Abstract
In leguminous plants such as pea (Pisum sativum), alfalfa (Medicago sativa), barrel medic (Medicago truncatula), and chickpea (Cicer arietinum), 4′-O-methylation of isoflavonoid natural products occurs early in the biosynthesis of defense chemicals known as phytoalexins. However, among these four species, only pea catalyzes 3-O-methylation that converts the pterocarpanoid isoflavonoid 6a-hydroxymaackiain to pisatin. In pea, pisatin is important for chemical resistance to the pathogenic fungus Nectria hematococca. While barrel medic does not biosynthesize 6a-hydroxymaackiain, when cell suspension cultures are fed 6a-hydroxymaackiain, they accumulate pisatin. In vitro, hydroxyisoflavanone 4′-O-methyltransferase (HI4′OMT) from barrel medic exhibits nearly identical steady state kinetic parameters for the 4′-O-methylation of the isoflavonoid intermediate 2,7,4′-trihydroxyisoflavanone and for the 3-O-methylation of the 6a-hydroxymaackiain isoflavonoid-derived pterocarpanoid intermediate found in pea. Protein x-ray crystal structures of HI4′OMT substrate complexes revealed identically bound conformations for the 2S,3R-stereoisomer of 2,7,4′-trihydroxyisoflavanone and the 6aR,11aR-stereoisomer of 6a-hydroxymaackiain. These results suggest how similar conformations intrinsic to seemingly distinct chemical substrates allowed leguminous plants to use homologous enzymes for two different biosynthetic reactions. The three-dimensional similarity of natural small molecules represents one explanation for how plants may rapidly recruit enzymes for new biosynthetic reactions in response to changing physiological and ecological pressures.
INTRODUCTION
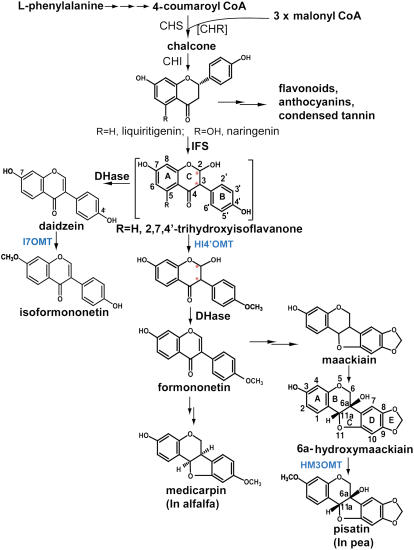
In sessile organisms such as plants, the need to rapidly evolve chemical defenses to changing environments is often met by the recruitment of enzymes into new metabolic pathways, resulting in the amazing diversity of plant secondary metabolism observed in nature (Pichersky and Gang, 2000). The isoflavonoid natural products of the Leguminosae act as antimicrobial phytoalexins during plant-fungal pathogen defense responses and as signaling molecules mediating bacterial or fungal symbioses (Dixon, 1999). Their biosynthesis diverges from the ubiquitous plant flavonoid pathway after the formation of committed flavanone intermediates, namely liquiritigenin or naringenin (Figure 1). The oxidative aryl ring migration and coupled introduction of a 2-OH moiety catalyzed by the cytochrome P450 isoflavone synthase (IFS) (Hashim et al., 1990) together with the subsequent 4′-O-methylation of the IFS product, 2-hydroxyisoflavanone, comprise critical catalytic steps at the entry point into the formation of a structurally and functionally diverse group of isoflavonoid phytoalexins, including isoflavans, coumestans, and pterocarpans (Wong, 1975; Dewick, 1993; Dixon, 1999; Aoki et al., 2000).
Figure 1.
Biosynthesis of 6aR,11aR-Medicarpin and 6aR,11aR-Pisatin.
CHS, chalcone synthase; CHR, chalcone reductase; CHI, chalcone isomerase; HI4′OMT, 2-hydroxyisoflavanone 4′-O-methyltransferase; HM3OMT, 6a-hydroxymaackiain 3-O-methyltransferase; I7OMT, isoflavone 7-O-methyltransferase; DHase, isoflavanone dehydratase. The double arrows indicate multiple steps in the biosynthetic pathway. The brackets around CHR signify that chalcone reductase is only necessary for forming liquiritigenin. Red asterisks indicate the stereogenic carbons in the hydroxyisoflavanone substrate of HI4′OMT and its resultant product.
Methylation of the C4′ hydroxyl group of 2-hydroxyisoflavanone by a 4′-specific O-methyltransferase, HI4′OMT, recently identified in licorice (Glycyrrhiza echinata) (Akashi et al., 2003), yields 2-hydroxy-4′-O-methoxy-isoflavanone that undergoes dehydration to yield formononetin (7-hydroxy-4′-methoxyisoflavone) (Akashi et al., 2000; Aoki et al., 2000; Liu and Dixon, 2001; Akashi et al., 2005) (Figure 1). HI4′OMT is homologous to the previously characterized 6a-hydroxymaackiain 3-O-methyltransferase (HM3OMT) from pea (Pisum sativum; Preisig et al., 1989). Pea HM3OMT catalyzes the final O-methylation step in pea phytoalexin biosynthesis, converting the pterocarpan 6a-hydroxymaackiain into the antimicrobial end product pisatin (Preisig et al., 1989, 1991; Wu et al., 1997). This reaction is critical for resistance of pea against the fungal pathogen Nectria hematococca, virulent isolates of which possess a specific detoxifying cytochrome P450 enzyme, pisatin demethylase, located on a dispensable chromosome (VanEtten et al., 1980; Ciuffetti and VanEtten, 1996; Wasmann and VanEtten, 1996).
Among several legumes, including pea, alfalfa (Medicago sativa), barrel medic (Medicago truncatula), and chickpea (Cicer arietinum), only pea species accumulate naturally the 3-O-methylated pterocarpanoid pisatin. However, feeding the pisatin precursor 6a-hydroxymaackiain to barrel medic suspension culture results in the formation of the 3-O-methylated pea product pisatin (see below). The high level of amino acid sequence similarity of barrel medic HI4′OMT with pea HM3OMT suggests that the two enzymes may possess dual activity for either 3- or 4′-O-methylation depending upon substrate availability. Therefore, HI4′OMTs represent fascinating examples of likely serendipitous functional redundancy in phylogenetically related plants that hold special significance for the evolution of defense responses in the Leguminosae. Here, we report the biochemical characterization of HI4′OMT from M. truncatula and the molecular basis of the dual functionality of HI4′OMT by determining the steady state kinetic parameters of HI4′OMT for each substrate and by elucidating the structures of HI4′OMT at high resolution with each substrate bound.
RESULTS
3-O-Methylation of Pterocarpans and Functional Identification of M. truncatula HI4′OMT/HM3OMT
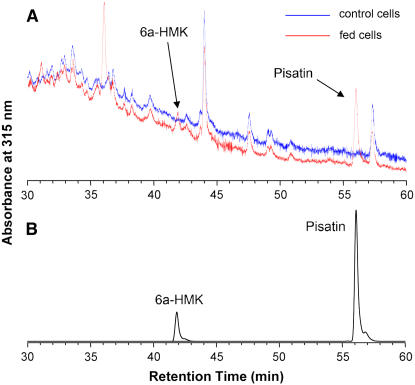
Like the forage legume alfalfa, M. truncatula (barrel medic) primarily accumulates the pterocarpanoid phytoalexin medicarpin (Figure 1). To test whether barrel medic also possesses the biosynthetic activity capable of 3-O-methylation of pterocarpans, such as 6a-hydroxymaackiain, leading to pisatin as found in pea, M. truncatula cell suspension cultures were fed 20 μM 6a-hydroxymaackiain for 36 h. Chromatographic analysis of phenolic extracts from the treated and control cells revealed the presence of a metabolite with the identical UV spectrum and retention time as an authentic pisatin standard (Figure 2). This feeding study provides analytical evidence that barrel medic cells contain an enzyme or enzymes capable of regiospecifically methylating a compound they do not make, namely 6a-hydroxymaackiain, resulting in the accumulation of a pea-specific phytoalexin, namely pisatin. In addition to detecting pisatin, an additional product identical in UV spectrum to the 6a-hydroxymaackiain substrate but differing in elution time was also detected in cell extracts. This compound is most likely the glycosylated conjugate of the fed 6a-hydroxymaackiain precursor, and experiments to ascertain this are under way.
Figure 2.
Biotransformation of Exogenously Fed 6a-Hydroxymaackiain into Pisatin Using M. truncatula Cell Suspension Cultures.
(A) Portion of the HPLC-UV profile of the phenolic extracts from 6a-hydroxymaackiain (6a-HMK) fed cells (red line) and control cells (blue line). One of the new peaks appearing at ∼36 min is presumably resulting from sugar conjugation of 6a-HMK.
(B) Resolution of authentic standards of 6a-hydroxymaackiain and pisatin as in (A).
To identify putative O-methyltransferases responsible for this unexpected activity, the pea HM3OMT sequence (GenBank accession number U69554) was used for BLAST searching of M. truncatula EST libraries. This bioinformatic analysis led to the identification of a full-length cDNA clone spanning 1.478 kb (Mt HI4′OMT; GenBank accession number AY942158), with 90% amino acid sequence identity to pea HM3OMT, 87% sequence identity to licorice HI4′OMT (accession number AB091684), and 51% sequence identity to alfalfa isoflavone 7-OMT (accession number U97125). Not unexpected given the high degree of sequence identity, phylogenetic analysis indicated that the HI4′ OMTs from different legumes and the pea HM3OMT all clustered together and that this cluster was distinct from several other functionally characterized O-methyltransferases belonging to the same type I plant OMT family (Zubieta et al., 2001; Noel et al., 2003) (Figure 3).
Figure 3.
Sequence Alignment and Phylogenetic Analysis of Phenylpropanoid Metabolizing OMTs.
(A) Sequence alignment of HI4′OMT from M. truncatula, Ps HM3OMT from P. sativum, Ge HI4′OMT from Glycyrrhiza echinata, and Ms I7OMT from M. sativa. Active site residues of HI4′OMT are in red boxes. The experimentally determined secondary structure of HI4′OMT is depicted at the top of the alignment. α-Helices are depicted as gold cylinders and β-strands as blue arrows.
(B) Unrooted neighbor-joining phylogenetic tree constructed with HI4′OMTs described above, the putative Lj HI4′OMT from Lotus japonicus (accession number BAC58013), Ms COMT from M. sativa (alfalfa caffeic acid 3-O-methyltransferase, accession number P28002), Ms CCOMT (alfalfa caffeoyl-CoA O-methyltransferase; accession number T09399), and Ms ChOMT (alfalfa chalcone 2′-O-methyltransferase; accession number AAB48058).
The protein encoded by the Mt HI4′OMT gene was expressed in Escherichia coli and purified with a hexa-His N-terminal tag. To obtain the hydroxyisoflavanone substrate that is not commercially available and is chemically unstable, 2,7,4′-trihydroxyisoflavanone was prepared biosynthetically using recombinant M. truncatula IFS incubated with a racemic mixture of 2R/2S-liquiritigenin (7,4′-dihydroxyflavanone) substrates (Figure 1). In this reaction, only the 2S-isomer served as an IFS substrate (data not shown), consistent with previous reports of IFS substrate specificity (Hagmann and Grisebach, 1984; Kochs and Grisebach, 1986). However, the 2,7,4′-trihydroxyisoflavanone product resulting from this IFS-catalyzed in vitro reaction existed as a pair of stereoisomers based upon rapid purification using chiral HPLC (data not shown). Given the instability of these hydroxyisoflavanone products, it was impossible to establish the stereochemistry of this mixture. This biosynthetically derived mixture of minimally two stereoisomers of 2,7,4′-trihydroxyisoflavanone was then used for kinetic analyses and also soaked into preexisting crystals of HI4′OMT complexed to the demethylated product of S-adenosyl-l-Met (SAM), S-adenosyl-l-homo-Cys (SAH) (see next section).
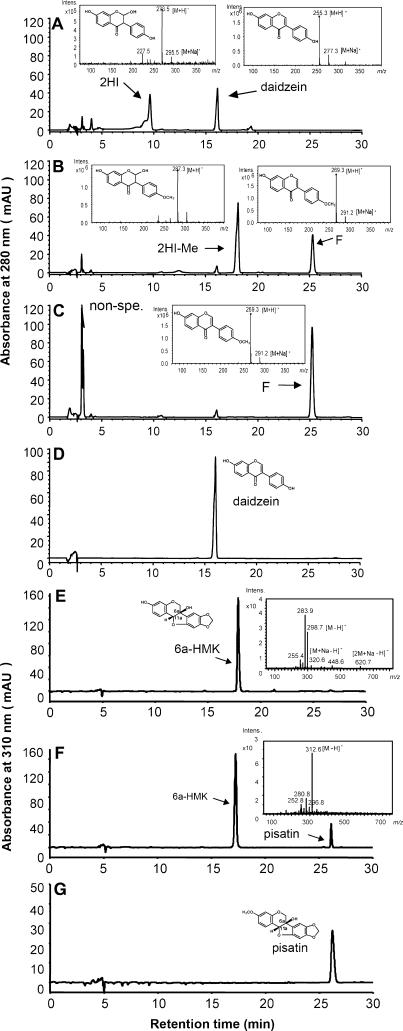
Incubation of the recovered recombinant protein with 2,7,4′-trihydroxyisoflavanone obtained from the in vitro reaction of M. truncatula IFS with 7,4′-dihydroxyflavanone and the methyl donor SAM resulted in the accumulation of a methylated intermediate that was readily dehydrated to formononetin, indicative of 4′-OMT activity (Figures 4A to 4C). Incubation of the recombinant protein with the pea compound 6a-hydroxymaackiain and SAM resulted in production of pisatin (Figures 4F and 4G). HI4′OMT was not active with di- or trihydroxylated isoflavones, including daidzein (Figure 4D) and genistein, or with the pterocarpans (–)-medicarpin and maackiain (data not shown).
Figure 4.
HPLC-UV Electrospray Ionization–Mass Spectrometry Profiles of Products Formed by Incubation of HI4′OMT with 2,7,4′-Trihydroxyisoflavanone and 6a-Hydroxymaackiain.
(A) Control reaction of inactive enzyme, obtained by incubation in a boiling water bath for several minutes, with 2,7,4′-trihydroxyisoflavanone (2HI). Portions of the remaining substrate underwent spontaneous dehydration to daidzein. The early unlabeled peaks arise from the solvents used to dissolve the compounds and residual contaminants from the in vitro reactions.
(B) Products from the incubation of HI4′OMT with 2,7,4′-trihydroxyisoflavanone. The major product is 2,7-dihydroxy-4′-methoxyisoflavanone (2HI-Me), portions of which spontaneously dehydrate to formononetin (F). The rapid conversion of 2,7,4′-trihydroxyisoflavanone into 4′-methylated products by enzyme prevented spontaneous dehydration to daidzein. The early unlabeled peaks arise from the solvents used to dissolve the compounds and residual contaminants from the in vitro reactions.
(C) Dehydrated product from the reaction shown in (B) after the addition of HCl. 2,7-Dihydroxy-4′-methoxyisoflavanone was completely converted into the isoflavone formononetin. The large unknown peak(s) (non-spe.) recorded in the early stages of chromatographic separation are commonly seen when extracting scaled-up reactions using ethyl acetate with residual contamination by the water phase containing UV absorbing material.
(D) Reaction of HI4′OMT with daidzein (7,4′-dihydroxyisoflavone). Absorbance measured at 280 nM.
(E) Control reaction of inactive (boiled) enzyme with 6a-hydroxymaackiain (6a-HMK).
(F) Product from the incubation of HI4′OMT with 6a-hydroxymaackiain.
(G) Pisatin authentic standard.
The insets show total ion mass spectra of the corresponding compounds recorded using either positive ([A] to [C]) or negative mode ([E] and [F]). In the spectra of (E), 298.7 mass-to-charge ratio (m/z) is the corresponding mass of 6a-hydroxymaackiain; 283.9 m/z is a fragment of 298.7. There is a 298.7 plus Na [M+Na-H]- and [2 M+Na-H]- in the larger mass range, confirming that 298.7 is the true mass.
The steady state kinetics of recombinantly expressed barrel medic HI4′OMT catalyzed methylation of 2,7,4′-trihydroxyisoflavanone and 6a-hydroxymaackiain were measured using various concentrations of substrates (the maximum concentration of 2,7,4′-trihydroxyisoflavanone or 6a-hydroxymaackiain achievable, ∼250 μM, was limited by their low solubility in the 100% methanol used for preparation of the stock solution and the final reaction buffer used for kinetic analysis) at a fixed concentration of SAM of 312.5 μM in the presence of AdoHcy nucleosidase to eliminate product inhibition by SAH (Hendricks et al., 2004). Methanol concentrations were held constant at ∼5% derived from the stock solution of substrate and additional methanol to compensate at low substrate concentrations. HI4′OMT exhibited an apparent Vmax= 35.9 ± 4.0 nmol·mg protein−1·min−1 and an apparent Km = 73.3 ± 20.2 μM for 2,7,4′-trihydroxyisoflavanone and an apparent Vmax= 17.9 ± 2.8 nmol·mg−1·min−1 and an apparent Km = 62.1 ± 25 μM for 6a-hydroxymaackiain. Apparent values were obtained because of the difficulties involved in obtaining saturating conditions for substrates. The kcat/Km (specificity constant) for 2,7,4′-trihydroxyisoflavanone is 334 ± 99.0 M−1·s−1, and for the pea compound, 6a-hydroxymaackiain, kcat/Km is 197 ± 85.0 M−1·s−1. Although the catalytic efficiency is slightly higher for 2,7,4′-trihydroxyisoflavanone (although arguably within experimental error of the measurements and calculations), HI4′OMT does exhibit nearly identical apparent affinity for both isoflavonoid compounds regardless of biological source. Finally, the steady state kinetic constants of HI4′OMT for the SAM cosubstrate (methyl donor) were measured at a fixed concentration of 2,7,4′-trihydroxyisoflavanone of 200 μM (near its solubility limit). HI4′OMT displays an apparent Vmax= 23.9 ± 3.0 nmol·mg−1·min−1 and apparent Km = 99.8 ± 32.4 μM. This latter value is within the range of previously reported estimates for isoflavonoid-specific OMTs (Wengenmayer et al., 1974; Preisig et al., 1989). While unlikely given the close correspondence of these values to previously reported values, it is possible that the apparent Vmax and Km could differ when using saturating concentrations of 6a-hydroxymaackiain.
Structural Determination by Protein X-Ray Crystallography
Recombinantly expressed and purified barrel medic HI4′OMT/HM3OMT crystallized from a polyethylene glycol precipitant containing 2.5 mM of the reaction product SAH. An initial x-ray crystal structure of the HI4′OMT/HM3OMT-SAH complex was solved by multiple-wavelength anomalous dispersion (MAD) phasing using seleno-Met (SeMet)–substituted protein and refined to 2.5-Å resolution. Subsequently, several HI4′OMT/HM3OMT-SAH-phenolic substrate complexes were generated by soaking HI4′OMT/HM3OMT-SAH crystals with the phenolic substrates 2,7,4′-trihydroxyisoflavanone, pea 6a-hydroxymaackiain, or the methylated pea product pisatin and the three-dimensional structures elucidated by molecular replacement (Table 1, Figure 5).
Table 1.
Crystallographic Data for HI4′OMT/HM3OMT
| λ1 | λ2 | λ3 | Trihydroxy-Isoflavanone | Hydroxy-Maackiain | Pisatin | |
|---|---|---|---|---|---|---|
| Data collection | ||||||
| Space group | P4(3)22 | P4(3)22 | P4(3)22 | P4(3)22 | ||
| Cell dimensions | ||||||
| a, b, c (A°) | 71.36 | 72.11 | 71.22 | 71.79 | ||
| 71.36 | 72.11 | 71.22 | 71.79 | |||
| 188.8 | 188.4 | 188.91 | 188.7 | |||
| α = β = γ (°) | 90 | 90 | 90 | 90 | ||
| Wavelength (Å) | 0.97932 | 0.97942 | 0.9855 | 0.97946 | 0.97897 | 0.97946 |
| Resolution range (Å) | 80–2.5 | 80–2.5 | 80–2.9 | 50–2.35 | 50–2.35 | 50–2.5 |
| Observations | 275,060 | 274,947 | 175,106 | 184,772 | 178,153 | 164,933 |
| Unique reflection | 32,213 | 32,187 | 20,705 | 20,221 | 21,559 | 17,937 |
| Completeness (%)a | 99.6 (99.4) | 99.6 (99.4) | 99.6 (99.6) | 93.3 (99.9) | 99.9 (100) | 99.9 (99.9) |
| I/σ | 7.46 (1.35) | 6.65 (1.16) | 4.13 (1.0) | 34.0 (7.5) | 23.5 (5.0) | 23.1 (6.2) |
| Rsym (%)ab | 12.0 (47.0) | 13.5 (52.2) | 19.9 (63.5) | 12.1 (58.2) | 11.7 (57.8) | 13.8 (55.3) |
| Number of Se sites | 5 | 5 | 5 | |||
| FOMc | 0.25 | |||||
| Refinement | ||||||
| Rcryst/Rfree (%)d | 20.4/23.7 | 21.8/26.7 | 20.7/24.5 | 23.4/27.8 | ||
| No. of atoms | ||||||
| Protein | 2,819 | 2,822 | 2,819 | 2,795 | ||
| Water molecules | 87 | 112 | 137 | 23 | ||
| SAH | 26 | 26 | 26 | 26 | ||
| Phenolic substrate | 20 | 22 | 23 | |||
| RMSD | ||||||
| Bonds (Å) | 0.016 | 0.007 | 0.006 | 0.009 | ||
| Angles (°) | 1.7 | 1.2 | 1.1 | 1.5 | ||
| Average B factors | ||||||
| Protein (Å)b | 28.4 | 34 | 35.3 | 26.7 | ||
| Water (Å)b | 24.1 | 21.9 | 31.0 | 18.6 | ||
| SAH (Å)b | 35.7 | 58.3 | 93.0 | 34.7 | ||
| Phenolic substrate (Å)b | 22.4 | 75.2 | 66.7 |
Number in parentheses refers to the highest shell.
Rsym = |Ih − <Ih>|/Ih, where <Ih> is the average intensity over symmetry equivalent reflections.
FOM, figure of merit.
Rcryst = Σ|Fobs − Fcalc|/ ΣFobs, where summation is over the data used for refinement, and Rfree was calculated using 5% of data excluded from refinement.
Figure 5.
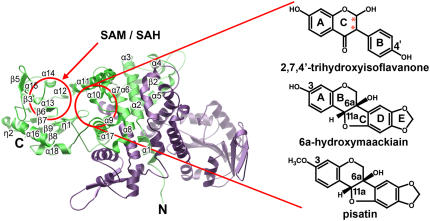
Ribbon Diagram of the HI4′OMT Homodimeric Quaternary Structure.
Monomer A is green, and monomer B is purple. Red circles indicate the corresponding SAM/SAH binding and phenolic substrate binding pockets. Some of the secondary structure elements shown in Figure 3 are labeled. For clarity, β1 and β4 are not shown, as they are obscured. The isoflavonoids complexed to HI4′OMT determined crystallographically in this study are indicated.
Previously, several crystal structures of phenylpropanoid and isoflavonoid-specific plant O-methyltransferases were determined with substrates and products bound. These catalytically and structurally similar enzymes were classified as plant type I OMTs (Noel et al., 2003), of which HI4′OMT and HM3OMT also belong. As originally noted in these previously published OMT crystal structures (Zubieta et al., 2001, 2002), HI4′OMT/HM3OMT also forms a well packed and substantially identical crystallographic dimer (Figure 5) that likely reflects the quaternary structure of the enzyme in solution and in cells. Each monomer consists of an N-terminal domain spanning 141 residues that primarily mediates dimerization but which partially constitutes the back wall of the active site cavity of the dyad-related neighboring polypeptide chain used for phenolic substrate recognition. A large C-terminal domain spanning 223 residues forms the core SAM/SAH binding module (Rossmann fold) that is shared with the type I, type II, and type III families of plant SAM-dependent small molecule methyltransferases (Noel et al., 2003) (Figure 5).
Structural Comparisons of HI4′OMT/HM3OMT with Other Plant Type I OMTs
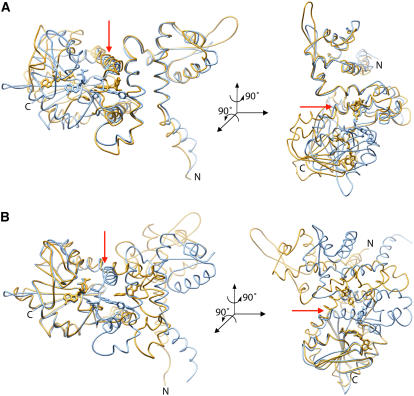
We then compared the three-dimensional structure of HI4′OMT to the previously solved structures of another type I plant small molecule methyltransferase, namely, alfalfa I7OMT (Zubieta et al., 2001; Noel et al., 2003), the enzyme responsible for 7-O-methylation of isoflavones in vitro (He and Dixon, 1996; He et al., 1998). This architectural comparison revealed large en-block conformational differences of the N-terminal domains relative to the C-terminal SAM/SAH binding domains in these otherwise highly similar (primary sequence identity 51%) plant small molecule OMTs. The global alignment of each structure displayed remarkably different overall topologies for both proteins with an overall root mean square deviation (RMSD) of 14.9 Å for equivalent Cα atoms of aligned amino acid residues. However, by individually aligning either the N-terminal domains or the portion of the C-terminal domains comprising the SAM/SAH binding pocket, the secondary structure and relationship of the secondary structure elements of each domain with each other are virtually identical (Figures 6A and 6B), suggesting that there is a global repositioning of the N-terminal and C-terminal domains with respect to each other in HI4′OMT compared with I7OMT.
Figure 6.
Backbone Architecture of HI4′OMT.
(A) Cα alignments of the N-terminal portions of HI4′OMT (gold) and Ms I7OMT (blue) (residues 15 to 87 of both enzymes) result in an RMSD of 1.02 Å. Orientation of the right panel is achieved after a 90° rotation around an axis perpendicular to the plane of the page and a second 90° rotation around the vertical axis shown in the left panel as a red arrow.
(B) Cα alignments of the C-terminal portions (residues 330 to 364 of HI4′OMT and residues 318 to 352 of Ms I7OMT) result in an RMSD of 0.782 Å. Orientation of the right panel is achieved after a 90° rotation around an axis perpendicular to the plane of the page and a second 90° rotation around the vertical axis shown in the left panel as a red arrow.
SAH/SAM Cosubstrate Binding Pockets
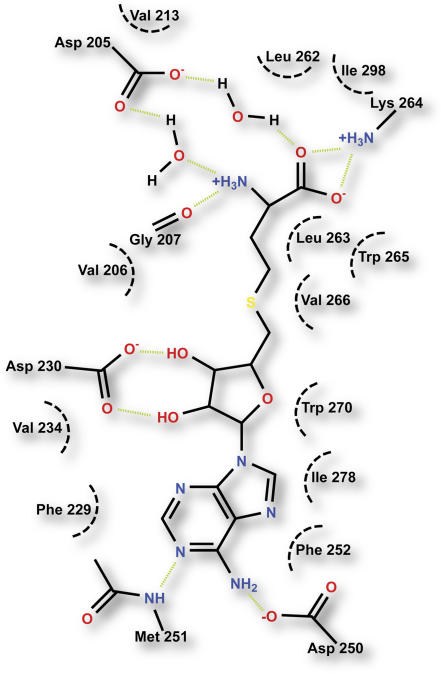
In the HI4′OMT/HM3OMT structures presented here, helix α14, β-strands β3, β4, and β6 and the connecting loops form a cleft where the demethylated product SAH resides (Figure 7). One wall of the SAH/SAM binding cleft is lined by the side chains of Asp-250, Met-251, Phe-252, Leu-263, Trp-265, Val-266, and Trp-270 and Ile-278, while the opposing wall is lined by the side chains of Val-206, Val-213, Phe-229, Val-234, and the apolar portions of Asp-230 and Gln-231 (data not shown). Together, both walls of the SAH/SAM binding site form a greasy crevice facilitating van der Waals interactions with SAH/SAM. The carboxyl moiety of Asp-230 forms hydrogen bonds with the 2′ and 3′ Rib hydroxyl groups of SAH, while the carboxyl group of Asp-250, the side chain amino moiety of Lys-264, and the peptide bond carbonyl oxygen of Gly-207 form a hydrogen bond network with the exocyclic amino group of the adenine ring and the free carboxyl and amino moieties of SAH, respectively. Finally, the carboxyl group of Asp-205 tethers the amino group and carboxyl moieties of SAH through water-mediated hydrogen bonds (Figure 7).
Figure 7.
Schematic Diagram of the SAM/SAH Binding Site of HI4′OMT.
Green dashed lines represent putative hydrogen bonds. Black dashed curves represent the hydrophobic portions of the side chains of residues contacting substrate/product. For clarity, Gln-231 mentioned in the text is not shown.
Stereoselective Binding Mode of HI4′OMT for Phenolic Substrates
In contrast with the previous prediction of 2R,3S configurations at C2 and C3 of the IFS product 2,7,4′-trihydroxyisoflavanone (Hashim et al., 1990; Sawada et al., 2002; Akashi et al., 2003), x-ray crystallographic analysis revealed that 2,7,4′-trihydroxyisoflavanone obtained from in vitro IFS reactions, HPLC purified under nonchiral resolving conditions, and then soaked into HI4′OMT crystals possesses the 2S,3R stereochemical configuration (Figure 8A). Moreover, in the HI4′OMT structures solved with either pea 6a-hydroxymaackiain or pea pisatin bound, the conformations of the 6aR,11aR-stereoisomers of these pea compounds observed in the phenolic binding pocket of the barrel medic HI4′OMT (Figure 8B) are identical to the conformation of 2S,3R-2,7,4′-trihydroxyisoflavanone described above. So while the pea compounds are chemically distinct from 2,7,4′-trihydroxyisoflavanone, all of the compounds share a nearly identical three-dimensional shape when bound to HI4′OMT that is complementary to the shape of its active site cavity, thus explaining the dual functionality of this enzyme in solution.
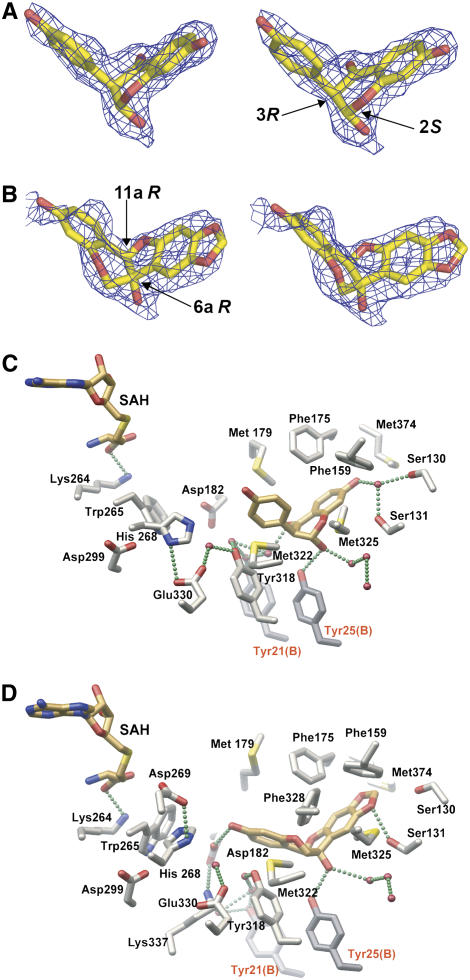
Figure 8.
Close-Up Views of the Complexes of HI4′OMT with 2,7,4′-Trihydroxyisoflavanone and 6aR,11aR-6a-Hydroxymaackiain.
(A) Stereo view of the electron density associated with the 2S,3R-stereoisomer of 2,7,4′-trihydroxyisoflavanone observed crystallographically in the HI4′OMT complex. The SIGMAA-weighted composite-omit electron density map was contoured at 1.2 σ. While the axial positions of the hydroxy-phenyl and hydroxyl moieties clearly represent higher-energy conformations, HI4′OMT presumably uses binding energy to selectively sequester the 2,7,4′-trihydroxyisoflavanone substrate.
(B) Stereo view of the electron density associated with 6aR,11aR-6a-hydroxymaackiain observed crystallographically in the HI4′OMT complex. The SIGMAA-weighted composite-omit electron density map was contoured at 0.6 σ due to lower occupancy of this bound pterocarpan. Given the lower occupancy of 6aR,11aR-6a-hydroxymaackiain obtained by soaking into HI4′OMT crystals with SAH already bound (no opportunity for methylation to occur), there is some deviation of the refined coordinates for 6aR,11aR-6a-hydroxymaackiain from the electron density.
(C) Close-up view of the HI4′OMT substrate/product binding site with SAH and 2S,3R-2,7,4′-trihydroxyisoflavanone shown. Bonds are color-coded by atom type, with SAH and isoflavanone carbon atoms in gold and protein carbon atoms in gray. Oxygen atoms are red, nitrogen atoms are blue, and sulfur atoms are yellow. Two amino acid residues from the dyad-related monomer B are labeled with red lettering. The putative hydrogen bonds are depicted as green spheres.
(D) Close-up view as in (C) illustrating the conformation and location of bound 6aR,11aR-6a-hydroxymaackiain. For clarity, the active site residues Asp-269, Phe-328, and Lys-337 that only participate in a hydrogen-bonding network in the HI4′OMT-SAH-6a-hydroxymaackiain complex shown in (D) were omitted in (C).
The 2S,3R configuration of 2,7,4′-trihydroxyisoflavanone bound in the catalytic cavity resides in a conformation in which the planes formed by the A and C rings, respectively, sit nearly perpendicular to the plane of the B ring linking the A and C rings. This apparently stably bound conformation of the HI4′OMT 2-hydroxyisoflavanone product fits snuggly in the Y-shaped phenolic substrate binding cavity, resulting in the proper positioning of the 4′-hydroxyl group on the B ring for transmethylation (Figure 8C).
The specific binding of 2S,3R-2,7,4′-trihydroxyisoflavanone is predominantly achieved through a complementary binding pocket formed by a contiguous van der Waals surface in a Y-shaped active site. This cavity consists of hydrophobic side chains, a large fraction of which are aromatic and Met residues. In particular, Met-179 and Met-322 form a thioether clamp that constrains the orientation of the B ring, while Met-325 and Met-374 form a second more widely spaced clamp for restraining the A and C rings. Most noticeable, the 2-hydroxyl group of 2S,3R-2,7,4′-trihydroxyisoflavanone serves as a key link for anchoring the bound molecule in the active site cavity (Figure 8C).
For the HI4′OMT structures solved with either 6a-hydroxymaackiain or pisatin bound, the plane formed by rings A and B of the pterocarpans orient nearly orthogonal to the C, D, and E rings, generating a sharply bent conformation that occupies the same Y-shaped binding cleft previously noted for 2S,3R-2,7,4′-trihydroxyisoflavanone. Moreover, the 6a-hydroxyl moiety of the pterocarpans acts much like the 2-hydroxyl group of 2,7,4′-trihydroxyisoflavanone in anchoring and orientating the methyl acceptor through a direct hydrogen bond with the hydroxyl moiety of Tyr-25 from the dyad-related polypeptide chain (Figure 8D). Negligible activity is observed when maackiain, the biosynthetic precursor of (6aR,11aR)-6a-hydroxymaackiain, bearing a similar stereoconfiguration but lacking the 6a-hydroxyl moiety, is employed in barrel medic HI4′OMT or pea HM3OMT assays (Preisig et al., 1989; Wu et al., 1997).
DISCUSSION
The large conformational differences of the N-terminal domains relative to the C-terminal SAM/SAH binding domains among previously solved plant type I OMTs and HI4′OMT results in a large cleft between the SAM/SAH binding surfaces and the phenolic substrate binding pockets. This cleft leaves a large solvent-exposed active site in HI4′OMT relative to the more compact arrangement of each domain observed previously in I7OMT (Zubieta et al., 2001). This open architecture of HI4′OMT results in a 7- to 10-Å distance between the methyl donor SAM, the catalytic base His-268, and the methyl accepting hydroxyl moiety of phenolic substrates bound against the wall formed by the N-terminal domain. Another plant type I OMT, namely, caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase (COMT) whose crystal structure was previously determined in our laboratory, possesses a similar open architecture (Zubieta et al., 2002).
Together, these current and past OMT crystal structures implicate a functionally important conformational change associated with phenolic substrate recognition, binding, catalysis, and product release in plant type I OMTs. Given the large accessible surface area buried in the plant type I OMT dimer (∼30% of the available surface area) and the extent to which the N-terminal domain participates in this oligomerization surface, the structural data suggest that the SAM/SAH binding C-terminal domain is capable of moving to bind substrates, turn them over, and then release products relative to the tightly packed N-terminal domains.
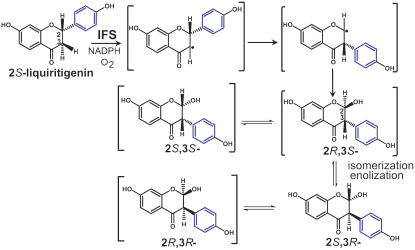
Based upon the crystal structures presented here, the 2S,3R-stereoisomer of 2,7,4′-trihydroxyisoflavanone is specifically recognized by HI4′OMT/HM3OMT (Figure 9). IFS, which is used to prepare 2,7,4′-trihydroxyisoflavanone, was previously shown to accept only 2S-flavanones as substrates (Hagmann and Grisebach, 1984; Kochs and Grisebach, 1986). Consistently, our studies here also demonstrated that the recombinant M. truncatula IFS only converted 2S-liquiritigenin (a 2S-flavanone) into 2,7,4′-trihydroxyisoflavanone when incubated with a racemic mixture of flavanone substrates (data not shown). The IFS-catalyzed facile suprafacial migration of the 2S aryl group was proposed to lead to a 3S aryl group configuration in the hydroxyisoflavanone product (Hashim et al., 1990; Hakamatsuka et al., 1998) (Figure 9). The 2R stereochemistry of the hydroxyl moiety at C2 was proposed based upon homology modeling of IFS with substrate docked into the active site and mechanistic comparisons with the known bacterial cytochrome P450 BM3 (Sawada et al., 2002).
Figure 9.
Proposed Stereochemistry of the IFS-Catalyzed Biosynthesis of 2,7,4′-Trihydroxyisoflavanone.
The aryl ring undergoing migration is blue. Small half-arrows depict electron migrations. Compounds in brackets are only proposed, as these stereoisomers have not been isolated and structurally confirmed. However, the skeletal rearrangement of the intermediates shown accounts for the formation of 2S,3R-2,7,4′-trihydroxyisoflavanone observed bound to Mt HI4′OMT following IFS-mediated in vitro generation, HPLC purification, and crystal soaking. To form each of the intermediate stereoisomers, each likely passes through an energetically and chemically accessible (double arrows) ring-opened form (data not shown for simplicity) that upon recyclization forms the depicted stereoisomers.
The demonstration of the 3R aryl group configuration bound to HI4′OMT/HM3OMT implicates a potentially facile skeletal rearrangement of the IFS product before HI4′OMT/HM3OMT-directed recognition and methylation. This observation implies that the putative 2R,3S stereoisomer of 2,7,4′-trihydroxyisoflavanone formed by IFS undergoes reversible transformation that ultimately leads to the formation of four diastereomeric structures in solution, each of which differs with respect to their ground state stability and conformation.
Finally, the close structural similarity of the 6a-hydroxylated 6aR,11aR-pterocarpan, 6a-hydroxymaackiain, with 2S,3R-2,7,4′-trihydroxyisoflavanone accounts for the observed dual activities of HI4′OMT toward two seemingly distinct isoflavonoid-derived substrates. Therefore, our biochemical and structural studies explain the recognition and stereospecific turnover of chemically distinct small molecules by highly similar plant type I OMTs. As such, this form of metabolic serendipity represents an example of possible short cuts to rapid evolutionary adaptation in organisms possessing a biosynthetically rich chemical repertoire for ecological interactions. The concept of substrate promiscuity in enzymes has been suggested to offer evolutionary advantages in organisms as they adapt to new environments and evolve new enzyme activities (O'Brien and Herschlag, 1999). In the case of chemical defenses in pea, critical evolutionary steps would be the appearance of the 6aR,11aR stereochemistry of the methylenedioxy ring bearing pterocarpans (as opposed to the 6aR,11aR stereochemistry in the Medicago pterocarpans; while the 6a and 11a stereocenters are inverted, the same R and S designations apply due to a change in priority rules) and introduction of the 6a-hydroxyl group which, interestingly, originates from water in contrast with the origin from molecular oxygen in the case of the 6aS,11aR pterocarpan glyceollin from soybean (Glycine max; Matthews et al., 1987).
The observation that barrel medic contains an enzyme with efficient in vitro catalytic activity for a compound (6a-hydroxy-6aR,11aR-pterocarpan) that it does not make is explained by the nearly identical three-dimensional structures for the 2S,3R-stereoisomer of 2,7,4′-trihydroxyisoflavanone and for the 6aR,11aR-stereoisomer of 6a-hydroxymaackiain bound to HI4′OMT. Given how similar the overall conformations of these two substrates are, it is difficult to imagine how limited mutational changes in HI4′OMT could lead to variants specific for only one of the substrates in question since changes are likely to affect activity against either substrate. Moreover, since both activities are necessary in pea for pisatin biosynthesis, it is possible that the related enzyme from pea, HM3OMT, carries out both methylations. Indeed, silencing of the Ps HM3OMT gene by antisense or sense constructs in pea hairy root cultures not only greatly reduced the accumulation of pisatin but also resulted in undetectable amounts of 6a-hydroxymaackianin. The lack of 6a-hydroxymaackianin accumulation was most likely due to silencing of the earlier 4′-O-methylation biosynthetic step possibly exploiting the dual activity of HI4′OMT/HM3OMT described here (Wu and VanEtten, 2004). Further genetic, biochemical, and structure-function studies of Ps HM3OMT will be necessary to conclusively answer this question. Nevertheless, the serendipitous exploitation of the intrinsic chemical restraints on small molecule conformations by plant biosynthetic machinery serves as a warning for functional annotation of natural product pathway genes based on limited in vitro data alone. Not only can catalytic promiscuity provide evolutionary advantages for the diversification of enzymes leading to new activities (O'Brien and Herschlag, 1999), but it may also be maintained to provide a plurality of advantageous functions in a single organism. Such metabolic serendipity may serve as an adaptive route by which plants adjust their specialized metabolic potential to respond to new environments.
METHODS
Materials and Chemicals
Pisatin and maackiain were generous gifts from Hans VanEtten (University of Arizona). (–)-Medicarpin and 6a-hydroxymaackiain were from our lab collection (R.A.D.). All other flavonoids and isoflavonoids used in the study were purchased from Indofine. SeMet, thrombin, SAM, and S-adenosyl-l-homo-Cys (SAH) were purchased from Sigma-Aldrich. Radiolabeled adenosyl-l-Met-S-(methyl-14C) was purchased from PerkinElmer Life Sciences. The pET28a(+) expression vector and Escherichia coli strain BL21(DE3) were purchased from Novagen. Ni2+-NTA resin was purchased from Qiagen. Benzamidine Sepharose and Superdex 200 FPLC columns were obtained from Amersham Bioscience.
Feeding of 6a-Hydroxymaackiain to Medicago truncatula Suspension Cultures and Metabolite Analysis
M. truncatula cells were maintained and subcultured in SH medium as previously described (Suzuki et al., 2005). Briefly, 150 μL of 2 mM 6a-hydroxymaackiain dissolved in methanol was added to 15 mL of 5-d-old subcultured cells. As a control, cells were also given only 150 μL of methanol. The resultant cultures were then incubated for 36 h, harvested by vacuum filtration, and subsequently frozen in liquid nitrogen. A portion of the culture media was also collected and frozen for metabolite extraction and analysis. For extractions, 0.5 g of frozen cells were thawed and extracted with 10 mL of acetone three times. The combined extracts were dried under a stream of N2, and the resultant residue was dissolved in 500 μL of methanol for HPLC analysis. For HPLC analysis, 30 μL of each redissolved sample was injected onto a C18 column (5 μ ODS2 250 × 4.6 mm; Waters Spherisorb) and eluted in 1% (v/v) phosphoric acid in water with a multistep acetonitrile gradient (Gradient I) at 5% (v/v) for 5 min, 5 to 10% (v/v) over 5 min, 10 to 17% (v/v) over 15 min, 17 to 23% (v/v) over 5 min, 23 to 50% (v/v) over 35 min, and finally 50 to 100% (v/v) over 4 min. The flow rate was set to 1 mL/min. Separated metabolites were monitored at 315 nm. Both 6a-hydroxymaackiain and pisatin standards were analyzed using the same HPLC protocol.
Isolation and Identification of M. truncatula cDNAs Encoding HI4′OMT/HM3OMT
Searches of The Institute for Genomic Research M. truncatula Gene Index were performed using nucleotide BLAST and the pea (Pisum satvium) HM3OMT gene sequence (GenBank accession number U69554) with the following parameters: blosum 62, expect 10, and description 20. All other parameters were set to their default values. The resulting EST clones were obtained from the M. truncatula yeast elicited cell culture library (Noble Foundation) and sequenced. Sequence analysis was performed using the ExPASy Molecular Biology Server tools, DNASTAR, and the ClustalW (1.82) method. The open reading frame of the full-length cDNA clone was amplified by PCR with forward primer 5′-GGAATTCATGGCTTTCAGTACCAACGGT-3′ and reverse primer 5′-CCTCGAGTCAAGGATAAACTTCGATGA-3′ to introduce EcoRI and XhoI restriction enzyme sites, respectively. After restriction enzyme digestion, the product was ligated into pET28a(+) in frame with a hexahistidine N-terminal tag. The construct was transformed into E. coli BL21(DE3). The recombinant protein was expressed in E. coli at 37°C using Terrific broth containing 50 μg/mL kanamycin until A600 = 1.0. Protein expression was induced at 22°C in the presence of 0.5 mM isopropylthio-β-galactoside, and cultures were then grown overnight. The recombinant protein was purified using a Ni-NTA agarose (Qiagen) affinity column, followed by digestion with thrombin to cleave the His tag and further purified by gel filtration chromatography on a Superdex 200 column equilibrated with a 25 mM HEPES buffer, pH 7.5, as described by Zubieta et al. (2001). SeMet-substituted protein was obtained from E. coli grown in minimal media with appropriate amino acids and SeMet added (Doublie, 1997). Expression and purification steps were as above.
Phylogenetic Analysis of Barrel Medic HI4′OMT
Protein sequences of several phenylpropanoid-specific OMTs were retrieved from the National Center for Biotechnology Information. These include Ps HM3OMT from P. sativum (accession number U69554), Ge HI4′OMT from Glycyrrhiza echinata (accession number AB091684), the putative Lj HI4′OMT from Lotus japonicus (accession number BAC58013), Ms I7OMT from Medicago sativa (accession number U97125), Ms COMT from M. sativa (alfalfa caffeic acid 3-O-methyltransferase; accession number P28002), Ms CCOMT (alfalfa caffeoyl-CoA O-methyltransferase; accession number T09399), and Ms ChOMT (alfalfa chalcone 2′-O-methyltransferase; accession number AAB48058). Together with the amino acid sequence of HI4′OMT from M. truncatula, multisequence alignment was performed using ClustalW (1.83) (Higgins et al., 1994) with parameters set as protein gap open penalty = 10.0, protein gap extension penalty = 0.2, protein matrix = Gonnet, protein/DNA ENDGAP = −1, and protein/DNA GAPDIST = 4. The resulting sequence alignment was subsequently subjected to phylogenetic tree construction with the program MEGA, version 3.1 (Kumar et al., 2004), using the neighbor-joining method. The statistical calculation of bootstrap values was carried out with the default sets of the MEGA program at 500 replicates and seed = 64,238.
Biosynthesis and Purification of 2,7,4′-Trihydroxyisoflavanone
A cDNA clone encoding IFS was isolated from a M. truncatula root cDNA library using soybean (Glycine max) IFS as probe and was designated Mt IFS1. The open reading frame of Mt IFS1 was amplified using the following primers: 5′-GAAGGAATTCATAATGTTGGTGGAAC-3′ and 5′-CTCAAGATGGTACCGGAGGAAAGAAG-3′, each of which contain restriction enzyme cut sites for EcoRI and KpnI, respectively. The PCR product was digested with EcoRI-KpnI and ligated into appropriately digested PICZ vector (Invitrogen) for expression in the yeast Pichia pastoris. As part of the PCR cloning strategy, the four nucleotides upstream of the ATG start codon were modified to conform to the yeast consensus for translation initiation. The PICZ-MtIFS1 plasmid was linearized with NsiI and transformed into yeast (P. pastoris) strain GS115 by electroporation as described in the EasySelect Pichia expression kit manual (Invitrogen). Transformants were selected on media containing 1000 μg/mL zeocin and confirmed to harbor the Mt IFS1 expression construct by genomic PCR.
Expression was performed according to the EasySelect Pichia expression kit manual. Yeast cells were collected and resuspended in 0.1 M potassium phosphate, pH 8.0, containing 0.4 M Suc, 14 mM β-mercaptoethanol, and 1× protease inhibitors (Complete EDTA-free Protease Inhibitor Cocktail tablets; Roche Diagnostics). Microsomes were isolated as described (Liu et al., 2003). The microsomal pellet was resuspended in 0.1 M potassium phosphate, pH 8.0, containing 0.4 M Suc and 0.5 mM reduced glutathione. Protein content was determined by Bradford assays using BSA as a standard (Bradford, 1976). The 2,7,4′-trihydroxyisoflavanone was generated by incubating 160 nmol (2RS)-liquiritigenin dissolved in DMSO (INDOFINE Chemical Company) with the microsomal fraction of Mt IFS1–expressing yeast (∼40 mg of protein) in the presence of 0.5 mM NADPH. After an overnight incubation at 16°C, reactions were extracted three times with ethyl acetate. Extracts were concentrated under N2, and the resultant dried material was dissolved in methanol. Aliquots were purified by preparative HPLC using a Phenomenex SYNERGI Polar-RP 80-Å column (4-μm particle size, 4.6 × 250 mm) and eluted in water with an increasing gradient of acetonitrile (Gradient II: 0 to 5 min, 20% [v/v]; 5 to 17 min, 20 to 38% [v/v]; 17 to 33 min, 38% [v/v]; and 33 to 34 min, 38 to 100% [v/v]) at a flow rate of 1 mL/min. The peak corresponding to 2,7,4′-trihydroxyisoflavanone was collected and residual acetonitrile removed under N2. Quantification of 2,7,4′-trihydroxyisoflavanone was determined after acid-mediated hydrolysis to daidzein as described (Sawada et al., 2002). Daidzein was quantified from HPLC traces by calculation of peak area and comparison to a standard curve. Chiral HPLC chromatography was performed as described (Akashi et al., 1999).
Activity Assays of Barrel Medic HI4′OMT
Enzyme assays were performed in 120 μL of 200 mM Tris-HCl, pH 8.0, containing 5 mM EDTA and 14 mm β-mercaptoethanol with 20 μg of purified protein, 400 μM SAM, and 100 μM phenolic substrate. The compounds used included the flavanones taxifolin and fustein, the isoflavones daidzein and genistein, the pterocarpans (–)-medicarpin, maackiain, coumestrol, and 6a-hydroxymaackiain, and the isoflavanone 2,7,4′-trihydroxyisoflavanone. Reactions were allowed to proceed for 30 min at 30°C. Products were partitioned with hexane:ethyl acetate (1:1), and a portion of the extract was hydrolyzed with 10% (v/v) HCl for 10 min at 55°C. The resulting products were extracted using ethyl acetate. After concentration under N2, the remaining material was dissolved in methanol and analyzed by HPLC using a YMC-Pack OD3-AQ column (S-5 μm particle size, 4.6 × 150 mm) and running a water/acetonitrile gradient as described above (Gradient II).
For MS analysis, an HP 1100 series II LC system (Hewlett-Packard) with a photodiode array detector was coupled to a Bruker Esquire ion-trap mass spectrometer (MSD trap XCT system) equipped with an electrospray ionization source. A reverse phase, C18, 5-μm, 4.6 × 250-mm column (Phenomenex) was used. The mobile phase consisted of 0.2% (v/v) formic acid (positive-ion mass spectra) or 0.1% (v/v) acetic acid (negative-ion mass spectra) using Gradient II as described above. The flow rate to the MSD trap was 0.5 mL/min (positive-ion spectra) or 0.8 mL/min (negative-ion spectra), and the temperature of the column was kept at 25°C. The positive-ion mass spectra were acquired for the enzymatic reactions with 2,7,4′-trihydroxyisoflavanone substrate, and negative-ion mass spectra were acquired for the enzymatic reactions with 6a-hydroxymaackiain. Positive-ion electrospray ionization was performed using an ion source voltage of 3.5 kV and a capillary offset voltage of 158.6 V. Nebulization was aided with a coaxial nitrogen sheath gas provided at a pressure of 50 p.s.i. Desolvation was aided using a counter current nitrogen flow set at a pressure of 11 p.s.i. and a capillary temperature of 350°C. Mass spectra were recorded over the range 50 to 1000 m/z. Negative-ion electrospray ionization was performed using an ion source voltage of 3.0 kV and a capillary offset voltage of 70.7 V. Nebulization and desolvation were aided as described above. Mass spectra were recorded over the range 50 to 2200 m/z. The ion trap mass spectrometer was operated under an ion current control of ∼10,000 with a maximum acquisition time of 200 ms for both positive-ion and negative-ion mass spectra.
Rates of HI4′OMT-mediated methylation of the substrates 6a-hydroxymaackiain and 2,7,4′-trihydroxyisoflavanone were determined using various concentrations of each substrate (1.25, 2.5, 5, 10, 20, 40, 80, 100, 150, 200, and 250 μM) with the total SAM concentration fixed at 312.5 μM and augmented with 0.0042 μCi of adenosyl-l-Met-S-(methyl-14C). The reactions were performed in a total volume of 60 μL of Tris-HCl buffer, pH 8.0, as described above at 30°C for 15 min. Ten micrograms of purified HI4′OMT and 1.5 μg of recombinant AdoHcy nucleosidase were simultaneously added to the reaction mixture to initiate transmethylation. The nucleosidase irreversibly cleaves SAH to adenine and S-ribosylhomocysteine, thus reducing SAH-mediated product inhibition of HI4′OMT (Hendricks et al., 2004). Duplicate assays were performed and averaged. Activities were quantified by liquid scintillation counting. Vmax and Km were determined by nonlinear regression analysis of the velocity concentration data fit to the Michaelis-Menten equation. The HI4′OMT molar concentration was calculated using the computed protein Mr of 40,753 D (http://us.expasy.org/tools/protpar) to calculated kcat. To measure the steady state kinetic constants of HI4′OMT for SAM, activities were determined with different concentrations of SAM (6.25, 12.5, 25, 50, 100, 200, and 400 μM) at a fixed concentration of 200 μM of 2,7,4′-trihydroxyisoflavanone in a total volume of 60 μL as described above. After partitioning by ethyl acetate, the products were hydrolyzed and detected by HPLC as described above. The product quantification was conducted based on the standard curve constructed from HPLC diode array determination using authentic formononetin.
Protein X-Ray Crystallography
The initial structure of M. truncatula HI4′OMT was determined by MAD phasing using SeMet-substituted protein. Crystals of SeMet HI4′OMT-SAH were grown at 4°C by the vapor diffusion method in hanging drops containing a 1:1 mixture of protein and crystallization buffer (14% [w/v] polyethylene glycol (PEG) 8000, 0.4 M NH4Ac, pH 5.5, and 2 mM DTT) with 2.5 mM SAH. The crystals grew in space group P4(3)22 with one chain per asymmetric unit and a solvent content of 59.5%. Unit cell dimensions for SeMet HI4′OMT crystals were as follows: a = 71.36 Å, b = 71.36 Å, and c = 188.8 Å. MAD data (Table 1) were collected at the Se K edge at 100 K on the FIP-BM30A beam line at the European Synchrotron Radiation Facility and reduced with the HKL suite (Otwinowski and Minor, 1997). MAD data were used in SOLVE (Terwilliger and Berendzen, 1999) to locate and refine the Se sites. SOLVE located and refined five Se sites (mean figure of merit: 0.25) out of the 11 anticipated Se positions. Density modification was performed in RESOLVE (mean figure of merit: 0.57) (Terwilliger, 1999), and 183 residues out of 364 were built automatically in RESOLVE. The model was extended to include a total of 356 residues by manual building using the O molecular modeling package (Jones et al., 1991), and the model was refined using CNS 1.1 (Brunger et al., 1998).
Crystals of HI4′OMT in complex with SAH and pisatin were obtained by cocrystallization of protein with 2.5 mM of SAH and ∼1 mM pisatin in a solution containing 6% (w/v) PEG 8000, 0.3 M ammonium acetate, and 2 mM DTT at 4°C. Pisatin was prepared in 100% DMSO (greater solubility than in methanol) at ∼5 mM final concentration and diluted into crystallization drops. All other native HI4′OMT-SAH phenolic complexes were obtained by first cocrystallization of HI4′OMT with SAH (2.5 mM) in solutions containing 8 to 14% (w/v) PEG 8000, 0.25 to 0.4 M ammonium acetate, pH 5.5, and 2 mM DTT at 4°C. Crystals were then soaked in the presence of ∼1 mM 2,7,4′-trihydroxyisoflavanone or 6a-hydroxymaackiain in the crystallization buffers for 24 h. Again as for pisatin, 2,7,4′-trihydroxyisoflavanone or 6a-hydroxymaackiain were prepared in 100% DMSO at ∼5 mM final concentration and diluted into crystallization drops. Diffraction data were collected from single crystals mounted in a cryoloop and flash frozen in a nitrogen stream at 105 K. All native diffraction data were collected at the Stanford Synchrotron Radiation Laboratory (SSRL), beam line 9 to 1 on a 30-cm Marresearch imaging plate system (marUSA) and indexed and scaled using HKL2000 (Otwinowski and Minor, 1997). Subsequent structures were solved by the molecular replacement method and refined using CNS 1.1 (Brunger et al., 1998).
During refinements, structure factors obtained from intensity data were used to generate SIGMAA-weighted 2Fo − Fc and Fo − Fc electron density maps with phases calculated from the structure of the current model. Inspection of the electron density maps and model building were performed in O (Jones et al., 1991). The quality of all models was assessed using the program PROCHECK (Laskowski et al., 1993). For the SeMet-HI4′OMT-SAH complex, 89.1, 7.8, and 3.1% of the residues were found in the most favored, the allowed, and the generously allowed regions of the Ramachandran plot, respectively, with a G factor of 0.1. No residues were found in the disallowed region.
For the HI4′OMT-SAH-2,7,4′-trihydroxyisoflavanone complex, 90.3, 7.8, and 1.9% of the residues were found in the most favored, the allowed, and the generously allowed regions of the Ramachandran plot, respectively, with a G factor of 0.35. No residues were found in the disallowed region. For the HI4′OMT-SAH-(6a)-hydroxymaackiain complex, 93.5, 5.6, and 0.9% of the residues were found in the most favored, the allowed, and the generously allowed regions of the Ramachandran plot, respectively, with a G factor of 0.34. For the HI4′OMT-SAH-pisatin complex, 91.8, 7.3, and 0.9% of the residues were found in the most favored, the allowed, and the generously allowed regions of the Ramachandran plot, respectively, with a G factor of 0.26. No residues were found in the disallowed region.
Coordinates and Accession Numbers
The atomic coordinates and structure factors, HI4′OMT-SAH (code 1ZHF), HI4′OMT-SAH-2,7,4′-trihydroxyisoflavanone (code 1ZG3), HI4′OMT-SAH-6a-hydroxymaackiain (code 1ZGA), and HI4′OMT-SAH-pisatin (code 1ZGJ), have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics (Rutgers University, New Brunswick, NJ) (http://www.rcsb.org/). Sequence data of the genes used in this article can be found in the GenBank/EMBL data libraries under the following accession numbers: HI4′OMT from Medicago truncatula (AY942158), Ps HM3OMT from Pisum sativum (U69554), Ge HI4′OMT from Glycyrrhiza echinata (AB091684), and Ms I7OMT from Medicago sativa (U97125).
Acknowledgments
We thank Michael B. Austin for valuable discussion on data processing, Daneel Ferreira for helpful discussions concerning IFS stereochemistry, and Hans VanEtten for the generous gift of maackiain and pisatin. This work was supported by the National Science Foundation under Grant 0236027 to J.P.N. C.-J.L. was supported in part by a senior postdoctoral fellowship from the Noble Foundation. The work done in the Brookhaven National Laboratory was supported by the Laboratory Directed Research and Development program to C.-J.L. under contract with the Department of Energy. B.E.D. was supported by a grant from the Oklahoma Center for the Advancement of Science and Technology to R.A.D. Portions of this research were carried out at SSRL, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program. J.P.N. is an investigator of the Howard Hughes Medical Institute.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Joseph P. Noel (noel@salk.edu).
Open Access articles can be viewed online without a subscription.
References
- Akashi, T., Aoki, T., and Ayabe, S. (1999). Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol. 121 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi, T., Aoki, T., and Ayabe, S. (2005). Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 137 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi, T., Sawada, Y., Aoki, T., and Ayabe, S. (2000). New scheme of the biosynthesis of formononetin involving 2,7,4′-trihydroxyisoflavanone but not daidzein as the methyl acceptor. Biosci. Biotechnol. Biochem. 64 2276–2279. [DOI] [PubMed] [Google Scholar]
- Akashi, T., Sawada, Y., Shimada, N., Sakurai, N., Aoki, T., and Ayabe, S. (2003). cDNA cloning and biochemical characterization of S-adenosyl-L-methionine: 2,7,4‘-trihydroxyisoflavanone 4’-o-methyltransferase, a critical enzyme of the legume isoflavonoid phytoalexin pathway. Plant Cell Physiol. 44 103–112. [DOI] [PubMed] [Google Scholar]
- Aoki, T., Akashi, T., and Ayabe, S. (2000). Flavonoids of leguminous plants: Structure, biological activity, and biosynthesis. J. Plant Res. 113 475–488. [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T., et al. (1998). Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D54, 905–921. [DOI] [PubMed]
- Ciuffetti, L.M., and VanEtten, H.D. (1996). Virulence of a pisatin demethylase-deficient Nectria haematococca MPVI isolate is increased by transformation with a pisatin demethylase gene. Mol. Plant Microbe Interact. 9 787–792. [Google Scholar]
- Dewick, P.M. (1993). Isoflavonoids. In The Flavonoids: Advances in Research Since 1986, J.B. Harborne, ed (London: Chapman and Hall), pp. 117–238.
- Dixon, R.A. (1999). Isoflavonoids: Biochemistry, molecular biology and biological functions. In Comprehensive Natural Products Chemistry, U. Sankawa, ed (Amsterdam: Elsevier), pp. 773–823.
- Doublie, S. (1997). Preparation of selenomethionyl proteins for phasing determination. Methods Enzymol. 276 523–530. [PubMed] [Google Scholar]
- Hagmann, M., and Grisebach, H. (1984). Enzymatic rearrangement of flavanone to isoflavone. FEBS Lett. 175 199–202. [Google Scholar]
- Hakamatsuka, T., Mori, K., Ishida, S., Ebizuka, Y., and Sankawa, U. (1998). Purification of 2-hydroxyisoflavanone dehydratase from the cell cultures of Pueraria lobata. Phytochemistry 49 497–505. [Google Scholar]
- Hashim, M.F., Hakamatsuka, T., Ebizuka, Y., and Sankawa, U. (1990). Reaction mechamism of oxidative rearrangement of flavanone in isoflavone biosynthesis. FEBS Lett. 271 219–222. [DOI] [PubMed] [Google Scholar]
- He, X.-Z., and Dixon, R.A. (1996). Affinity chromatography, substrate/product specificity and amino acid sequence analysis of an isoflavone O-methyltransferase from alfalfa (Medicago sativa L.). Arch. Biochem. Biophys. 336 121–129. [DOI] [PubMed] [Google Scholar]
- He, X.-Z., Reddy, J.T., and Dixon, R.A. (1998). Stress responses in alfalfa (Medicago sativa L.) XXII. cDNA cloning and characterization of an elicitor-inducible isoflavone 7-O-methyltransferase. Plant Mol. Biol. 36 43–54. [DOI] [PubMed] [Google Scholar]
- Hendricks, C.L., Ross, J.R., Pichersky, E., Noel, J.P., and Zhou, Z.S. (2004). An enzyme-coupled colorimetric assay for S-adenosylmethionine-dependent methyltransferases. Anal. Biochem. 326 100–105. [DOI] [PubMed] [Google Scholar]
- Higgins, D., Thompson, J., Gibson, T., Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.-Y., Cowan, S.W., and Kjeldgaard, M. (1991). Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A49, 148–157. [DOI] [PubMed]
- Kochs, G., and Grisebach, H. (1986). Enzymic synthesis of isoflavones. Eur. J. Biochem. 155 311–318. [DOI] [PubMed] [Google Scholar]
- Kumar, S., Tamura, K., and Nei, M. (2004). MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5 150–163. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., MacArthur, M.W., Moss, D.S., and Throrton, J.M. (1993). PROCHECK: A program to check the stereochemical quality of protein strucutres. J. Appl. Crystallogr. 26 283–291. [Google Scholar]
- Liu, C.-J., and Dixon, R.A. (2001). Elicitor-induced association of isoflavone O-methyltransferase with endomembranes prevents formation and 7-O-methylation of daidzein during isoflavonoid phytoalexin biosynthesis. Plant Cell 13 2643–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C.-J., Huhman, D., Sumner, L.W., and Dixon, R.A. (2003). Regiospecific hydroxylation of isoflavones by cytochrome p450 81E enzymes from Medicago truncatula. Plant J. 36 471–484. [DOI] [PubMed] [Google Scholar]
- Matthews, D.E., Weiner, E.J., Matthews, P.S., and VanEtten, H.D. (1987). Role of oxygenases in pisatin biosynthesis and in the fungal degradation of maackiain. Plant Physiol. 83 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel, J.P., Dixon, R.A., Pickersky, E., Zubieta, C., and Ferrer, J.-L. (2003). Structural, functional and evolutionary basis for methylation of plant small molecules. Recent Adv. Phytochem. 37 37–58. [Google Scholar]
- O'Brien, P.J., and Herschlag, D. (1999). Catalytic promiscuity and the evolution of new enzymatic activities. Chem. Biol. 6 91–105. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z., and Minor, W. (1997). Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276 307–326. [DOI] [PubMed] [Google Scholar]
- Pichersky, E., and Gang, D.R. (2000). Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 5 439–445. [DOI] [PubMed] [Google Scholar]
- Preisig, C.L., Matthews, D.E., and VanEtten, H.D. (1989). Purification and characterization of S-adenosyl-L-methionine:6a-hydroxymaackiain 3-O-methyltransferase from Pisum sativum. Plant Physiol. 91 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig, C.L., VanEtten, H.D., and Moreau, R.A. (1991). Induction of 6a-hydroxymaackiain 3-O-methyltransferase and phenylalanine ammonia-lyase mRNA translational activities during the biosynthesis of pisatin. Arch. Biochem. Biophys. 290 468–473. [DOI] [PubMed] [Google Scholar]
- Sawada, Y., Kinoshita, K., Akashi, T., Aoki, T., and Ayabe, S. (2002). Key amino acid residues required for aryl migration catalysed by the cytochrome P450 2-hydroxyisoflavanone synthase. Plant J. 31 555–564. [DOI] [PubMed] [Google Scholar]
- Suzuki, H., Reddy, M.S., Naoumkina, M., Aziz, N., May, G.D., Huhman, D.V., Sumner, L.W., Blount, J.W., Mendes, P., and Dixon, R.A. (2005). Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta 220 696–707. [DOI] [PubMed] [Google Scholar]
- Terwilliger, T.C. (1999). Reciprocal-space solvent flattening. Acta Crystallogr. D55, 1863–1871. [DOI] [PMC free article] [PubMed]
- Terwilliger, T.C., and Berendzen, J. (1999). Automated MAD and MIR structure solution. Acta Crystallogr. D55, 849–861. [DOI] [PMC free article] [PubMed]
- VanEtten, H.D., Matthews, P.S., Tegtmeier, K.J., Dietert, M.F., and Stein, J.I. (1980). The association of pisatin tolerance and demethylation with virulence on pea in Nectria haematococca. Physiol. Plant Pathol. 16 257–268. [Google Scholar]
- Wasmann, C.C., and VanEtten, H.D. (1996). Transformation-mediated chromosome loss and disruption of a gene for pisatin demethylase decreases the virulence of Nectria haematococca on pea. Mol. Plant Microbe Interact. 9 793–803. [Google Scholar]
- Wengenmayer, H., Ebel, J., and Grisebach, H. (1974). Purification and properties of a S-adenosylmethionine: isoflavone 4′-O-methyltransferase from cell suspension cultures of Cicer arietinum L. Eur. J. Biochem. 50 135–143. [DOI] [PubMed] [Google Scholar]
- Wong, E. (1975). The isoflavonoids. In The Flavonoids, Part 2, J.B. Harborne, T.J. Mabry, and H. Mabry, eds (New York: Academic Press), pp. 743–800.
- Wu, Q., Preisig, C.L., and VanEtten, H.D. (1997). Isolation of the cDNAs encoding (+)6a-hydroxymaackiain 3-O-methyltransferase, the terminal step for the synthesis of the phytoalexin pisatin in Pisum satium. Plant Mol. Biol. 35 551–560. [DOI] [PubMed] [Google Scholar]
- Wu, Q., and VanEtten, H.D. (2004). Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen. Mol. Plant Microbe Interact. 17 798–804. [DOI] [PubMed] [Google Scholar]
- Zubieta, C., Dixon, R.A., and Noel, J.P. (2001). Crystal structures of chalcone O-methyltransferase and isoflavone O-methyltransferase reveal the structural basis for substrate specificity in plant O-methyltransferases. Nat. Struct. Biol. 8 271–279. [DOI] [PubMed] [Google Scholar]
- Zubieta, C., Kota, P., Ferrer, J.-L., Dixon, R.A., and Noel, J. (2002). Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 14 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]