Abstract
We previously have isolated an endosomal fraction from rat liver, termed receptor-recycling compartment (RRC), which is highly enriched in recycling receptors and in the transcytotic polymeric Ig receptor (pIgR). We now have analyzed the RRC fraction by immunoisolation and found that no uniquely transcytotic elements were present, because recycling receptors and the pIgR were coisolated on the same elements. In addition, RRC was very rich in proteins previously shown to be associated with recycling endosomes, such as rab 11, cellubrevin, and endobrevin, but relatively poor in early endosome antigen 1. As RRC contains mainly tubules and small vesicles, our results indicate that it is enriched in elements of a tubular endosomal compartment involved in receptor sorting. Biochemical analysis showed that the density of recycling receptors and transcytotic pIgR in RRC membranes was similar to that in early endosome membranes. This observation supports the idea that increasing membrane surface area by endosome tubulation is the main mechanism to ensure efficient receptor sorting and, at the same time, locates RRC in a common step of the endocytotic system before final receptor segregation into distinct recycling and transcytotic pathways.
Keywords: endocytosis, transcytosis, recycling
After endocytosis and delivery to early endosomes, material internalized by cells can follow multiple pathways. Many molecules, such as the epidermal growth factor receptor (EGFR), are transported to late endosomes. Other molecules, such as transferrin (Tf) and its receptor (TfR), are recycled to the plasma membrane. At least some recycling molecules travel from the early endosome to a recycling endosome consisting of tubules clustered around the centriole. The recycling endosome is a major site of sorting in the endocytotic pathway, as molecules can differentially pass through or be retained by it (1, 2). Polarized epithelial cells present an additional layer of complexity. After endocytosis at one surface of the cell, endocytosed material can be degraded, recycled to the original plasma membrane domain, or transcytosed to the opposite surface of the plasma membrane. Analysis of the transcytotic pathway of the polymeric Ig receptor (pIgR) and its ligand, IgA, in Madin-Darby canine kidney cells has shown that it involves ≥ 3 intermediate endosomal compartments. IgA endocytosed from the basolateral surface first enters the basolateral early endosome (BEE), which contains basolaterally endocytosed IgA, Tf, low density lipoprotein (LDL), and EGFR at high concentrations (3–5) (K. Dunn, personal communication). The second compartment consists of long 60-nm diameter tubules lying parallel to microtubules and oriented toward the apical cytoplasm (4). This “tubular compartment” contains IgA and transferrin at the same relative concentrations as the BEE, but is substantially depleted of LDL and EGFR, which are destined for the degradative pathway (5) (K. Dunn, personal communication). The tubular compartment is accessible to the membrane-bound markers of IgA and Tf endocytosed from either the apical or basolateral surface, which has led to its designation as an interconnected or common endosomal compartment (4). The final compartment in the transcytotic pathway consists of 100- to 150-nm diameter cup-shaped vesicles, which are distributed immediately below the apical plasma membrane (5). The cup-shaped vesicles are probably the equivalent of the apical recycling endosome (ARE) described in earlier work and is referred to here as ARE (3, 6). Electron microscopic analysis indicated that the ARE is enriched in the transcytotic marker, pIgR, and depleted in the recycling marker, TfR (5). Morphologic observations in rat liver also support the hypothesis that in hepatocytes there are three distinguishable homologous compartments in the transcytotic pathway (7–10). Despite the growing appreciation of the existence and importance of the tubular compartment and its involvement in receptor sorting for recycling or transcytosis, we are not aware of any reports of its isolation and characterization. Our knowledge of its existence, structure, and composition thus is limited to morphological and immunocytochemical observations. Although such techniques are extremely valuable, they are quite limited, for instance, in quantifying the composition of a compartment. Therefore, a deeper understanding of this tubular compartment, how it works in sorting and regulation of membrane traffic, and indeed of the organization of the endocytic and transcytotic pathways, will require its isolation or at least substantial enrichment by the techniques of cell fractionation. Such a fraction, which we analyze here, also will be very useful in probing its function, e.g., by in vitro reconstitution of traffic into and out of the compartment.
MATERIALS AND METHODS
Animals.
Male Sprague–Dawley rats (B & K Universal, Fremont, CA) were treated with 17-α-ethinyl estradiol (Sigma) to increase the number of hepatic LDL receptors (LDLRs), as described (11, 12). All animal experiments followed protocols approved by the University of California, San Francisco Committee on Animal Care.
Antibodies.
Antibodies to Tf and the pIgR secretory component were developed in this laboratory. Antibodies to the following antigens were generously provided: pIgR SC166 (J.-P. Kraehenbuhl, Institut Suisse de Recherche Experimentale sur le Cancer, Lausanne); LDLR 4A4 (J. Herz, Univ. of Texas, Dallas); TfR H68.4 (I. Trowbridge, Salk Institute, San Diego, CA); rab4 (I. Mellman, Yale Univ., New Haven, CT); rab11 121 (R. Parton, Univ. of Queensland, Australia); cellubrevin (R. Jahn, Yale Univ.); endobrevin (W. Hong, Institute of Molecular and Cellular Biology, Singapore); and early endosome antigen 1 (B.-H. Toh, Monash Univ., Victoria, Australia). Antibodies to the following were purchased commercially: IgA (ICN); apolipoprotein (apo) B-100 401-E22 (International Immunology, Murrieta, CA); and rab5 44–332 (QCB, Hopkinton, MA).
Isolation of the Endosome Fractions from Rat Liver: Separation of Membranes from Luminal Content. Morphological and partial biochemical characterization of highly purified endosome fractions has been described (13). This method takes advantage of the decrease in density of endosomes after internalization of a bolus of human LDL injected i.v. (14). By this method, three subcellular fractions can be obtained: compartment of uncoupling of receptor and ligand (CURL), multivesicular bodies (MVB), and a fraction rich in recycling receptors, which was termed RRC. In normal, nonestradiol-injected rats, the CURL and MVB fractions largely coisolate by this method and the purity of the RRC fraction, by morphological and biochemical criteria, tends to be more variable (15). To achieve more reproducible conditions, we treated the rats with estradiol in all of the experiments.
To separate the membranes of endosomes from their lipoprotein content and the rest of the luminal material, we stripped the endosomes by means of sodium carbonate treatment at high pH (16). Equal protein amounts of each fraction were resuspended in approximately 100 μl of 0.9% NaCl, containing a mixture of protease inhibitors (13). Na2CO3 was added to a final concentration of 100 mM and pH 11.5 (1 ml final volume). Samples were incubated at 0–4°C for 30 min and then spun for 2 h at 120,000 × gav in a Beckman TLA100.3 rotor, using adapters for Eppendorf tubes. The supernatants were taken for further analysis, and the pellets were rinsed twice with 1 ml of ice-cold H2O. Then, each pellet was resuspended in 100 μl of 0.9% NaCl (containing protease inhibitors) by repeated aspiration through a 25-gauge needle and sonicated, 3 × 15 sec each (1-min intervals), in a Branson bath sonifier.
Immunoisolation Using Magnetic Beads.
Binding of magnetic beads to antibodies was performed according to the manufacturer’s instructions (Dynal). All incubations were carried out in 1.5-ml Eppendorf tubes subjected to continuous slow rotation. After the incubations, beads containing the bound antibodies, or the bound population of endosomal elements, were collected by using a magnetic device (Dynal). To bind the endosomal elements to the beads, intact RRC was incubated in PBS, pH 7.4, containing 1% FBS and 2 mM EDTA, with the primary antibody-coated magnetic beads at 5–10 μg RRC protein/mg beads (1 h to overnight, at 4°C). Then, beads were washed 15–20 min ×4, in 1% FBS-PBS/EDTA, and 5 min ×2 in PBS alone. The unbound population of elements (remaining in the supernatant) either was mixed with different primary antibody-coated magnetic beads (for a second immunoisolation) or was centrifuged to pellet these elements. This centrifugation was done at 150,000 × gav for 1 h in a Sorvall RP100⋅AT4–236 rotor, using adapters for Eppendorf tubes. An equal aliquot of starting material for immunoisolation also was centrifuged as above. Before centrifugations, an equal volume of 8% sucrose was added to maintain the intactness of the elements and dilute the extra proteins coming from the FBS. All samples finally were resuspended in equal volumes of Laemmli sample buffer (17), heated to 100°C for 3 min, and loaded onto gels to determine the distribution of proteins between the bound and unbound populations of elements. To control for the specificity of the interactions, a bacterial glutathione S-transferase fusion protein containing the cytoplasmic domain of the pIgR was included in some of the incubations. This GST fusion protein, kindly provided by T. Weimbs (Univ. of California, San Francisco), contains the target domain for the anti-pIgR cytoplasmic domain antibody, SC166.
Gel Electrophoresis, Western Blotting, and Analytical Procedures.
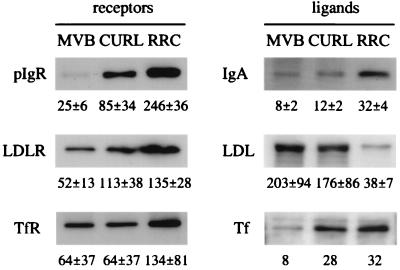
Polypeptides were separated by SDS/PAGE and subjected to Western blotting as described (18). Image analysis was performed with an imagequant Personal Densitometer apparatus (Molecular Dynamics). Only values determined by volume integration from bands of the same gel and exposure were compared. The values reported correspond to fold enrichments of the proteins detected in the endosome fractions over the total liver homogenate. For all quantitations, the amount of protein loaded was estimated to maintain the signal intensity in the linear range for the corresponding antigen in all samples compared. The protein concentration of the samples was measured as described (19), with BSA as standard. As markers of endosome membrane for either the recycling or the transcytotic pathway in hepatocytes, we compared the distributions of LDLR, TfR, and pIgR in endosomes and endosome membranes. As integral membrane proteins, these receptors remained virtually entirely in the membrane after Na2CO3 treatment. In addition, all were comparatively more enriched in RRC than in the other endosome fractions (see Fig. 1). To determine and compare the density of these receptors in the membrane of each endosome fraction, we took into account the efficiency of luminal content removal from each fraction. For this purpose, we used human apo B-100, the main protein of the internalized LDL, as a marker of the remaining luminal content. Whereas complete LDL removal after the Na2CO3 treatment never was achieved, one easily can extrapolate graphically to a projected zero LDL remaining (0% apo B-100), and thereby estimate the receptor’s relative density in the membranes.
Figure 1.
Representative Western blots showing distribution of proteins in the endosome fractions and enrichment over the liver homogenate. The same amount of protein (3–10 μg, depending on the antigen) was loaded on each of the compared lanes of endosome fractions. The values are from quantifications by densitometric scanning of Western blots and indicate the fold of protein enrichment, in each fraction, over the liver homogenate. The reported value corresponds to the mean ± SEM of ≥ 3 different experiments, except for Tf (two).
RESULTS
RRC Does Not Contain a Population of Transcytotic Elements: Recycling Receptors and the Transcytotic pIgR Coisolate on the Same Elements. Of our three previously isolated endosomal subfractions, the CURL appears to be enriched in elements corresponding to the BEE, whereas the MVB contains predominantly late endosomes. The RRC is highly enriched in recycling receptors, e.g., LDLR and TfR, and poor in ligands and receptors headed for degradation, such as LDL and EGFR (13, 20). RRC is also very rich in the pIgR and IgA, which are directed toward the transcytotic pathway (18). However, the relative density of these receptors in the membranes of the endosome fractions has not been analyzed. Using specific antibodies in Western blotting, we determined the distribution of various proteins in the three fractions. The proteins analyzed include the transmembrane glycoprotein receptors pIgR, LDLR, and TfR, and their respective ligands, IgA, apo B-100 (LDL), and Tf. As shown in Fig. 1, RRC was most enriched in the transmembrane receptors, though the enrichment of pIgR was greater than that of the recycling receptors, LDLR and TfR. RRC was also relatively abundant in IgA and Tf, but had very low levels of LDL, which follows the degradative pathway and is abundant in CURL and especially in MVB (as shown by ref. 13). These data confirm the suggested involvement of RRC in transcytosis and recycling (18, 20–23).
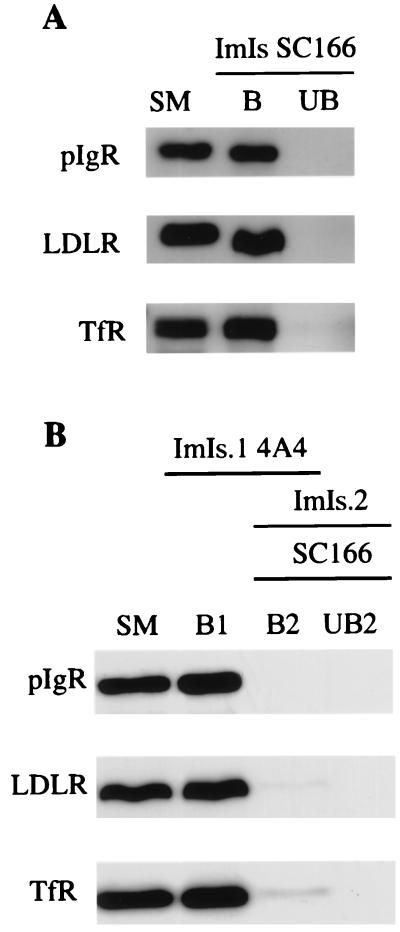
We therefore tested the hypothesis that RRC may include separate transcytotic and recycling elements. We immunoabsorbed RRC with antibody-coated magnetic beads, followed by a characterization of the corresponding bound and unbound populations. We first carried out an immunoisolation of the RRC fraction using magnetic beads coated with antibodies against the cytoplasmic domain of the pIgR, SC166. When antibodies against recycling receptors, LDLR and TfR, were tested by Western blotting, virtually all of these receptors were found associated with the elements immunoabsorbed on the basis of their content of pIgR (Fig. 2A). The efficiency of our immunoisolations (using the pIgR or other antigens; see below) was always close to 100%, i.e., almost all of the positive elements were recovered by the antibody-coated magnetic beads, because the amount of the pIgR in the bound population was comparable to that in the starting material. Several controls indicated that these immunoisolations were specific. Thus, immunoisolation of RRC using the SC166 antibody was done in the presence of a bacterial glutathione S-transferase fusion protein containing the target domain for the antibody. In addition, RRC was immunoabsorbed by using irrelevant antibodies. In both cases, virtually all elements containing pIgR remained in the unbound population, indicating a high degree of specificity for the conditions used (data not shown).
Figure 2.
Immunoisolations of RRC show that distinct transcytotic elements were not detected; rather, recycling receptors and the pIgR were coisolated on the same elements. All immunoisolations were carried out by using 5 μg of RRC protein per 1 mg of antibody-coated magnetic beads. The entire sample from each population of elements was loaded onto the gels. (A) Immunoisolation (ImIs) was carried out by using magnetic beads coated with the anti-pIgR SC166 antibody. (B) The first immunoisolation (ImIs.1) was done by using magnetic beads coated with the anti-LDLR 4A4 antibody. The second immunoisolation (ImIs.2) was done by using magnetic beads coated with the anti-pIgR SC166 antibody. Starting material (SM), bound (B), and unbound (UB) populations of elements were analyzed by Western blotting using either the SC166 antibody or the antibodies against the recycling receptors, LDLR and TfR.
These data indicate that the elements of RRC containing recycling receptors are associated with the elements containing pIgR. However, it was still possible that some elements containing pIgR lack recycling receptors. To test for the presence of these transcytotic elements in RRC, we first immunoabsorbed RRC using magnetic beads coated with the anti-LDLR antibody (4A4), which recognizes the cytoplasmic domain of LDLR. That way, we depleted recycling elements from RRC, which would be expected to be LDLR positive. Then, we immunoabsorbed the remaining unbound material with magnetic beads coated with the SC166. As shown in Fig. 2B, the corresponding Western blots indicate that all of the pIgR-reactive elements already were immunoabsorbed in the first step (B1), together with those for the LDLR and the TfR. The same result, not shown here, was found by immunoabsorption for the TfR in the first step (instead of the LDLR). Therefore, we did not detect a specific population of transcytotic elements in RRC, that is, elements that contain pIgR, but lack recycling receptors. On the contrary, our data show that RRC is biochemically rather uniform, in that all elements contain both recycling receptors and the transcytotic pIgR.
RRC Is Enriched in Proteins Associated with Recycling Endosomes: Enrichment of RRC in Elements of a Tubular Endosomal Compartment.
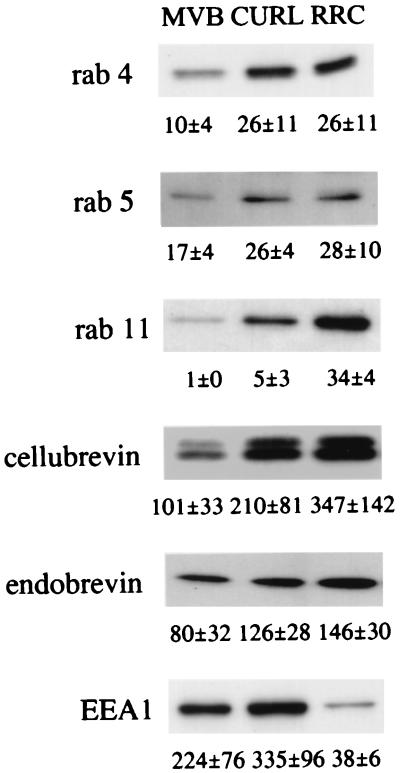
Our findings suggested that RRC may be enriched in the tubular endosomal compartment, as this contains both recycling and transcytosing proteins. We therefore investigated the enrichment in RRC of components of the membrane traffic machinery associated to recycling endosomes. As shown in Fig. 3, whereas cellubrevin, endobrevin, and early endosome antigen 1 were highly enriched in endosomes over the homogenate (depending on the fraction, but generally by factors of ≥ 100), the rab proteins were enriched less than 20- to 30-fold. Neither rab 4 nor rab 5, which are involved in plasma membrane and early endosome dynamics, were particularly abundant in RRC compared with CURL. In contrast, enrichment of rab 11, which is known to be involved in endocytic recycling (24–26), was 7-fold higher in RRC than in CURL. In addition, RRC’s relative abundance of cellubrevin (and less dramatically of endobrevin), as compared with CURL and MVB, further supports the reported involvement of RRC in the recycling step of the endocytic pathway (22). In contrast, in comparison to CURL or MVB, RRC contains a lower level of early endosome antigen 1, which is an effector of rab 5 and primarily is associated with early endosomes (27). These results indicate that RRC is enriched in elements involved in recycling rather than in early endosomes.
Figure 3.
Representative Western blots showing distribution of proteins involved in membrane traffic in the endosome fractions and enrichment over the liver homogenate. The same amount of protein (3–10 μg, depending on the antigen) was loaded on each of the compared lanes of endosome fractions. The values are from quantifications by densitometric scanning of Western blots and indicate the fold of protein enrichment, in each fraction, over the liver homogenate. The reported value corresponds to the mean ± SEM of ≥ 3 different experiments. EEA1, early endosome antigen 1.
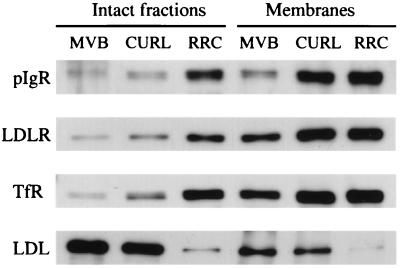
In RRC Membranes, the Density of Recycling Receptors and the Transcytotic pIgR Is Similar to That in Early Endosome Membranes: Evidence of Receptor Sorting by Default. As previously described (13), the morphological differences among CURL, MVB, and RRC account for their differing proportions of membranes and luminal contents. This is shown in Fig. 4, where the amount of the luminal marker, LDL (detected with the antibody to apo B-100), in the intact fractions is greatest in MVB, somewhat less in CURL, and much less in RRC. We then wanted to quantitate the composition of the membranes alone, in particular, the relative density of recycling receptors and the transcytotic pIgR in the membranes. As a way to correct for these large differences in luminal content, we isolated the membranes of each fraction by means of high pH sodium carbonate treatment (16). As shown in Fig. 4 in a representative experiment, the enrichments of the receptors in the three endosome fractions increased substantially in the carbonate-stripped membranes as opposed to the intact endosomes. However, carbonate treatment did not completely deplete luminal content, as determined by the presence of residual LDL.
Figure 4.
Representative Western blots showing distribution of the transcytotic pIgR, the recycling receptors, LDLR and TfR, and apo B-100 (LDL) in intact endosome fractions and in endosome membranes. The same amount of protein (1 μg) was loaded on each lane. Endosome membranes were isolated by means of high-pH sodium carbonate treatment, by which 60–70% of the contents was removed.
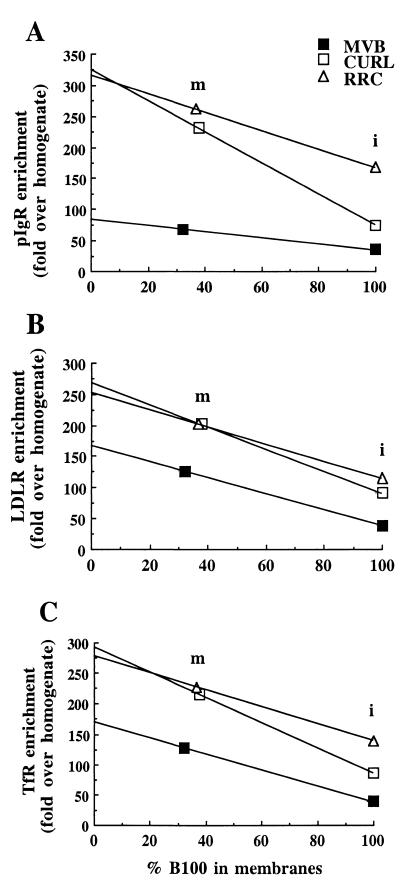
To reliably compare the density of the receptors in the membranes themselves under conditions where no luminal contents would be present, we need to correct for this technical limitation. Thus, we can graphically extrapolate to 0% apo B-100, i.e., the hypothetical condition where removal of luminal contents is complete. In all cases, that is, for the recycling LDLR and TfR, and for the transcytotic pIgR, the lines for CURL and RRC nearly converge when extrapolated to 0% apo B-100, which indicates that the density of these receptors in the membranes of the two fractions is nearly identical (Fig. 5). Note that these data do not provide the absolute density of receptors in the membrane, and therefore do not allow comparisons of the absolute densities among different receptors. Rather, the data enable us to compare the relative density of a particular receptor in the membranes of the three different endosomal fractions. These findings strongly suggest that these receptors are not sorted in the plane of the membrane between CURL and RRC fractions, and thus most likely between the BEE and the tubular compartment in vivo. Rather, the major difference between BEE and tubular compartment could be formation of the tubular geometry, whose large surface and small volume leads to segregation of soluble contents from membranes, therefore supporting the model of receptor sorting by default. In contrast, the density of LDLR and TfR in the MVB membranes remained less than two-thirds of that in the two other fractions, and less than one-third for pIgR, suggesting that a sorting event in the plane of the membrane takes place during MVB formation. This might involve, at least in part, the budding of smaller vesicles into the lumen of the MVB, with these intravesicular vesicles being relatively depleted of recycling and transcytotic receptors. On the other hand, it is noteworthy that pIgR behaves similarly to the recycling receptors, suggesting that in RRC, and therefore in the tubular compartment, pIgR has not been yet sorted from the recycling receptors.
Figure 5.
Graphic representations showing the density of receptors versus the percentage of LDL remaining in the membranes. The x axis is the % apo B-100 (LDL) remaining in the membranes (m), taking the intact (noncarbonate stripped) fraction (i) as 100% for each endosome fraction. The y axis is the relative density of each receptor, expressed as fold enrichment over the total homogenate.
DISCUSSION
Previous identification and analysis of epithelial endosomal compartments has been confined largely to morphological and immunocytochemical approaches. Although these powerful techniques have been elegantly applied to this problem, definitive confirmation of the existence of any new compartment, as well as analysis of its composition and properties, requires the isolation of such compartment by cell fractionation. Indeed, the combination of morphological and biochemical approaches has been a hallmark of many important advances in the field of membrane trafficking (28, 29).
Recent morphologic analyses have suggested that IgA that is transcytosing from the basolateral to the apical surface moves through three compartments: BEE, tubular compartment, and ARE. The tubular compartment has been recognized only very recently, and therefore its properties and even its very existence as a distinct compartment are not well established (4, 5). Isolation of a fraction enriched in the tubular compartment therefore would be a significant step in confirming its existence, and in furthering our understanding of the endocytic and transcytotic pathways in epithelial cells. A critical step in the discovery of the tubular compartment as an entity distinct from the ARE was the demonstration that the tubular compartment contained high concentrations of both recycling receptors and the transcytotic pIgR, whereas the ARE contained high amounts of pIgR, but was depleted in recycling receptors. The tubular compartment also lacks molecules destined for the degradative pathway (5) (K. Dunn, personal communication). Therefore, the tubular compartment should be considered a recycling compartment, but not an exclusively transcytotic compartment.
The discovery of the tubular compartment used cultured Madin-Darby canine kidney cells, which are particularly useful to morphologic analysis, but are less well suited for cell fractionation. Instead, we have taken advantage of a method previously developed in our laboratory to obtain highly purified endosomal subfractions, including CURL, MVB, and RRC. The RRC was enriched in both recycling and transcytotic receptors, and so it seemed likely that it contained separate recycling and transcytotic elements. However, our immunoisolation experiments establish that virtually all elements in the RRC contain both transcytotic pIgR as well as recycling receptors. Elements that contained only transcytotic pIgR were essentially not detected, indicating that ARE, or another purely transcytotic compartment, is not present in RRC. Moreover, we studied by Western blotting the distribution of proteins shown as involved in the regulation of endosome trafficking. Our results are clearly indicative of a recycling nature for RRC. Our findings agree in part with two recent reports in which a detailed analysis on the distribution of several proteins in these endosome fractions was carried out (21, 30). The comparative analysis among the three fractions guided those authors to conclude that RRC is a fraction involved in both recycling and transcytosis (21). Unlike our present work, these previous studies did not use immunoisolation or other techniques to determine whether recycling and transcytotic proteins actually are contained on identical elements and could not have discerned whether the RRC contained separate recycling and transcytotic elements. Therefore, the distinction between the tubular compartment involved in recycling and the ARE involved only in transcytosis was not considered in these earlier studies. Finally, our exclusive analysis of the protein enrichments in the endosome fractions over the liver homogenate gave us a valuable indication of the abundance and relevance of recycling elements in RRC. When these data are considered in the context of the recent division of the transcytotic pathway into three distinct compartments, the simplest interpretation is that the RRC largely corresponds to the tubular compartment.
Thus, our results suggest that both transcytosed and recycled receptors are in fact present on the same compartment before final delivery to the canalicular/apical plasma membrane domain by elements containing putatively only the pIgR/IgA complexes. Our findings are compatible with the description in hepatocytes of pericentriolar tubules emerging from a juxtanuclear compartment and mostly containing pIgR/IgA complexes on their way to the canalicular plasma membrane (10). Whether these pericentriolar tubules are physically connected to the juxtanuclear compartment cannot be addressed by our approach of cell fractionation. In fact, our current understanding of the nature of the recycling endosome in nonpolarized cells, or the tubular compartment in polarized epithelial cells, has been very limited because such isolated compartments had not been recognized previously. Altogether, our data greatly bolster the argument for the existence and importance of the tubular compartment as a sorting station in both rat liver and cultured Madin-Darby canine kidney cells (5, 31).
Our isolation of a fraction highly enriched in the tubular compartment already has allowed us to address a long-standing question in our understanding of the endocytic pathways: the nature of the mechanism by which proteins enter the so-called tubular compartment. One type of model is that membrane proteins are actively segregated into the tubules, or excluded from them (32). Alternatively, membrane proteins could enter the tubule nonselectively (33). As the RRC and other fractions that we isolate contain variable amounts of contents, such as LDL, we removed most of these contents by stripping with a high-pH carbonate wash. Remarkably, when we compared the CURL fraction (enriched in BEE) and the RRC fraction (enriched in the tubular compartment), we found that the fractions did not differ in their enrichment in three transmembrane receptors (pIgR, LDLR, and TfR). Hence, it appears that these proteins were neither actively included nor excluded from the tubular compartment as it formed from the BEE. Rather, the proteins appear to enter the tubules nonselectively, though we cannot completely exclude other models. This conclusion is also compatible with previous results obtained by morphological methods (5). However, biochemical analysis of cell fractions prepared from tissue permits independent and more precise quantitation and avoids any potential for artifacts associated with using transfected cells in culture.
A very different situation was observed with the MVB fraction, where the density of all three receptors was consistently lower than in the CURL and RRC fractions. The most likely explanation is that the MVB fraction contains many internalized membrane vesicles, which are selectively de-enriched in pIgR, LDLR, and TfR, and instead probably contain proteins destined for the degradative pathway, such as EGFR (34).
As in any subcellular fractionation procedure, it is possible that elements are altered during the homogenization and fractionation process. For example, the tubular compartment may be in continuity with the BEE, either transiently or even in a relatively stable way. This continuity may have been destroyed during homogenization, and the tubules sheared off from the BEE. However, it seems unlikely that this process would materially alter our basic conclusions.
Acknowledgments
Special thanks go to Drs. J. Herz, W. Hong, R. Jahn, J.-P. Kraehenbuhl, I. Mellman, R. Parton, B.-H. Toh, I.S. Trowbridge, and T. Weimbs for their generous gifts of reagents. We thank K. Dunn, S. Hansen, F. Luton, and S. van Ijzendorn for comments during the preparation of the manuscript. This work was supported by National Institutes of Health Grants R01AI25144 and R01AI36953. M.V. was the recipient of a fellowship from the Ministry of Education and Culture (Spain).
ABBREVIATIONS
- ARE
apical recycling endosome
- BEE
basolateral early endosome
- apo
apolipoprotein
- CURL
compartment of uncoupling of receptor and ligand
- EGFR
epidermal growth factor receptor
- LDL
low density lipoprotein
- LDLR
LDL receptor
- MVB
multivesicular bodies
- pIgR
polymeric Ig receptor
- RRC
receptor-recycling compartment
- Tf
transferrin
- TfR
Tf receptor
References
- 1.Robinson M S, Watts C, Zerial M. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G, Katz L A, Mostov K E. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Futter C E, Gibson A, Allchin E H, Maxwell S, Ruddock L J, Odorizzi G, Domingo D, Trowbridge I S, Hopkins C R. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson A, Futter C E, Maxwell S, Allchin E H, Shipman M, Kraehenbuhl J-P, Domingo D, Odorizzi G, Trowbridge I S, Hopkins C R. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barroso M, Sztul E. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geuze H J, Slot J W, Strous G J A M, Peppard J, von Figura K, Hasilik A, Schwartz A L. Cell. 1984;37:195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe C A, Connolly T P, Hubbard A L. J Cell Biol. 1985;101:2113–2123. doi: 10.1083/jcb.101.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr V A, Scott L J, Hubbard A L. J Biol Chem. 1995;270:27834–27844. doi: 10.1074/jbc.270.46.27834. [DOI] [PubMed] [Google Scholar]
- 10.Hemery I, Durand-Schneider A M, Feldmann G, Vaerman J P, Maurice M. J Cell Sci. 1996;109:1215–1227. doi: 10.1242/jcs.109.6.1215. [DOI] [PubMed] [Google Scholar]
- 11.Chao Y S, Windler E E, Chen G C, Havel R J. J Biol Chem. 1979;254:11360–11366. [PubMed] [Google Scholar]
- 12.Kovanen P T, Brown M S, Goldstein J L. J Biol Chem. 1979;254:11367–11373. [PubMed] [Google Scholar]
- 13.Belcher J D, Hamilton R L, Brady S E, Hornick C A, Jaeckle S, Schneider W J, Havel R J. Proc Natl Acad Sci USA. 1987;84:6785–6789. doi: 10.1073/pnas.84.19.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havel R J, Eder H A, Bragdon J H. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäckle S, Runquist E, Brady S, Hamilton R L, Havel R J. J Lipid Res. 1991;32:485–498. [PubMed] [Google Scholar]
- 16.Fujiki Y, Hubbard A L, Fowler S, Lazarow P B. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Enrich C, Jäckle S, Havel R J. Hepatology. 1996;24:226–232. doi: 10.1002/hep.510240136. [DOI] [PubMed] [Google Scholar]
- 19.Peterson G L. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Jäckle S, Runquist E A, Miranda-Brady S, Havel R J. J Biol Chem. 1991;266:1396–1402. [PubMed] [Google Scholar]
- 21.Pol A, Ortega D, Enrich C. Biochem J. 1997;323:435–443. doi: 10.1042/bj3230435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havel R J, Hamilton R L. Hepatology. 1988;8:1689–1704. doi: 10.1002/hep.1840080637. [DOI] [PubMed] [Google Scholar]
- 23.Enrich, C., Pol, A., Calvo, M., Pons, M. & Jäckle, S. (1999) Hepatology, in press. [DOI] [PubMed]
- 24.Ullrich O, Reinsch S, Urbé S, Zerial M, Parton R G. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini D D. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casanova J E, Wang X, Kumar R, Bhartur S G, Navarre J, Woodrum J E, Altschuler Y, Ray G S, Goldenring J R. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen A, Lippé R, Christoforidis S, Gaullier J M, Brech A, Callaghan J, Toh B-H, Murphy C, Zerial M, Stenmark H. Nature (London) 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 28.Pierre P, Mellman I. Curr Opin Immunol. 1998;10:145–153. doi: 10.1016/s0952-7915(98)80242-2. [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Tellam J, Livingstone C, Slot J W, Gould G W, James D E. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pol A, Ortega D, Enrich C. Biochem J. 1997;327:741–746. doi: 10.1042/bj3270741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Ijzendoorn S C D, Hoekstra D. Trends Cell Biol. 1999;9:144–149. doi: 10.1016/s0962-8924(99)01512-3. [DOI] [PubMed] [Google Scholar]
- 32.Geuze H J, Slot J W, Schwartz A L. J Cell Biol. 1987;104:1715–1723. doi: 10.1083/jcb.104.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn K W, McGraw T E, Maxfield F R. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKanna J A, Haigler H T, Cohen S. Proc Natl Acad Sci USA. 1979;76:5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]