Abstract
Interaction of syntaxin 1 with the α1D subunit of the voltage-gated L type Ca2+ channel was investigated in the pancreatic β cell. Coexpression of the enhanced green fluorescent protein-linked α1D subunit with the enhanced blue fluorescent protein-linked syntaxin 1 and Western blot analysis together with subcellular fractionation demonstrated that the α1D subunit and syntaxin 1 were colocalized in the plasma membrane. Furthermore, the α1D subunit was coimmunoprecipitated efficiently by a polyclonal antibody against syntaxin 1. Syntaxin 1 also played a central role in the modulation of L type Ca2+ channel activity because there was a faster Ca2+ current run-down in cells incubated with antisyntaxin 1 compared with controls. In parallel, antisyntaxin 1 markedly reduced insulin release in both intact and permeabilized cells, subsequent to depolarization with K+ or exposure to high Ca2+. Exchanging Ca2+ for Ba2+ abolished the effect of antisyntaxin 1 on both Ca2+ channel activity and insulin exocytosis. Moreover, antisyntaxin 1 had no significant effects on Ca2+-independent insulin release trigged by hypertonic stimulation. This suggests that there is a structure–function relationship between the α1D subunit of the L type Ca2+ channel and the exocytotic machinery in the pancreatic β cell.
Syntaxin 1 plays a central role in exocytosis from pancreatic β cells (1–3). Thus, it has been demonstrated that exposure of permeabilized mouse pancreatic β cells to antibodies against syntaxin 1 significantly inhibits Ca2+-induced insulin secretion (1). Furthermore, synthetic peptides, corresponding to the H3 region of the carboxyl-terminal domain of syntaxin 1, markedly inhibit Ca2+-dependent insulin secretion from permeabilized pancreatic β cells (2).
In neurons, syntaxin interacts not only with other synaptic proteins but also with voltage-dependent Ca2+ channels (4, 5). Several studies indicate that the interaction between syntaxin and voltage-dependent Ca2+ channels results in a functional modulation of the Ca2+ current. Thus, electrophysiological studies have shown that syntaxin modulates N, LC, and Q type Ca2+ currents in Xenopus oocytes, coexpressing voltage-gated Ca2+ channels and syntaxin (6–8). It also has been reported that intracellular application of a synthetic peptide fragment of the α1C subunit diminished exocytosis from the pancreatic β cell via interference with the interaction of the L type Ca2+ channel with the exocytotic machinery. However, analysis of mRNAs encoding the α1C and α1D subunits implies that the Ca2+-conducting subunits of the L type Ca2+ channel in the pancreatic β cell mainly consist of the α1D subunit (9, 10).
We now show that syntaxin 1 colocalizes and associates with the α1D subunit of the voltage-gated L type Ca2+ channel and thereby modulates not only Ca2+ channel activity but also insulin release in a Ca2+-dependent manner.
MATERIALS AND METHODS
Preparation of Islets and Single β cells.
Islets of Langerhans and single pancreatic β cells were isolated from adult obese mice (gene symbol ob/ob) as described previously (11).
Coexpression of α1D Subunit–Enhanced Green Fluorescent Protein (EGFP) and Enhanced Blue Fluorescent Protein (EBFP)–Syntaxin 1 and Fluorescence Microscopy.
The cDNA for hamster α1D3a (provided by J. Dillon, New England Medical Center, Boston) was flanked with the human cytomegalovirus (CMV) promoter and the bovine growth hormone poly(A) site to enable expression in mammalian cells. A HindIII site was introduced by site-directed mutagenesis at the nucleotides coding for the last amino acid and the stop codon, which allowed in-frame fusion with the EGFP cDNA and generation of pCMV α1D3a EGFP. To create pCMV EBFP-syntaxin 1A, a SmaI site was introduced into the EBFP cDNA at the nucleotides coding for Asp-235 and Glu-237, generating pCMV EBFP0. The cDNA for rat syntaxin 1A (gift from R. H. Scheller, Stanford University, Stanford, CA) was removed from pRcCMV syntaxin 1A by digestion with EcoRV and XbaI and fused in-frame with the EBFP cDNA of the SmaI/XbaI-opened pCMV EBFP0. Cultured single pancreatic β cells were cotransfected with pCMV α1D3a EGFP and pCMV EBFP-syntaxin 1A overnight by the lipofectamine technique. The cotransfected cells were monitored for EGFP/EBFP fluorescence 48 h after the start of transfection by using a Leica Fluovert FU microscope (Leica, Deerfield, IL) and PL Fluotar 100/1.32 oil lens (Leitz) equipped with a LSR AstroCam TE3/A/S charge-coupled device camera (LSR, Cambridge, U.K.). Filter settings for EGFP fluorescence measurements were: excitation, HQ470/40; dichroic mirror, Q495LP; and emission, HQ525/50. Filter settings for EBFP fluorescence measurements were: excitation, D399/22; dichroic mirror, 420DLCP; and emission, E430LP. Colocalization of α1D3a EGFP and EBFP-syntaxin 1A was studied by applying the deconvolution method on a stack of 15 images (200-nm vertical distance) using the iterative Tikhonov–Miller restoration procedure (Huygens System 2; Scientific Volume Imaging, Hilversum, The Netherlands).
Density Gradient Subcellular Fractionation.
Subcellular fractionation, using a linear sucrose gradient, was performed as described previously (12).
Gel Electrophoresis and Western Blot Analysis.
Proteins (90 μg/slot) from individual fractions were separated in discontinuous gels consisting of a stacking gel (3% acrylamide) and a separating gel, SDS-polyacrylamide gradient gel (6.6–13.3% acrylamide). The separated proteins then were electroblotted to hydrophobic polyvinylidene difluoride membrane (Hybond-P). The blots were blocked by incubation for 2 h with 7.5% nonfat milk powder in tris(hydroxymethyl)aminomethane-buffered saline and then incubated overnight at 4°C with polyclonal anti-α1D subunit (1:200; Alomone Labs, Jerusalem), anti-NKα1 (1:1,000; courtesy of M. Caplan, Yale University, New Haven, CT), and monoclonal antisyntaxin (clone HPC-1, 1:500; Sigma), respectively. After washing with tris(hydroxymethyl)aminomethane-buffered saline, the blots were incubated with the secondary antibodies [horseradish peroxidase-conjugated goat anti-mouse IgG or horseradish peroxidase-conjugated goat anti-rabbit IgG; 1:50,000 in tris(hydroxymethyl)aminomethane-buffered saline; Bio-Rad] at room temperature for 1 h. The immunoreactive bands were visualized with ECL Plus Western blotting detection system (Amersham Pharmacia).
Immunoprecipitation.
The pooled plasma membrane fractions (from no. 4 to no. 9) from mouse pancreatic islets of Langerhans were solubilized in 1% CHAPS after 2 h of incubation in the buffer containing 1 mM CaCl2, 170 mM tris(hydroxymethyl)aminomethane-HCl, pH 7.4. The CHAPS extract was subjected to immunoprecipitation with polyclonal antisyntaxin 1 (Alomone Labs), nonimmune rabbit IgG, and protein A-Sepharose (Amersham Pharmacia) or with monoclonal antisyntaxin 1 (Sigma) and nonimmune mouse IgG precoupled to protein G-Sepharose (Amersham Pharmacia). To investigate possible binding of syntaxin 1 to polyclonal antisyntaxin 1, monoclonal antisyntaxin 1, or nonimmune IgG coupled to protein A- or G-Sepharose and of α1D subunit to syntaxin 1, SDS/PAGE and Western blot analyses were performed.
Patch-Clamp and Amperometry Recordings. Whole-cell Ca2+ currents were recorded as described previously (13) in pancreatic β cells incubated with monoclonal antisyntaxin 1 (1:200; Sigma), monoclonal antitubulin (1:200; Sigma), normal extracellular solution, or monoclonal antisyntaxin 1 (1:200) preabsorbed with glutathione S-transferase (GST)–syntaxin 1A (240 μg/ml diluted antibody), respectively, for 1 h.
Amperometry recordings were performed as described previously (13). Six to 12 h before the experiment, cells were preincubated in 5-hydroxy-dl-tryptophan (5-HTP; Sigma). 5-HTP was prepared as stock solutions with a concentration of 25 mM and then added to the culture medium at a final concentration of 1 mM. 5-HTP is converted to serotonin in the β cell, and it is well established that serotonin is loaded primarily into secretory vesicles, being cosecreted with insulin by exocytosis (14). Cells were stimulated by a transient (30-s) pulse of extracellular solution, with the K+ concentration elevated to 50 mM or with 500 mOsM sucrose by using a flow-injector (Transjector, Eppendorf, Germany).
Cell Permeabilization and Radioimmunoassay.
Cells were electropermeabilized as described previously (15). After permeabilization, the cell suspension was spun down, resuspended, and incubated with intracellular buffer with or without monoclonal antisyntaxin (1:200) or mouse IgG (1:200) in the absence of both Ca2+ and Ba2+ or in the presence of 10 μM Ca2+ and/or 100 μM Ba2+ at 37°C for 10 min. Insulin release was measured by standard radioimmunoassay (15).
RESULTS
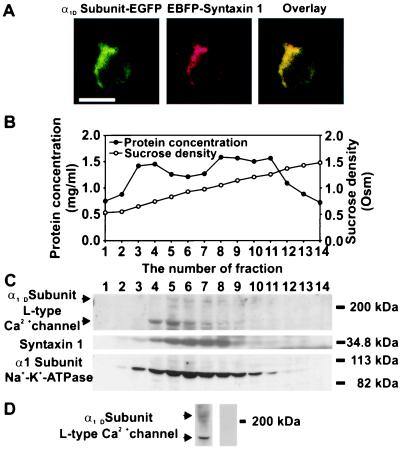
Localization of the α1D Subunit and Syntaxin 1 in Pancreatic β Cells. Coexpression of the EGFP-linked α1D subunit and EBFP-linked syntaxin 1 together with the deconvolution of fluorescence images showed the profiles of α1D subunit-EGFP and EBFP-syntaxin 1 in the same β cell (Fig. 1A). Green (Fig. 1A Left) and red (Fig. 1A Center) represent the digital pseudocolor for the fluorescence emitted from EGFP and EBFP, respectively. The two fluorescence signals were observed mainly in the plasma membrane region of the cell. The yellow color (Fig. 1A Right) obtained by overlaying the EGFP signal with the EBFP signal indicated colocalization of the expressed α1D subunit-EGFP with EBFP-syntaxin 1 in the plasma membrane.
Figure 1.
Localization of the α1D subunits of the L type Ca2+ channel and syntaxin 1 in the pancreatic β cell. (A) Fluorescence images of the coexpressed α1D subunit-EGFP with EBFP-syntaxin 1 in the same pancreatic β cell. The cotransfected cells were monitored for EGFP/EBFP fluorescence 48 h after the start of transfection. The green color is used as the digital pseudocolor for the fluorescence emitted from EGFP (Left) and the red is used as the digital pseudocolor for the fluorescence emitted from EBFP (Center). The yellow color (Right), obtained after overlaying the deconvoluted EGFP and EBFP signals, indicated the colocalization of the expressed α1D-EGFP with EBFP-syntaxin 1 in the plasma membrane. (Bar = 10 μm.) (B) Distribution of protein and sucrose density in 14 fractions collected from a linear sucrose density gradient (0.53–1.48 M). The postnuclear supernatants were loaded onto a linear sucrose density gradient, centrifuged at 120,000 × g, and fractions were collected. The sucrose density was measured by refractometry. (C) Subcellular distribution of the two isoforms (upper arrow, 210 kDa; lower arrow, 180 kDa) of the α1D subunit, syntaxin 1, and Na+-K+-ATPase α1 subunit in 14 fractions from mouse pancreatic islets. The immunoreactive bands were visualized with ECL Plus Western blotting detection system. The experiments were repeated three times. (D) Specific immunoreactivity of the α1D subunit of the L type Ca2+ channel in the plasma membrane fraction (no. 6) from mouse islets. Polyclonal antibody against the α1D subunit recognized two major bands of proteins (Left) with molecular masses of 210 (upper arrow) and 180 kDa (lower arrow). Specific interaction of the antibody with the α1D subunit was inhibited fully by preabsorption (Right) with the antigen peptide CND1.
To confirm further the colocalization of the α1D subunit and syntaxin 1 in the plasma membrane and to characterize the membrane fractions to be used in immunoprecipitation experiments, the subcellular distribution pattern, using a linear sucrose gradient (Fig. 1B), of the α1D subunit and syntaxin 1 was visualized by Western blot analysis. The antibody against the α1D subunit recognized two bands of proteins with molecular masses of 210 and 180 kDa (Fig. 1C Top), respectively, concentrated mainly in fractions 4–8. The distribution of immunoreactivity for syntaxin 1 was similar to that of the α1D subunit and, thus, predominately located in fractions 4–8 (Fig. 1C Middle) corresponding to the plasma membrane. The Na+-K+-ATPase α1 (Nkα1) subunit was used as a marker for the plasma membrane (Fig. 1C Bottom) (16).
That the antibody against the α1D subunit specifically recognized two bands of proteins (Fig. 1 C Top and D Left) with molecular masses of 210 and 180 kDa, respectively, is in agreement with previous findings (17). To test further the specificity of the antibody against the α1D subunit, the blot was incubated with anti-α1D subunit antibodies preabsorbed with the antigen peptide, CND1. After preabsorption, no signal from the α1D subunit could be detected (Fig. 1D Right).
Association of Syntaxin 1 with the α1D Subunits in the Plasma Membrane of Pancreatic β Cells.
To test for association of syntaxin 1 with the α1D subunit in the plasma membrane, immunoprecipitation was performed with a polyclonal antibody against the intracellular domain of syntaxin 1 and a mAb to the extracellular domain of syntaxin 1. As shown in Fig. 2, the polyclonal antibody against the intracellular domain of syntaxin 1 not only immunoprecipitated syntaxin 1 (Fig. 2, lane 1, lower blot), but also pulled down the two isoforms [Fig. 2, upper arrow (210 kDa) and lower arrow (180 kDa)] of the α1D subunit (Fig. 2, lane 1, upper blot). In contrast, the mAb to the extracellular domain of syntaxin 1 only immunoprecipitated syntaxin 1 (Fig. 2, lane 3, lower blot) without a detectable associated α1D subunit (Fig. 2, lane 3, upper blot). Nonimmune rabbit or mouse IgG could not pull down either syntaxin 1 (Fig. 2, lanes 2 and 4, lower blots) or the α1D subunit (Fig. 2, lanes 2 and 4, upper blots).
Figure 2.
Association of syntaxin 1 with the α1D subunit of the voltage-gated L type Ca2+ channel in plasma membrane of mouse pancreatic β cells. The pooled plasma membrane fractions (four to eight) were solubilized in CHAPS and then subjected to immunoprecipitation with polyclonal antisyntaxin 1 (lane 1), nonimmune rabbit IgG (lane 2), monoclonal antisyntaxin 1 (lane 3), and nonimmune mouse IgG (lane 4). Syntaxin 1 (lower blots) and the α1D subunit (upper blots) were visualized by SDS/PAGE and Western blotting and the ECL Plus detection system. Syntaxin 1 bound to both the polyclonal antisyntaxin 1 (lane 1) and the monoclonal antisyntaxin 1 (lane 3). The two isoforms (upper arrow, 210 kDa; lower arrow, 180 kDa) of the α1D subunit were pulled down only by the polyclonal antisyntaxin 1 (lane 1). The experiments were repeated two times.
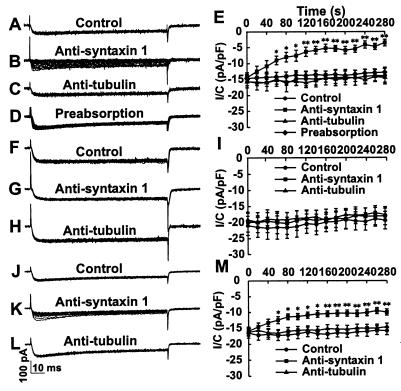
Effects of Antisyntaxin 1 on L type Ca2+ Currents in Pancreatic β Cells. Pancreatic β cells were incubated with normal extracellular solution in the absence (Fig. 3A) or presence (Fig. 3C) of the mAb against tubulin (1:200) and with the monoclonal antisyntaxin 1 (1:200) preabsorbed with GST-syntaxin 1A (Fig. 3D, 240 μg/ml diluted antibody), respectively. Typical L type Ca2+ current traces evoked by repetitive depolarizing voltage pulses from a holding potential of −70 mV to 0 mV (100 ms, 0.05 Hz) were not significantly different between the three different groups of cells, as shown by the 15 superimposed current traces. In contrast, the same voltage protocol resulted in a gradual decrease in the Ca2+ currents (Fig. 3B) after incubation with the mAb against syntaxin 1 (1:200). Compiled data, as shown in Fig. 3E, show that L type Ca2+ currents ran down significantly faster in cells incubated with antisyntaxin 1 compared with control cells or cells incubated with antitubulin.
Figure 3.
Effect of antisyntaxin 1 on L type Ca2+ currents in mouse pancreatic β cells. (A, C, and D) Typical whole-cell patch-clamp recordings with 15 superimposed L type Ca2+ current traces, evoked by depolarizing voltage steps from −70 mV to 0 mV (100 ms, 0.05 Hz). Currents were similar after incubation of pancreatic β cells with extracellular solution, antitubulin (1:200), and antisyntaxin 1 (1:200) preabsorbed with GST-syntaxin 1A (240 μg/ml diluted antibody), respectively, for 1 h. (B) After incubation with antisyntaxin 1 (1:200), the Ca2+ currents decreased with the application of the depolarizing command voltage pulses. (E) Compiled data on changes in current amplitude showed that L type Ca2+ currents ran down faster in cells incubated with antisyntaxin 1 (■, n = 10) compared with the control cells (●, n = 9), cells incubated with antitubulin (▴, n = 10) and cells incubated with antisyntaxin 1 preabsorbed with GST-syntaxin 1A (♦, n = 11). The recordings were made in 10 mM Ca2+ at room temperature. (F–H) Representative whole-cell patch-clamp recordings using the same voltage step protocol, but exchanging Ca2+ for Ba2+, showed no difference in the obtained currents after the incubation of pancreatic β cells with extracellular solution, antisyntaxin 1, and antitubulin, respectively. (I) Time courses of L type Ca2+ currents in cells incubated with antisyntaxin 1 (■, n = 8) were not significantly different in amplitude from those in control cells (●, n = 8) and cells incubated with antitubulin (▴, n = 8). (J and L) Typical whole-cell patch-clamp recordings with 15 superimposed L type Ca2+ current traces, evoked by the above-mentioned voltage-step protocol, showed similar currents after the incubation of pancreatic β cells in extracellular solution containing 10 mM Ca2+/10 mM Ba2+ with and without antitubulin (1:200) for 1 h. (K) After incubation with antisyntaxin 1 (1:200) in the presence of 10 mM Ca2+/10 mM Ba2+, the Ca2+ currents decreased gradually after application of the depolarizing command voltage pulses. (M) Compiled data on changes in current amplitude showed that L type Ca2+ currents ran down faster in cells incubated with antisyntaxin 1 (■, n = 18) compared with control cells (●, n = 17) and cells incubated with antitubulin (▴, n = 18). The data are presented as means ± SEM. Statistical significance was evaluated by one-way ANOVA followed by least significant difference (LSD) test. ∗, P < 0.05; ∗∗, P < 0.01.
Previous studies have shown that the H3 region (N type Ca2+ channel-binding domain) of syntaxin is responsible for Ca2+-induced insulin release (2) and interacts with the α1B subunit of the N type Ca2+ channel in a Ca2+-dependent manner (18). To test whether the inhibitory effect of antisyntaxin 1 on the L type Ca2+ channel was Ca2+-dependent, Ca2+ was replaced with equimolar concentrations of Ba2+ (10 mM) in the extracellular solution. As shown in Fig. 3 F–H, 15 superimposed Ca2+ current traces evoked by the same voltage protocol showed similar currents after the incubation of pancreatic β cells with extracellular solution, antitubulin, and antisyntaxin 1, respectively. Compiled data show that the time courses of whole-cell L type Ca2+ currents in cells incubated with antisyntaxin 1 did not differ significantly from those in control cells or in cells incubated with antitubulin (Fig. 3I). Thus, the blocking effect of antisyntaxin 1 on Ca2+ channel activity disappeared after replacement of extracellular Ca2+ with Ba2+. Furthermore, 10 mM Ca2+ together with 10 mM Ba2+ in the extracellular solution could partly restore the effect of antisyntaxin 1 on Ca2+ currents. As seen in Fig. 3 J and L, the same voltage protocol as above produced similar superimposed current traces in the presence of antitubulin and under control conditions. By contrast, antisyntaxin 1 treatment promoted a significantly faster run-down of Ca2+ currents when recorded in 10 mM Ca2+ together with 10 mM Ba2+ (Fig. 3K), although this effect was smaller than that in extracellular solution containing 10 mM Ca2+ alone (Fig. 3B). Compiled data (Fig. 3M) show that L type Ca2+ currents decreased significantly faster in cells incubated with antisyntaxin 1 in comparison with control cells or cells incubated with antitubulin.
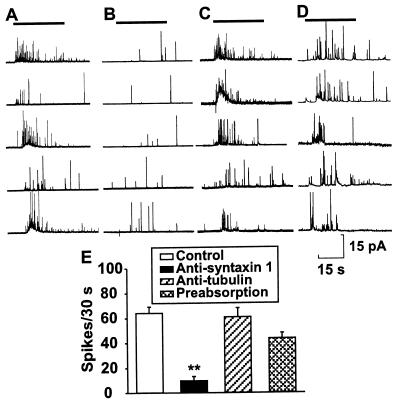
Effects of Antisyntaxin 1 and Anti-α1D Subunit of the L type Ca2+ Channel on Exocytosis of Insulin from Pancreatic β Cells. Fig. 4 shows amperometric recordings from single cells under control conditions (Fig. 4A) or when pretreated with antisyntaxin 1 (1:200, Fig. 4B), antitubulin (1:200, Fig. 4C), or antisyntaxin 1 (1:200) preabsorbed with GST-syntaxin 1 (Fig. 4D, 240 μg/ml diluted antibody), during a short pulse of 50 mM K+. Each amperometric spike represents the oxidation current of serotonin molecules released from individual vesicles. A pulse of 50 mM K+ potently and rapidly induced a similar magnitude of secretion in cells preincubated with extracellular solution (64.3 ± 5.0 spikes/30 s, n = 6), antitubulin (61.0 ± 7.3 spikes/30 s, n = 6), and antisyntaxin 1 preabsorbed with GST-syntaxin 1A (43.7 ± 4.5 spikes/30 s, n = 6), whereas preincubation with antisyntaxin (9.8 ± 3.4 spikes/30 s, n = 6) dramatically decreased the number of secretory events (P < 0.01) (Fig. 4E).
Figure 4.
Effect of monoclonal antisyntaxin 1 on exocytosis in single mouse pancreatic β cells preloaded with 5-HTP. Typical examples of the secretory response after stimulation with 50 mM K+ (as indicated by horizontal bars) in the control group (A), after treatment with antisyntaxin 1 (1:200) (B), after treatment with antitubulin (1:200) (C), and after treatment with antisyntaxin 1 (1:200) preabsorbed with GST-syntaxin 1A (D). (E) Compiled data of the number of spikes during a 30-s period under each experimental condition. The data are presented as means ± SEM (n = 6). Statistical significance was evaluated by one-way ANOVA followed by least significant difference test. ∗∗, P < 0.01 vs. control, antitubulin, and antisyntaxin 1 preabsorbed with GST-syntaxin 1A.
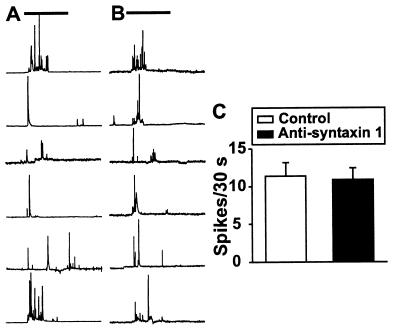
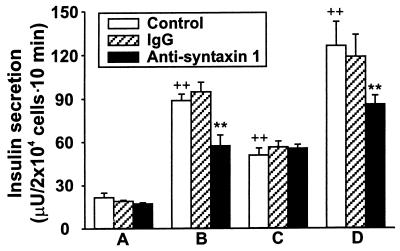
Our data with an antisyntaxin 1-induced decrease in secretory response evoked by depolarization by K+ in the intact pancreatic β cell using amperometric measurements raised the question of whether this effect resulted from a direct effect on the exocytotic machinery or whether it was partly a consequence of the decreased activity of the voltage-dependent Ca2+ channel. To address this question, we also tested the effect of monoclonal antisyntaxin 1 on Ca2+-independent insulin secretion. In single mouse pancreatic β cells preloaded with 5-HTP, hypertonic solution (500 mOsM sucrose) was employed to trigger Ca2+-independent exocytosis. Hypertonic solution-triggered exocytosis is independent of extracellular Ca2+ concentration, the Ca2+ channel blocker Cd2+, and manipulation of intracellular Ca2+ stores (19). Fig. 5 A and B shows typical examples of the secretory response after application of 500 mOsM sucrose (as indicated by horizontal bars) to the control group (Fig. 5A) and to the cells (Fig. 5B) treated with antisyntaxin 1 (1:200). As seen from the compiled data (Fig. 5C) of the number of spikes during a 30-s period of hypertonic stimulation, there is no significant difference between control and antisyntaxin 1-treated groups (11.4 ± 1.8 vs. 10.9 ± 1.6, n = 10, P > 0.05). Furthermore, insulin release was assessed in electroporated pancreatic β cells challenged by 10 μM Ca2+ and/or 100 μM Ba2+. Capacitance measurements have shown that Ba2+ can be used instead of Ca2+ to stimulate exocytosis in RINm5F insulin-secreting cells (20). The use of permeabilized cells eliminates ion fluxes through ion channels and, thus, allows analysis of direct effects on the exocytotic machinery. As shown in Fig. 6, the antisyntaxin 1 treatment (1:200) produced no significant influence on basal insulin secretion in the absence of both Ca2+ and Ba2+, as compared with control and IgG treatment (1:200). However, insulin release from permeabilized pancreatic β cells treated with antisyntaxin 1 in the presence of 10 μM Ca2+ was attenuated significantly in comparison with that evoked from cells treated with IgG or preincubated in control buffer. Interestingly, Ba2+ (100 μM)-induced insulin release was not significantly different between cells preincubated with antisyntaxin 1, IgG, or normal intracellular buffer. The inhibitory effect of antisyntaxin on insulin release was partly restored by adding 10 μM Ca2+ to the solution containing 100 μM Ba2+.
Figure 5.
Effect of monoclonal antisyntaxin 1 on exocytosis induced by application of hypertonic solution in single mouse pancreatic β cells preloaded with 5-HTP. Typical examples of the secretory response after application of 500 mOsM sucrose (as indicated by horizontal bars) in the control group (A) and in the cells treated with antisyntaxin 1 (1:200) (B). (C) Compiled data of the number of spikes during a 30-s period under each experimental condition. The data are presented as means ± SEM (n = 10). Statistical significance was evaluated by Student’s t test. There is no significant difference between the two groups.
Figure 6.
Effect of mAb against syntaxin 1 on insulin release from electropermeabilized mouse pancreatic β cells. Permeabilized cells were incubated with normal intracellular buffer, monoclonal antisyntaxin 1 (1:200), and mouse IgG (1:200) in the absence of both Ca2+ and Ba2+ (A), in the presence of 10 μM Ca2+ (B) or 100 μM Ba2+ (C), and in the presence of 10 μM Ca2+ together with 100 μM Ba2+ (D). The released insulin was determined by radioimmunoassay. The data are presented as means ± SEM (n = 6). Statistical significance was evaluated by one-way ANOVA followed by least significant difference test. ∗∗, P < 0.01 vs. the absence of both Ca2+ and Ba2+, ++, P < 0.01 vs. control and IgG groups in the presence of 10 μM Ca2+.
Whereas treatment with antisyntaxin 1 reduced the secretory response in intact cells by approximately 80%, the same treatment led to a reduction by about 40% (10 μM Ca2+) and 30% (10 μM Ca2+ plus 100 μM Ba2+) in permeabilized cells. Thus, it appears that part of the inhibitory effect on insulin secretion from intact β cells depends on reduced L type Ca2+ currents induced by antisyntaxin treatment.
Anti-α1D was used to test whether the α1D subunit of the voltage-gated Ca2+ channel functions as a structural base for docking/priming/fusion of insulin-containing granules with the plasma membrane. Insulin release, measured by radioimmunoassay, in permeabilized β cells incubated with anti-α1D subunit (1:100) did not differ significantly from that in control cells or cells treated with IgG (1:100) (data not shown). These negative findings do not exclude that the α1D subunit has a structural function in insulin exocytosis but, rather, may suggest that anti-α1D does not recognize the α1D subunit in situ and/or that the epitope on the α1D subunit recognized by the antibody is not an active site in exocytosis.
DISCUSSION
We now show that syntaxin 1 and the α1D subunit of the L type Ca2+ channel colocalize in the β cell plasma membrane by means of coexpression of the EGFP-linked α1D subunit with the EBFP-linked syntaxin 1 and Western blot analysis together with subcellular fractionation. These data provide the molecular basis for a modulatory effect of syntaxin 1 on L type Ca2+ channel activity. Interestingly, the polyclonal antibody against the intracellular domain of syntaxin 1 efficiently coimmunoprecipitated the α1D subunit from the plasma membrane fractions, whereas the mAb against the extracellular domain of syntaxin 1 did not. In this context, it is interesting to note that microinjection of the fragment 1–267 of syntaxin 1, lacking only the transmembrane domain, had no effect on the coexpressed LC type Ca2+ channel, but the expressed full-length syntaxin 1 significantly modified the coexpressed LC type Ca2+ channel activity in the Xenopus oocyte (7). The binding of the mAb against the extracellular domain of syntaxin 1 thus may mask the syntaxin-interaction site at the α1D subunit and/or result in a conformational change of the interaction site of syntaxin, thereby preventing protein–protein interaction between syntaxin 1 and the α1D subunit. Indeed, the interaction site of syntaxin 1 with α1B and α1C subunits of the Ca2+ channel has been shown to be close to the extracellular domain and far from the intracellular domain of syntaxin 1 (21).
In agreement with the coimmunoprecipitation data, our electrophysiological results clearly demonstrate the presence of a functional interaction between syntaxin 1 and the L type Ca2+ channel to maintain functional L type Ca2+ channel activity. Cells incubated with antisyntaxin 1 in 10 mM extracellular Ca2+ showed a significant decrease in L type Ca2+ currents. That this effect on the L type Ca2+ channel reflected a specific interaction between antisyntaxin 1 and syntaxin 1 was suggested from the experiments demonstrating no effect when cells were incubated in normal extracellular solution in the presence of antitubulin or antisyntaxin 1 preabsorbed with syntaxin 1. Antisyntaxin recognizes a part of the carboxyl terminus (residues 181–288) of syntaxin 1 (22, 23), which, in turn, has been demonstrated to overlap, or to be situated nearby, the site at which the syntaxin molecule interacts with the α1B subunit of the N type Ca2+ channel in a Ca2+-dependent manner (18, 21). Our results suggest that syntaxin 1 also forms a complex with the α1D subunit of the L type Ca2+ channel and thereby prevents Ca2+ channel run-down. This effect is likely to be Ca2+-dependent because antisyntaxin 1 failed to affect Ca2+ channel activity when Ca2+ was exchanged for Ba2+. In the presence of Ba2+ alone, the Ca2+-dependent interaction between syntaxin and the α1D subunit can no longer take place and Ca2+-mediated Ca2+ channel run-down is not activated (24, 25). Therefore, antisyntaxin 1 is without inhibitory effect on the Ba2+ current. The effect of antisyntaxin 1 on the Ca2+ channel was restored partly by the addition of Ca2+ to the Ba2+ solution. Noteworthy is that binding of antisyntaxin 1 to syntaxin 1 occurred independent of Ca2+ because Western blot analysis shows that antisyntaxin 1 binds to syntaxin 1 in the absence of Ca2+.
The H3 region of the syntaxin 1 molecule, corresponding to amino acids 191–285, has been shown to be important in the interactions of syntaxin with other proteins that participate in the secretory process (2). This region was demonstrated to be involved in Ca2+-induced secretion in permeabilized β cells (2). In the present study we investigated the effects of antisyntaxin 1 on both intact and permeabilized mouse β cells, showing a more pronounced inhibitory effect in intact cells. Thus, whereas insulin release from permeabilized cells treated with antisyntaxin was decreased by approximately 40%, challenged by 10 μM Ca2+ alone, antisyntaxin 1 reduced the secretory response by about 80% in intact cells. In accordance with Ca2+ channel data, Ba2+-induced exocytosis was not inhibited by antisyntaxin 1. Furthermore, there is no significant difference in Ca2+-independent insulin release, evoked by hypertonic stimulation, between antisyntaxin-treated and control groups. The antisyntaxin 1 treatment did not influence basal insulin secretion from permeabilized mouse β cells in the absence of both Ca2+ and Ba2+. It, therefore, is reasonable to assume that the more pronounced inhibitory effect in intact cells was due to the decreased activity of the L type Ca2+ channel by treatment with antisyntaxin 1. In agreement with the patch-clamp data, addition of Ca2+ to the Ba2+ buffer regained the inhibitory effect of antisyntaxin on insulin release from permeabilized cells. The coexpressed α1C subunit and syntaxin 1 have been demonstrated to interact with each other in the Xenopus oocyte (7, 8). The in vitro binding of a synthetic peptide fragment (LC753–893) of the α1C subunit to GST-syntaxin 1 also has been documented (8). Moreover, it has been shown that the synthetic peptide of the α1C subunit interfered with the interaction between the native LC type Ca2+ channel and the exocytotic machinery in the pancreatic β cell (8). However, it is worth noting that there is a much higher level of mRNA encoding the α1D subunit than the α1C subunit in the pancreatic β cell (9, 10). Our preliminary data indicate a stronger α1D subunit signal than α1C subunit signal in the plasma membrane of the pancreatic β cell (data not shown). Therefore, it is likely that the α1D subunit is the major α1 subunit interacting with syntaxin in the pancreatic β cell.
Interestingly, insulin release from control permeabilized cells evoked by 10 μM Ca2+ plus 100 μM Ba2+ was approximately equal to the sum of insulin release evoked by either divalent cation alone. This suggests that Ba2+ has a different binding site than Ca2+ in the activation of the exocytotic process. It indeed has been demonstrated that Ba2+ triggers exocytosis in permeabilized chromaffin cells by an effect on the exocytotic machinery that is different from that induced by Ca2+ (26). Furthermore, depletion of intracellular Ca2+ stores by thapsigargin fully blocks Ca2+-triggered exocytosis, but only slightly affects Ba2+-induced insulin secretion in this cell line (20).
We have shown that syntaxin 1 interacts with the α1D subunit of the voltage-gated L type Ca2+ channel and that binding of antisyntaxin 1 to syntaxin 1 interferes with the regulation of both the voltage-gated L type Ca2+ channel and the exocytotic machinery in insulin-secreting pancreatic β cells. This may reflect an interference with the assembly of the α1D subunit, syntaxin 1, and synaptic vesicle proteins, which are located in the insulin-containing granule, into a functioning core complex. This suggests that the LD type Ca2+ channel has a similar structure–function relationship with the exocytotic machinery as has been described previously for the neuronal N type Ca2+ channel (27). Thus, the ability of syntaxin to modulate overall neuroendocrine secretion seems to have a general explanation at the molecular level.
Acknowledgments
We are grateful to Dr. William A. Catterall for providing GST-syntaxin 1. This work was supported by grants from the Swedish Medical Research Council (03X-09891, 03X-09890, 03XS-12708, 72X-12549, and 19X-00034), the Swedish Diabetes Association, the Nordic Insulin Foundation Committee, Funds of Karolinska Institutet, Berth von Kantzows Foundation, Juvenile Diabetes Foundation International, the Swedish Society for Medical Research, and the Novo Nordisk Foundation.
ABBREVIATIONS
- EBFP
enhanced blue fluorescent protein
- EGFP
enhanced green fluorescent protein
- GST
glutathione S-transferase
- CMV
cytomegalovirus
- 5-HTP
5-hydroxy-dl-tryptophan
References
- 1.Martin F, Moya F, Gutierrez L M, Reig J A, Soria B. Diabetologia. 1995;38:860–863. doi: 10.1007/s001250050364. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Salinas E, Vazquez J, Soria B, Reig J A. Biochem J. 1996;320:201–205. doi: 10.1042/bj3200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagamatsu S, Fujiwara T, Nakamichi Y, Watanabe T, Katahira H, Sawa H, Akagawa K. J Biol Chem. 1996;271:1160–1165. doi: 10.1074/jbc.271.2.1160. [DOI] [PubMed] [Google Scholar]
- 4.el Far O, Charvin N, Leveque C, Martin-Moutot N, Takahashi M, Seagar M J. FEBS Lett. 1995;361:101–105. doi: 10.1016/0014-5793(95)00156-4. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. J Biol Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 6.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 7.Wiser O, Bennett M K, Atlas D. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 8.Wiser O, Trus M, Hernández A, Renström E, Barg S, Rorsman P, Atlas D. Proc Natl Acad Sci USA. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashima Y, Pugh W, Depaoli A M, Takeda J, Seino S, Bell G I, Polonsky K S. Diabetes. 1993;42:948–955. doi: 10.2337/diab.42.7.948. [DOI] [PubMed] [Google Scholar]
- 10.Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson J H, Bell G I. Proc Natl Acad Sci USA. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellman B. Ann N Y Acad Sci. 1965;131:541–558. doi: 10.1111/j.1749-6632.1965.tb34819.x. [DOI] [PubMed] [Google Scholar]
- 12.Chibalin A V, Katz A I, Berggren P-O, Bertorello A M. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 13.Brown H, Larsson O, Bränström R, Yang S-N, Leibiger B, Leibiger I, Fried G, Moede T, Deeney J T, Brown G R, et al. EMBO J. 1998;17:5048–5058. doi: 10.1093/emboj/17.17.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aspinwall C A, Brooks S A, Kennedy R T, Lakey J R. J Biol Chem. 1997;272:31308–31314. doi: 10.1074/jbc.272.50.31308. [DOI] [PubMed] [Google Scholar]
- 15.Efanov A M, Zaitsev S V, Berggren P-O. Proc Natl Acad Sci USA. 1997;94:4435–4439. doi: 10.1073/pnas.94.9.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hundal H S, Maxwell D L, Ahmed A, Darakhshan F, Mitsumoto Y, Klip A. Mol Membr Biol. 1994;11:255–262. doi: 10.3109/09687689409160435. [DOI] [PubMed] [Google Scholar]
- 17.Hell J W, Westenbroek R E, Warner C, Ahlijanian M K, Prystay W, Gilbert M M, Snutch T P, Catterall W A. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng Z H, Rettig J, Cook T, Catterall W A. Nature (London) 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 19.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 20.Richmond J E, Codignola A, Cooke I M, Sher E. Pflügers Arch. 1996;432:258–269. doi: 10.1007/s004240050132. [DOI] [PubMed] [Google Scholar]
- 21.Sheng Z H, Rettig J, Takahashi M, Catterall W A. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 22.Bennett M K, Garcia-Arraras J E, Elferink L A, Peterson K, Fleming A M, Hazuka C D, Scheller R H. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 23.Inoue A, Obata K, Akagawa K. J Biol Chem. 1992;267:10613–10619. [PubMed] [Google Scholar]
- 24.Hofer G F, Hohenthanner K, Baumgartner W, Groschner K, Klugbauer N, Hofmann F, Romanin C. Biophys J. 1997;73:1857–1865. doi: 10.1016/S0006-3495(97)78216-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuhmann K, Romanin C, Baumgartner W, Groschner K. J Gen Physiol. 1997;110:503–513. doi: 10.1085/jgp.110.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldman E, Levine M, Raveh L, Pollard H B. J Biol Chem. 1989;264:7914–7920. [PubMed] [Google Scholar]
- 27.Mochida S, Sheng Z H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]