Abstract
Inhibitors of apoptosis (IAPs) are a family of proteins that bear baculoviral IAP repeats (BIRs) and regulate apoptosis in vertebrates and Drosophila melanogaster. The yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe both encode a single IAP, designated BIR1 and bir1, respectively, each of which bears two BIRs. In rich medium, BIR1 mutant S. cerevisiae underwent normal vegetative growth and mitosis. Under starvation conditions, however, BIR1 mutant diploids formed spores inefficiently, instead undergoing pseudohyphal differentiation. Most spores that did form failed to survive beyond two divisions after germination. bir1 mutant S. pombe spores also died in the early divisions after spore germination and became blocked at the metaphase/anaphase transition because of an inability to elongate their mitotic spindle. Rather than inhibiting caspase-mediated cell death, yeast IAP proteins have roles in cell division and appear to act in a similar way to the IAPs from Caenorhabditis elegans and the mammalian IAP Survivin.
Apoptosis is implemented by a mechanism highly conserved among metazoans. A number of mammalian cell death proteins resemble those from insects and nematodes both in structure and function, and some, such as members of the Bcl-2, caspase, and inhibitor of apoptosis (IAP) families are able to act in heterologous organisms (1), suggesting an ancient origin of the effector and control mechanisms of cell death.
Although examples of cell-suicide mechanisms have been described in single-celled organisms (reviewed in ref. 2), it is not yet known whether any similarity exists between the mechanisms of cell death in metazoans and unicellular organisms. In single-celled organisms, homologs of cell death molecules may be involved in cell death or may have unrelated roles. In either case, analysis of such proteins may reveal clues as to how they function. Although no homologs of Bcl-2 or caspases have been identified within single-celled organisms, by using low-stringency database searches we have identified homologs of IAP proteins in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe (3). We designated these genes BIR1 and bir1, respectively, because they bear a pair of BIR (baculovirus IAP repeat) motifs (4) within their amino termini.
IAP proteins are a family of cell death inhibitors identified in baculoviruses, where they prevent defensive apoptosis of the host cell and thereby promote viral replication (5). Function of these baculoviral IAPs is conserved, because they also were able to inhibit apoptosis of mammalian cells (6), and most of the cellular homologs of IAPs identified in Drosophila and vertebrates are also cell death inhibitors (7–10). Nevertheless, it is possible that some IAPs have other functions, as at least one IAP from Caenorhabditis elegans is probably not involved in apoptosis but is required for cytokinesis during the first cell divisions after fertilization (11).
Here we describe the phenotype of BIR1 and bir1 deletion mutants and localization of the proteins they encode.
MATERIALS AND METHODS
S. cerevisiae Culture and Transformation. Isogenic wild-type strains JK9 (Mata/Matα, his4/his4, leu2/leu2, ura3/ura3, trp1/trp1) and MH272 (Mata/Matα his3/his3, leu2/leu2, ura3/ura3, trp1/trp1, ade2/ade2) were used. Cells were grown in rich medium (YPD: 1% yeast extract/2% bactopeptone/2% glucose) at 30°C to late log phase and transferred to presporulation medium (YEPA: 1% yeast extract/2% bactopeptone/0.2 M potassium acetate) for a further 24 hr and then resuspended in sporulation medium (SM: 30 mM potassium acetate/0.3 mM raffinose), incubated for up to 12 days, and prepared for tetrad dissection (12) or microscopy.
PCR-mediated gene disruption (13) was used to delete the BIR1 gene. A DNA fragment incorporating the complete HIS3 gene from S. cerevisiae was amplified from plasmid DNA by PCR using oligonucleotide primers including 50 nt corresponding to the 5′ and 3′ ends of the S. cerevisiae BIR1 gene. The yeast strain MH272 was transformed with this construct, and His+ transformants were checked by Southern blotting to confirm the identity of the yeast strain YAU1 (Mata/Matα his3/his3, leu2/leu2, ura3/ura3, trp1/trp1, ade2/ade2, BIR1/HIS3∷bir1).
To create a homozygous mutant strain, YAU1 was sporulated, and after tetrad dissection, haploid Δbir1 strains of each mating type were identified and mixed on plates of rich medium. Zygotes then were identified and isolated. The homozygous diploid YAU11 (Mata/Matα, his3/his3, leu2/leu2, ura3/ura3, trp1/trp1, ade2/ade2, HIS3∷bir1/HIS3∷bir1) can sporulate, albeit poorly, confirming its diploid status.
Pseudohyphal Growth Experiments.
YAU11 cells were transformed with gene fragments encompassing the URA3 gene, the LEU2 gene, the ADE2 gene, and the TRP1 gene to create the heterotrophic bir1 mutant strain YAU1100 (Mata/Matα, Δbir1/Δbir1). The YAU1100 cells were transferred to nitrogen-poor SLAD medium (14) and compared with an isogenic, heterotrophic wild-type strain. Both the wild-type and bir1 mutant strain grew with an elongated morphology characteristic of pseudohyphae, demonstrating that BIR1 is not required in the signaling pathway for pseudohyphae formation. Some S. cerevisiae mutants maintain a constitutively active pseudohyphal state. This includes the elm1 (14) and tec1 (15) mutants. To test whether the BIR1 gene could suppress this signaling pathway, elm1–1 and tec1 mutants were transformed with the multicopy vector pYX212-BIR1. No change was observed in the cells overexpressing BIR1 relative to untransformed cells or cells transformed with the empty plasmid pYX212.
Electron Microscopy.
S. cerevisiae cells were cultured for 28 hr in SM and then fixed in 1% paraformaldehyde/1% glutaraldehyde in 0.04 M phosphate buffer (PB), pH 6.8, and incubated for 5 min at 20°C and then 30 min on ice. For staining, cells were washed three times in PB, incubated in 1% sodium metaperiodate for 15 min, washed, and incubated in 50 mM ammonium chloride for 15 min at 20°C. The cells were postfixed at 20°C in 0.5% potassium permanganate, 5 min as suspension, and then fixed as a pellet for an additional 20 min at 20°C. After washing they were en bloc stained in 0.5% uranyl acetate overnight at 4°C. The stained cells dehydrated in 50, 70, and 95% ethanol and, finally, in three changes of 100% ethanol. Cells were infiltrated with LR White resin as follows: ethanol/resin (1:2) for 1 hr, rotating, ethanol/resin (1:1) for 1 hr, ethanol/resin (1:1) overnight, and then two changes of pure resin for 1 hr. Cells then were embedded and polymerized at 55°C for 2 days. Sections were cut and collected onto 0.25% formvar-coated grids. Uranyl acetate and lead citrate were used for contrasting, and the sections were examined by using a JEOL 1200EX transmission electron microscope.
S. pombe Culture and Spore Preparation.
Strains were derivatives of 972 h− and 975 h+. Wild-type diploids (leu1–32/leu1–32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 h+/h−) and haploids (leu1–32 ura4-D18 ade6–704 h−) were used. Usual procedures for the propagation and manipulation of S. pombe were used (16). Large-scale sporulation cultures were expanded in yeast extract media plus amino acids and then transferred to Edinburgh minimal medium lacking a nitrogen source (ammonium chloride) plus amino acids. The resulting asci were digested at 37°C in glucuronidase (Sigma), washed extensively in water, and germinated in EMM plus amino acids.
S. pombe Constructs. The bir1 knockout construct was assembled by PCR amplification of 1-kb arms surrounding the ORF, which then were subcloned into pBluescript. A ura4 marker was subcloned between these arms. This construct was linearized and transformed into wild-type haploid and diploid cells by using a lithium acetate-transformation procedure. Southern blots of DNA digested with HindIII and ClaI were used to screen for knockouts.
The bir1 ORF lacking the predicted intron of the genomic sequence was amplified by PCR from an S. pombe cDNA library (17) and subcloned into the pRep3X expression vector (18). A version of the bir1 ORF lacking a stop codon also was amplified by PCR and fused to the GFP(S65T) ORF, and this fusion was subcloned into pRep3X.
S. pombe Microtubule Staining.
Germinated spores were fixed in 3.7% formaldehyde/0.25% glutaraldehyde and processed for microtubule staining as described (19) with a 1:100 dilution of primary antibody TAT-1 (a gift of Keith Gull, University of Manchester) and 1:100 dilution of Cy3-labeled anti-mouse IgG (Sigma).
RESULTS
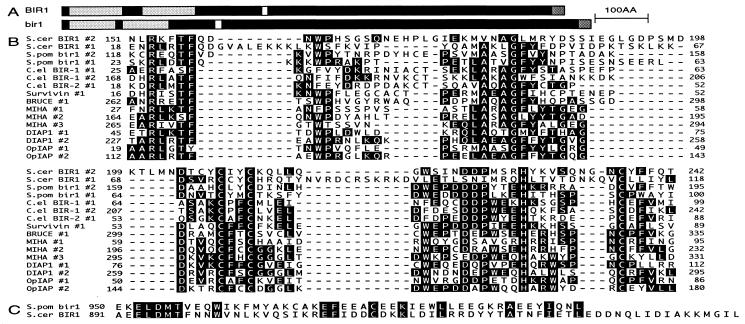
S. cerevisiae and S. pombe Both Have Conserved BIR Motif Proteins. Nucleic acid databases of the yeasts S. cerevisiae and S. pombe were searched for ORFs containing conserved residues characteristic of the BIR motifs of metazoan IAP proteins. A 954-aa ORF was identified from S. cerevisiae (YJR089w) encoding two BIR motifs near its N terminus (Y12 to I116 and D145 to F240) and a putative nuclear localization sequence (K386 to R393) (Fig. 1A). A similar ORF was identified in S. pombe (cosmids SPAC2C6.16 and SPCC962.02c). The predicted spliced product encodes a protein of 997 aa, which contains two N-terminal BIR motifs (R17 to A98 and Q117 to F193), a putative nuclear localization sequence (P288 to K295), and a putative nucleotide-binding domain (G622 to S629) (Fig. 1A). These yeast BIR motifs contain a large proportion of the key conserved residues common to all BIRs, including the invariant cysteine and histidine residues (Fig. 1B). In addition to having two BIR motifs at their N termini and being similar in length, they also share a conserved domain at their C termini (Fig. 1C).
Figure 1.
BIR motif containing proteins of S. cerevisiae and S. pombe. (A) Domain structure of BIR1 and Bir1. BIR1 and Bir1 are similar in length and contain two BIR motifs at their N termini, a putative nuclear localization signal near their centers, and a conserved domain at their C termini. (B) The BIR motifs of BIR1 (S. cerevisiae), Bir1 (S. pombe), both C. elegans IAP homologs, Survivin (human), BRUCE (human), and anti-apoptotic IAPs such as MIHA (mouse), DIAP1 (Drosophila), and OpIAP (baculovirus) share a number of key conserved residues. IAPs that also contain RING finger motifs, such as MIHA and OpIAP, are more closely related to each other than they are to the longer class of BIR motifs. (C) Conserved residues within the C-terminal motif of BIR1 and Bir1.
Comparison of the BIRs of various IAPs place these yeast BIR motifs in a subfamily along with those of two C. elegans IAPs and the mammalian IAPs Survivin (20) and BRUCE (21) (Fig. 1B). BIR motifs from this class of IAPs are longer than other BIR motifs, because of the presence of larger, variable gaps between the universally conserved GLY and first CYS residues. Notably, a conserved intron is present at the same position just 5′ of the nucleotides encoding the invariant GLY residue within the BIRs in S. pombe bir1 (BIR #2), both C. elegans IAPs, and survivin (Fig. 1B). The absence of a similarly placed intron within BIR1 is not surprising, given the paucity of intron-containing genes in S. cerevisiae.
BIR1 Is Required for Both Efficient Meiotic Division and Spore Germination in S. cerevisiae. One copy of the ORF of BIR was deleted in diploid S. cerevisiae by homologous recombination. These diploid heterozygotes were induced to sporulate, and the growth phenotypes of the wild-type and Δbir1 haploid daughter cells were assayed. Although the BIR1 gene was not required for vegetative growth of haploid cells, spore formation was delayed in the diploid heterozygotes, and less than 25% of these cells formed complete tetrads.
Homozygous mutant Δbir1/Δbir1 diploid cells also underwent normal vegetative growth in rich medium (1% yeast extract, 2% peptone, 100 mg/liter adenine sulfate/2% dextrose), but their ability to form complete tetrads was diminished greatly. In wild-type cells some complete tetrads were visible after 1 day in sporulation medium (SM), and the process was largely complete after 3 days. In marked contrast, the mutant Δbir1/Δbir1 cells did not generate complete tetrads until 6–12 days on sporulation medium, and even then these represented less than 5% of the culture (Fig. 2A).
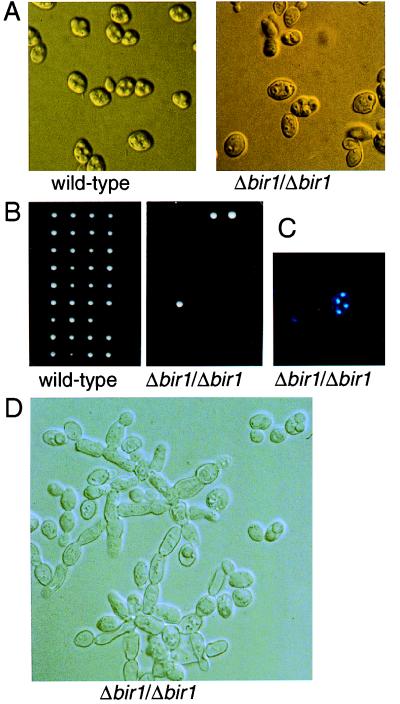
Figure 2.
Loss of BIR1 renders diploid S. cerevisiae incompetent for sporulation. Wild-type or Δbir1/Δbir1 mutant yeast cells were incubated in SM. For wild-type cells, spore formation was almost complete after 3 days (A Left). Even after 12 days, few mature spores appeared in the Δbir1/Δbir1 culture (A Right). (B) The few tetrads formed from the Δbir1/Δbir1 cells after 12 days on SM were dissected and allowed to germinate and form colonies on rich medium with glucose. (C) DAPI staining to visualize the nucleus of each of the spores in the Δbir1/Δbir1 tetrads. (D) Pseudohyphae in the Δbir1/Δbir1 culture after 12 days on SM.
When complete tetrads isolated from the Δbir1/Δbir1 mutant cells were dissected and replated, more than 90% of the haploid Δbir1 spores failed to complete germination (Fig. 2B). 4′,6-Diamidino-2-phenylindole (DAPI) staining of complete tetrads invariably showed one nucleus per haploid spore, but partial aneuploidy of these nuclei cannot be ruled out (Fig. 2C). The mutant Δbir1 spores initiated mitosis, but died at the two- to five-cell stage.
Loss of BIR1 Induces Inappropriate Pseudohyphal Development. In the absence of a rich nitrogen source, diploid S. cerevisiae cells begin either of two developmental programs: under conditions of starvation, they undergo meiosis and form a tetrad of spores, whereas if a rich carbon source such as glucose is readily available, they develop into pseudohyphae (22). Diploid Δbir1/Δbir1 mutant cells were indistinguishable from wild-type diploids when grown in rich medium, except for the presence of a small proportion of cells in these cultures (<5%) that displayed characteristics of pseudohyphal development such as elongation of cells and unipolar cell division. The proportion of pseudohyphal cells was increased in cultures in presporulation or sporulation medium. After 12 days of culture in sporulation medium, pseudohyphal structures represented 10–20% of the culture (Fig. 2D). In contrast, parental wild-type cells did not develop pseudohyphae when cultured in rich, presporulation or sporulation medium.
Formation of pseudohyphae was not simply due to the failure of Δbir1/Δbir1 cells to sporulate because pseudohyphal development of Δbir1/Δbir1 cells was observed in rich (YPAD) cultures under conditions in which the corresponding wild-type cells neither arrested nor initiated sporulation. Furthermore, other sporulation defectives [e.g., Δtom22 cells, which are compromised in sporulation because of mitochondrial defects (23)] do not undergo pseudohyphal development as a default pathway when sporulation is blocked. Despite the inappropriate pseudohyphal development in Δbir1 mutants, neither loss of the BIR1 gene nor overexpression of BIR1 on a multicopy plasmid could suppress constitutive pseudohyphal growth in elm1 or tec1 yeast mutants (T.L., unpublished data).
Appearance of bir1 Mutant Yeast by Electron Microscopy. Cultures of wild-type and Δbir1/Δbir cells were harvested after 28 hr in SM and were fixed, mounted, and sectioned for electron microscopy analysis. In wild-type samples the majority of cells (≈80%) contained a large vacuole in their center and a number of smaller lipid bodies and vesicles at their periphery (Fig. 3A), indicative of cells that have initiated meiosis I and are undergoing formation of the spore wall (24). About 10% of cells had undergone either one or two meiotic divisions, and the spores produced within complete tetrads appeared normal (Fig. 3B).
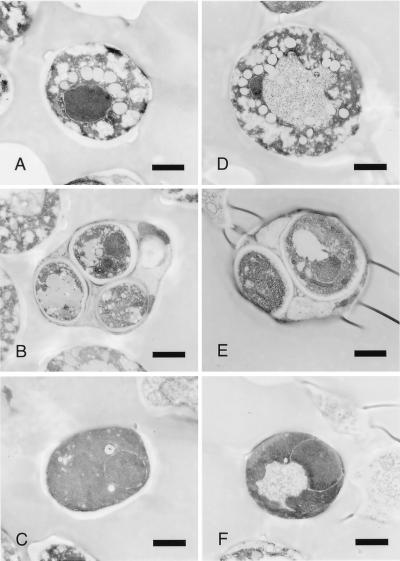
Figure 3.
Electron micrographs of wild-type and (Δbir1/Δbir1) S. cerevisiae 28 hr after transfer to SM. (A) Typical, highly vacuolated cells, which comprised 81% of the wild-type cells. (B) Wild-type asci/spores, which comprised 11% of the wild-type cells, half of which had three or four nuclei. (C) Dark, unvacuolated wild-type cells (8% of the population). (D) Vacuolated mutant cells (47% of the population). (E) Mutant spores comprised 3% of the population, and less than 1% had more than two nuclear profiles. (F) Typical, darkly staining bir1 mutant cells (50% of the mutant cells). (Bar = 1 μm.)
Almost half of Δbir1/Δbir cells had a morphology more typical of vegetative cells that have not initiated meiosis, their cytoplasm being more dense with few if any vesicles or lipid bodies, and the vacuole being small if present (Fig. 3F). The few (≈3%) cells that had undergone either one or two meiotic divisions looked similar to wild type (Fig. 3E).
Bir1 of S. pombe Is Required for Spindle Elongation. To examine the function of the S. pombe bir1 gene, a construct that replaced the ORF of bir1 with the ura4 marker by homologous recombination was transformed into both haploid and diploid S. pombe cells (Fig. 4A). No haploid bir1∷ura4 mutants were obtained, but multiple strains of heterozygous diploid bir1∷ura4/bir1+ strains were isolated that grew and looked normal. When bir1∷ura4/bir1+ heterozygotes were induced to sporulate, they underwent meiosis and formed asci. Dissection of these asci revealed that only the two wild-type spores from each ascus were viable, whereas the ura+ mutant spores arrest in cell cycle and/or die during their first or second division after germination.
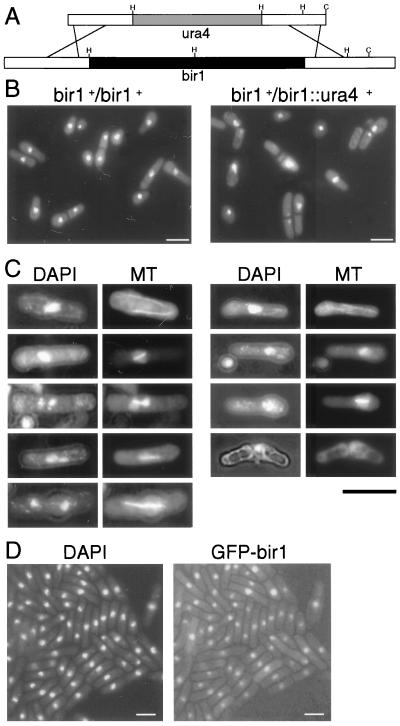
Figure 4.
Failure of the metaphase/anaphase transition in S. pombe bir1 mutants. (A) bir1 knockout construct consisting of two 1-kb arms of genomic fragments flanking the coding region of bir1 and a ura4 marker inserted between these arms. HindIII sites “H” and ClaI sites “C” were used to screen for homologous integrants by Southern blotting. (B) Large-scale spore preparations derived from wild-type and bir1∷ura4/bir1+ heterozygote diploids were germinated in minimal media for 12 hr and stained with DAPI. Wild-type spores germinated normally, with a single, central nucleus in all cells. bir1∷ura4 spores germinated but failed to complete mitotic divisions, with septa frequently cutting through the nucleus destroying it or forming on one side of the nucleus creating cytoplasts. (C) Tubulin staining of wild-type and bir1∷ura4 germinating spores. Cytoplasmic microtubules were present in interphase wild-type cells (Top Left). Mitotic cells showing formation and elongation of spindles were present in the culture at normal frequencies. In bir1∷ura4 spores (Right), microtubules were evident in interphase cells. Mitotic cells possessed only short spindles. In >2,000 cells examined, no evidence for spindle elongation was observed. These cells underwent cytokinesis, often forming a cell plate through the nucleus, killing the cell and, thereby, destroying microtubular structures. (D) Bir1-GFP localizes to the nucleus of vegetative S. pombe. Green immunofluorescence from Bir1-GFP fusion protein overlaps the DAPI-stained portions of the nucleus. (Bars = 10 μm.)
To study the terminal phenotype of bir1∷ura4 cells, we sporulated the heterozygous diploid parent in a liquid culture of minimal medium lacking an added nitrogen source and prepared a pure preparation of spores free of vegetative cells. These were inoculated into minimal medium lacking uracil. Under these conditions, only the bir1∷ura4-containing spores are capable of efficient germination. Samples were taken at various time points after germination, fixed, and stained with DAPI. The bir1∷ura4 cells arrested with a single nucleus frequently bisected by a medial septum (Fig. 4B). This appearance, termed a “cut phenotype,” is characteristic of an inability to successfully complete mitosis before the onset of cytokinesis and is seen in a number of mutants defective in aspects of mitotic progression (reviewed in ref. 25).
In wild-type controls, cells with interphase arrays and mitotic spindles were observed by indirect immunofluorescence. Conversely, bir1∷ura4 cells contained only interphase arrays or short mitotic spindles (Fig. 4C). As aberrant mitosis progressed, leading to the terminal cut phenotype, microtubule staining was lost, as usually occurs in dead cells. The absence of cells with elongated spindles indicates that the deletion of bir1 results in an inability to progress from metaphase to anaphase.
Bir1 Localizes to the Nucleus in S. pombe and S. cerevisiae. An episomal plasmid construct expressing a green fluorescent protein (GFP) fused to the C terminus of the bir1 ORF was transfected into S. pombe haploid cells. GFP fluorescence was observed primarily within the nucleus, overlapping the chromatin (Fig. 4D). Notably, the transfected cells expressing the highest amounts of bir1-GFP fusion displayed some of the morphological characteristics similar to those seen in the bir1∷ura4 knockout haploids. Furthermore, passaging of bir1-GFP lines frequently resulted in a loss of GFP expression, suggesting that the bir1-GFP fusion may be nonfunctional and, when overexpressed, becomes toxic because of interference with the product of the wild-type allele, or that Bir1 cannot be tolerated at high levels.
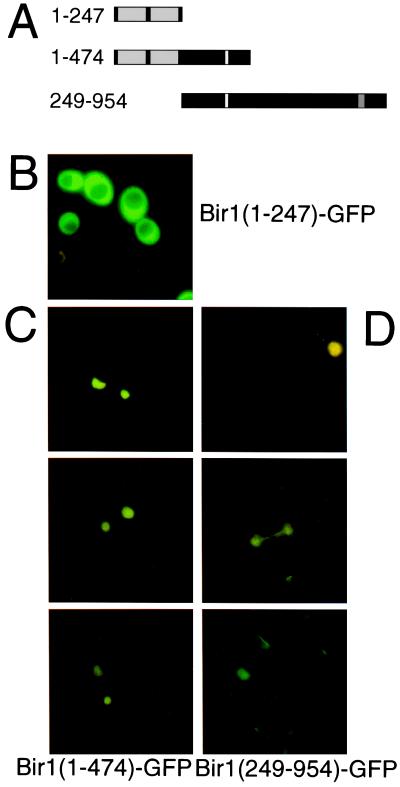
In S. cerevisiae the case was even more extreme: full-length BIR1-GFP could not be overexpressed in this species of yeast (T.B. and T.L., unpublished data). To determine whether the putative nuclear localization sequence directs BIR1 into the nucleus, we fused domain fragments of the S. cerevisiae protein to GFP and ectopically expressed them in S. cerevisiae cells grown on rich glucose medium (Fig. 5A). BIR1 (1–247)-GFP, a fusion protein bearing the BIR domains alone, remained in the cytoplasm of vegetatively growing yeast cells (Fig. 5B). However BIR1(1–474)-GFP, a fusion protein that includes a putative nuclear localization sequence (KKKRKFKR393), was found in the nucleus and distributed uniformly through the nucleoplasm (Fig. 5C).
Figure 5.
Expression and nuclear localization of S. cerevisiae Bir1-domain fragments fused to GFP. (A) Three GFP fusion constructs were designed to carry the BIR domains (shaded), the putative nuclear localization sequence (white), and/or the conserved C-terminal domain (shaded). The yeast strain YAU11 (Δbir1/Δbir1) was transformed with plasmids encoding Bir1(1–247)-GFP (B), Bir1(1–474)-GFP (C), or Bir1(249–954)-GFP (D). Expression of GFP fusions in mid-log-phase cells was analyzed by fluorescence microscopy.
The BIR1(249–954)-GFP fusion protein containing the carboxyl-terminal domain of BIR1 also was targeted to the nucleus (Fig. 5D), but this protein was associated with a fibrillar structure within the nucleus of dividing cells resembling the spindle of anaphase cells. Treatment of these cells with nocodazole, which disrupts microtubule-base structures, led to a uniform fluorescence of the nucleoplasm (data not shown), suggesting that this domain of the fusion protein can mediate association with spindle fibers.
DISCUSSION
Although the phenotypes of Δbir1 mutant S. cerevisiae and bir1 mutant S. pombe were not identical, and we have not been able to complement the yeast IAPs with each other or any other IAP, there were similarities between the structure and function of BIR1 and bir1, suggesting that they act similarly in cell division. The primary defect of Δbir1 S. cerevisiae diploid cells was an almost fatal inefficiency in both the first meiotic division of spore formation and the early mitotic divisions after spore germination. Neither Northern analysis of mRNAs nor hybridization of labeled cDNAs to arrays containing the majority of the ORFs of the S. cerevisiae genome (S. cerevisiae Gene Filters from Research Genetics, Huntsville, AL) revealed clear differences in transcriptional induction or repression between the wild-type and mutant cells during induction of sporulation (data not shown). Despite this normal transcription pattern, more than 90% of the Δbir1/Δbir cells failed to complete the first round of meiotic division, instead remaining as large, rounded diploid cells that had a morphology similar to vegetative cells.
Of the spores that did form from Δbir1/Δbir asci, the majority were inviable. Electron microscopy analysis did not reveal any obvious morphological differences between the few Δbir spores that did form and those from wild-type asci. Although DAPI staining showed there was no gross maldistribution of the DNA, partial aneuploidy because of chromosomal loss during earlier mitotic or meiotic divisions may have occurred.
The Δbir1 phenotype closely resembles that of the cell division mutant chl1 (26). CHL1, a helicase associated with the kinetochore, is required for efficient mitotic chromosome segregation, normal levels of meiotic recombination, and spore viability (26, 27). Diploid S. cerevisiae homozygous for chl1 mutations are viable, but are highly mitotically unstable and become aneuploid at 1% per cell division. As a result, spores from chl1 mutants have low viability because of frequent nullizygosity. Thus, it is possible that BIR1 has a similar role in chromosome segregation during cell division. Such a role is consistent with the nuclear and spindle location of BIR1-GFP.
S. pombe bir1 was required for divisions immediately after germination. Haploid mutant spores derived from S. pombe bir1∷ura4/bir1+ heterozygote diploids were unable to progress more than two divisions after germination. Because these cells die after germination and we were unable to isolate haploid Δbir1 mutants by transfecting the knockout construct into haploids, it appears that this gene is also essential for vegetative growth. Unlike the S. cerevisiae Δbir1/+ heterozygotes, S. pombe bir1∷ura4/bir1+ heterozygotes underwent normal meiotic division and formed asci that were indistinguishable from wild type. Because these mutants are heterozygotes, however, it is not yet possible to say whether bir1 also acts in meiosis. The finding that the two wild-type (ura−) spores from the asci of bir1∷ura4/bir1+ heterozygotes were viable suggests the cell division defects observed in the two ura+ spores from each of these asci are cell autonomous.
Tubulin staining of the S. pombe haploid cells deleted for bir1 showed they were capable of undergoing spindle pole body duplication and nucleation of a short metaphase spindle, but were unable to undergo the metaphase-to-anaphase transition. Although cytokinesis proceeded in these cells, a cell plate was formed frequently that cut through the arrested nucleus. Thus, it appears that the lethal phenotype of bir1 mutants results from an inability to extend the mitotic spindle. The capacity for the C terminus of S. cerevisiae BIR1 to localize to the spindle suggests a similar role for the two IAP proteins in spindle function in both species of yeast.
Other IAPs bearing BIRs that resemble those from the yeasts may also have roles in spindle function. Of the vertebrate IAP genes, the sequence and exon structure of the yeast IAPs is most closely related to that of the mammalian IAP Survivin. survivin expression increases during the G2/M phase of cell cycle, and Survivin localizes to the mitotic spindle in vivo and cosediments with polymerized tubulin (28). RNA-mediated gene interference of one of the IAPs from C. elegans, caused abnormalities in cytokinesis during embryonal cell divisions (11).
Although it is not clear whether vertebrate and insect IAPs function primarily by blocking caspase activation signals (29–31) or by binding directly to caspases (32–35), most are thought to inhibit a caspase-dependent apoptotic mechanism. Survivin has been reported to bind directly to, and inhibit, caspases 3 and 7 (35), but its homologs in C. elegans, bir-1 and bir-2, appear to have no role in the control of cell death, and the phenotype caused by inactivation of one of the C. elegans IAPs can be suppressed partially by transgenic expression of survivin (11). Because neither S. pombe nor S. cerevisiae appears to encode caspases, and neither has been shown to use a cell suicide program, Bir1 and BIR1 are unlikely to function by inhibiting cell death mechanisms resembling those in metazoans.
The structural and functional similarities between human Survivin, cerevisiae BIR1, pombe Bir1, and the BIR-bearing proteins from C. elegans suggest that they share conserved roles in cell division.
Acknowledgments
We thank M. Hall for yeast strains, J. Hegemann, and J. Leighton for plasmids and A. Andrianopoulos, M. Blacketer, C. Koehler, S. Gratzer, and E. Speliotes for discussions. This work was supported by grants from the National Health and Medical Research Council (RegKey 973002, MOC 9734857 to T.L. and W.E.H.I.), the Anti-Cancer Council of Victoria (D.L.V. and A.G.U.), and Australian Postgraduate Research Awards (T.B. and A.G.U.).
ABBREVIATIONS
- IAP
inhibitor of apoptosis
- BIR
baculoviral IAP repeat
- SM
sporulation medium
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Vaux D L, Strasser A. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaux D L, Korsmeyer S J. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Uren A G, Coulson E J, Vaux D L. Trends Biochem Sci. 1998;23:159–162. doi: 10.1016/s0968-0004(98)01198-0. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum M J, Clem R J, Miller L K. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook N E, Clem R J, Miller L K. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins C J, Uren A G, Hacker G, Medcalf R L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:13786–13790. doi: 10.1073/pnas.93.24.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckett C S, Nava V E, Gedrich R W, Clem R J, Vandongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 8.Hay B A, Wassarman D A, Rubin G M. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 9.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Chertonhorvat G, Farahani R, Mclean M, Ikeda J E, Mackenzie A, et al. Nature (London) 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 10.Uren A G, Pakusch M, Hawkins C J, Puls K L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser A G, James C, Evan G I, Hengartner M O. Curr Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 12.Dawes I W, Hardie I D. Mol Gen Genet. 1974;131:281–289. doi: 10.1007/BF00264859. [DOI] [PubMed] [Google Scholar]
- 13.Wach A, Brachat A, Albertisegui C, Rebischung C, Philippsen P. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Blacketer M J, Koehler C M, Coats S G, Myers A M, Madaule P. Mol Cell Biol. 1993;13:5567–5581. doi: 10.1128/mcb.13.9.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrias V, Andrianopoulos A, Gimeno C J, Timberlake W E. Mol Microbiol. 1996;19:1255–1263. doi: 10.1111/j.1365-2958.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 16.Moreno S, Klar A, Nurse P. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 17.Craig R, Norbury C. J Cell Sci. 1998;111:3609–3619. doi: 10.1242/jcs.111.24.3609. [DOI] [PubMed] [Google Scholar]
- 18.Forsburg S L. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krien M J E, Bugg S J, Palatsides M, Asouline G, Morimyo M, O’Connell M J. J Cell Sci. 1998;111:967–976. doi: 10.1242/jcs.111.7.967. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini G, Adida C, Altieri D C. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 21.Hauser H P, Bardroff M, Pyrowolakis G, Jentsch S. J Cell Biol. 1998;141:1415–1422. doi: 10.1083/jcb.141.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kron S J, Styles C A, Fink G R. Mol Biol Cell. 1994;5:1003–1022. doi: 10.1091/mbc.5.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lithgow T, Junne T, Suda K, Gratzer S, Schatz G. Proc Natl Acad Sci USA. 1994;91:11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu S, Derisi J, Eisen M, Mulholland J, Botstein D, Brown P O, Herskowitz I. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 25.Yanagida M. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/s0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]
- 26.Liras P, McClusker J, Mascioli S, Haber J E. Genetics. 1978;88:651–671. [PMC free article] [PubMed] [Google Scholar]
- 27.Amann J, Kidd V J, Lahti J M. J Biol Chem. 1997;272:3823–3832. doi: 10.1074/jbc.272.6.3823. [DOI] [PubMed] [Google Scholar]
- 28.Li F Z, Ambrosini G, Chu E Y, Plescia J, Tognin S, Marchisio P C, Altieri D C. Nature (London) 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 29.Manji G A, Hozak R R, Lacount D J, Friesen P D. J Virol. 1997;71:4509–4516. doi: 10.1128/jvi.71.6.4509-4516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vucic D, Kaiser W J, Harvey A J, Miller L K. Proc Natl Acad Sci USA. 1997;94:10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vucic D, Kaiser W J, Miller L K. Mol Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assamunt N, Salvesen G S, Reed J C. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 34.Roy N, Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm I, Wang Y, Sausville E, Scudiero D A, Vigna N, Oltersdorf T, Reed J C. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]