoimmune mice was from self-duplication rather than stem cell differentiation (18). Although the possibility of neogenesis from ductal progenitor cells or transdifferentiation from exocrine cells was not ruled out, the proliferating host β cells in the chimeric new-onset diabetic NOD recipients were most likely from the replication of residual host β cells, because the time period for the regeneration was <30 days, and this regeneration was not observed at the same time period in the late-stage diabetic NOD mice that had too few residual islet β cells.
We also observed that, although chimeric as well as some nonchimeric new-onset diabetic NOD mice given anti-CD3 conditioning only showed reversal of hyperglycemia, the chimeric but not the nonchimeric mice showed an increase in β cell quantity. By comparing the kinetics of insulitis reduction, β cell proliferation, and β cell quantity increase in the chimeric recipients and nonchimeric mice, we noticed that a marked reduction of insulitis after HCT is associated with a vigorous proliferation and a marked increase of β cell quantity. This indicates that elimination of insulitis plays an important role in augmenting β cell regeneration. It was reported that newly formed β cells were more susceptible to cytokine-induced cell death (17, 39). Therefore, we speculate that anti-CD3 conditioning alone may result in control of insulitis via induction of Treg cells in some diabetic mice as described previously (10). This prevents further damage of the existing β cells and even improves their function, but this does not allow the survival of newly generated β cells because of the effect of the inflammatory cytokines from the existing insulitis. Therefore, some diabetic NOD mice given anti-CD3 conditioning alone showed reversal of hyperglycemia, but no β cell regeneration. On the other hand, elimination of insulitis in chimeric recipients allows the survival of newly generated β cells and leads to an increase of islet β cell quantity. Because BM-derived stem cells were shown to initiate pancreatic regeneration (32), donor BM cells in the chimeric recipients may also directly augment the proliferation and survival of islet β cells.
The anti-CD3/CD8 conditioning regimen is a radiation-free conditioning regimen that induces chimerism without causing GVHD in diabetic NOD mice. Although administration of donor BM and costimulatory blockade were reported to induce chimerism in nonirradiated mice (40, 41), it did not work in nonirradiated autoimmune NOD mice because of a generalized tolerance defect in the mice (42). Our recent studies showed that the mechanisms of GVHD prevention in anti-CD3-conditioned recipients include confinement of donor CD8+ T cells to host lymphohematological tissues as well as tolerization of the residual donor CD8+ T cells (43).
This radiation-free GVHD preventive anti-CD3/CD8 conditioning regimen not only allows induction of chimerism and reversal of diabetes in new-onset diabetic NOD mice but also allows induction of chimerism and immune tolerance to donor islets in late-stage diabetic NOD mice [supporting information (SI) Fig. 6]. Therefore, induction of chimerism via anti-CD3/CD8 conditioning may be a potential curative therapy for type 1 diabetes.
Materials and Methods
Mice.
Female NOD/LtJ (H-2g7), FVB/N (H-2q), and EGFP-transgenic FVB/N(H-2q) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained in a pathogen-free room at City of Hope Research Animal Facilities (Duarte, CA).
Flow-Cytometric Analysis and Cell Sorting.
The following anti-mouse mAbs were purchased from BD Biosciences PharMingen (San Diego, CA) and eBioscience (San Diego, CA): CD3ε (145-2C11), TCRβ (H57-597), CD4 (RM4-5), CD8α (53-6.7), B220 (RA3-6B2), CD11b/Mac-1 (M1/70), and Gr-1(RB6–8C5). FACS was performed with a four-laser MOFLO immunocytometry system (Dako Cytomation, Fort Collins, CO), and data were analyzed with FLOWJO software (Tree Star, San Carlos, CA) as described previously (27).
Anti-CD3 and Anti-CD8 mAb Treatment and HCT.
Anti-CD3 (145-2C11) and anti-CD8 (116-13.1) antibodies were produced as described previously (27). Diabetic NOD/LtJ mice were implanted with insulin pellets following the vendor's instruction (Linshin, Toronto, ON, Canada), then injected i.v. with anti-CD3 and anti-CD8 mAb at a dose of 20 μg/g of body weight each. Five days after antibody injection, mice were injected with donor BM (100 × 106) in combination with donor CD4+ T cell-depleted SPL cells (100 × 106). The donor CD4+ T cell-depleted SPL cells were injected again 5 days after injection. Thereafter, the recipients were monitored for clinical signs of GVHD and blood glucose levels as described previously (27).
i.p. Glucose Tolerance Test.
After overnight fasting, NOD mice were injected i.p. with glucose (2 mg/g of body weight). Blood samples were collected from tail vein into heparinized capillary tubes. Blood glucose levels were determined with a glucometer. Plasma insulin levels were measured by using a commercial mouse insulin ELISA kit (Mercodia, Winston Salem, NC).
Histopathology of Skin, Small Intestine, and Pancreatic Islets.
Histopathologic specimens from skin, small intestine, and pancreata of mice were fixed in formalin before embedding in paraffin blocks. Tissue sections were stained with hematoxylin and eosin, and insulin staining was done by using Tech-mate 1000 autostainer (Ventana, Tucson, AZ) as described previously (27).
Measurement of β Cell Surface and Scoring of Insulitis.
Formalin-fixed pancreatic tissues were embedded in paraffin. Five sections of 5-μm thickness were cut with a distance of 75 μm between each section. All five sections were immunostained for insulin and hematoxylin and were visualized with an Olympus IX50 fluorescent microscope and equipped with an Olympus DP7e CCD camera (Olympus America, Melville, NY). The images were analyzed by using Olympus Microsuite B35V image analysis software in the Measure-Area mode. The ratio of total insulin-stained areas versus total hematoxylin-stained areas of all five sections was calculated and compared.
Insulin-secreting islets were scored as follows: 1, no insulitis (free of infiltration); 2, periinsulitis (a few to many inflammatory cells outside or in the immediate vicinity of the islets); 3, insulitis (a clear and often extensive islet infiltrate that shows direct lymphocyte–β cell contact). The percentage of no insulitis, periinsulitis, and insulitis among total islets in each mouse was calculated.
BrdU Labeling of Proliferating β Cells.
Mice were injected i.p. daily with BrdU (Sigma, St. Louis, MO) in PBS at a dose of 50 μg/g of body weight for 2 weeks as described previously (18, 32). Formalin-fixed pancreatic tissues were embedded in paraffin. Five sections of 5-μm thickness were cut with a distance of 75 μm between each section. Sections were stained with mouse anti-BrdU mAb (Roche, Indianapolis, IN) and guinea pig anti-insulin (Linco Research, St. Charles, MO). The secondary antibodies were FITC-conjugated anti-mouse-IgG and Texas red-conjugated anti-guinea pig IgG (Jackson ImmunoResearch, West Grove, PA). Nuclei were stained with DAPI (Sigma). All insulin+ or insulin+BrdU+ cells in the section were counted. Photos were taken by using an Olympus BX51 fluorescent microscope equipped with a Pixera cooled CCD camera.
Statistical Analysis.
Statistical significance was evaluated by using the log-rank test with Prism version 3.0 (GraphPad, San Diego, CA) and an unpaired two-tailed Student's t test.
Supplementary Material
Acknowledgments
We thank Stephen Scott in our department, Lucy Brown at the City of Hope Flow Cytometry Facility, Sofia Loera at the City of Hope Anatomic Pathology Laboratory, and Clive Wasserfall of the University of Florida for their excellent technical assistance. We also thank Dr. Richard Ermel and his staff at the City of Hope Research Animal Facility for providing excellent animal care. These studies were supported by Juvenile Diabetes Foundation and National Institutes of Health Grant R21 DK71002 (to D.Z.).
Abbreviations
- NOD
nonobese diabetic
- BM
bone marrow
- HCT
hematopoietic cell transplantation
- Treg
regulatory T
- GVHD
graft-versus-host disease
- SPL
spleen.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611101104/DC1.
References
- 1.Castano L, Eisenbarth GS. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 2.Rossini AA. Diabetes. 2004;53:267–275. doi: 10.2337/diabetes.53.2.267. [DOI] [PubMed] [Google Scholar]
- 3.Roep BO, Atkinson M, von Herrath M. Nat Rev Immunol. 2004;4:989–997. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 4.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Pugliese A, Miceli D. Diabetes Metab Res Rev. 2002;18:13–25. doi: 10.1002/dmrr.261. [DOI] [PubMed] [Google Scholar]
- 6.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 7.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. J Exp Med. 1999;190:963–972. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson MA. Diabetes. 2005;54:1253–1263. doi: 10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- 9.Chatenoud L, Thervet E, Primo J, Bach JF. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 11.Chatenoud L, Primo J, Bach JF. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 12.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 13.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 14.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. J Clin Invest. 2003;111:409–418. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson MA, Rhodes CJ. Diabetologia. 2005;48:2200–2202. doi: 10.1007/s00125-005-1957-2. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Brown J, Martinez OI, Melton DA. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 19.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 20.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 21.Sykes M, Nikolic B. Nature. 2005;435:620–627. doi: 10.1038/nature03728. [DOI] [PubMed] [Google Scholar]
- 22.Shizuru J. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 324–343. [Google Scholar]
- 23.Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Diabetes. 2004;53:376–383. doi: 10.2337/diabetes.53.2.376. [DOI] [PubMed] [Google Scholar]
- 24.Beilhack GF, Scheffold YC, Weissman IL, Taylor C, Jerabek L, Burge MJ, Masek MA, Shizuru JA. Diabetes. 2003;52:59–68. doi: 10.2337/diabetes.52.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA, Greiner DL. Blood. 2000;95:2175–2182. [PubMed] [Google Scholar]
- 26.Li H, Kaufman CL, Boggs SS, Johnson PC, Patrene KD, Ildstad ST. J Immunol. 1996;156:380–388. [PubMed] [Google Scholar]
- 27.Liang Y, Huang T, Zhang C, Todorov I, Atkinson M, Kandeel F, Forman S, Zeng D. Blood. 2005;105:2180–2188. doi: 10.1182/blood-2004-06-2411. [DOI] [PubMed] [Google Scholar]
- 28.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 29.Ildstad ST, Chilton PM, Xu H, Domenick MA, Ray MB. Blood. 2005;105:2577–2584. doi: 10.1182/blood-2004-04-1340. [DOI] [PubMed] [Google Scholar]
- 30.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa N, List JF, Habener JF, Maki T. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 32.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 33.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 34.Brockenbrough JS, Weir GC, Bonner-Weir S. Diabetes. 1988;37:232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- 35.Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 36.Nishio J, Gaglia JL, Turvey SE, Campbell C, Benoist C, Mathis D. Science. 2006;311:1775–1778. doi: 10.1126/science.1124004. [DOI] [PubMed] [Google Scholar]
- 37.Chong AS, Shen J, Tao J, Yin D, Kuznetsov A, Hara M, Philipson LH. Science. 2006;311:1774–1775. doi: 10.1126/science.1123510. [DOI] [PubMed] [Google Scholar]
- 38.Suri A, Calderon B, Esparza TJ, Frederick K, Bittner P, Unanue ER. Science. 2006;311:1778–1780. doi: 10.1126/science.1123500. [DOI] [PubMed] [Google Scholar]
- 39.Meier JJ, Ritzel RA, Maedler K, Gurlo T, Butler PC. Diabetologia. 2006;49:83–89. doi: 10.1007/s00125-005-0069-3. [DOI] [PubMed] [Google Scholar]
- 40.Seung E, Mordes JP, Rossini AA, Greiner DL. J Clin Invest. 2003;112:795–808. doi: 10.1172/JCI18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 42.Markees TG, Serreze DV, Phillips NE, Sorli CH, Gordon EJ, Shultz LD, Noelle RJ, Woda BA, Greiner DL, Mordes JP, Rossini AA. Diabetes. 1999;48:967–974. doi: 10.2337/diabetes.48.5.967. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Lou J, Li N, Todorov I, Lin C, Cao Y, Contag C, Kandeel F, Forman S, Zeng D. J Immunol. 2007;178:838–850. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.