Abstract
PTEN is a recently identified tumor suppressor inactivated in a variety of cancers such as glioblastoma and endometrial and prostate carcinoma. It contains an amino-terminal phosphatase domain and acts as a phosphatidylinositol 3,4,5-trisphosphate phosphatase antagonizing the activity of the phosphatidylinositol 3-OH kinase. PTEN also contains a carboxyl-terminal domain, and we addressed the role of this region that, analogous to the amino-terminal phosphatase domain, is the target of many mutations identified in tumors. Expression of carboxyl-terminal mutants in PTEN-deficient glioblastoma cells permitted the anchorage-independent growth of the cells that otherwise was suppressed by wild-type PTEN. The stability of these mutants in cells was reduced because of rapid degradation. Although the carboxyl-terminal region contains regulatory PEST sequences and a PDZ-binding motif, these specific elements were dispensable for the tumor-suppressor function. The study of carboxyl-terminal point mutations affecting the stability of PTEN revealed that these were located in strongly predicted β-strands. Surprisingly, the phosphatase activity of these mutants was affected in correlation with the degree of disruption of these structural elements. We conclude that the carboxyl-terminal region is essential for regulating PTEN stability and enzymatic activity and that mutations in this region are responsible for the reversion of the tumor-suppressor phenotype. We also propose that the molecular conformational changes induced by these mutations constitute the mechanism for PTEN inactivation.
PTEN was identified recently as a tumor-suppressor gene located on human chromosome 10q23.3 (1, 2). Deletions or somatic mutations of PTEN occur with high frequency in malignant gliomas (3, 4) and endometrial cancer (5) and with a lower rate in other malignancies such as prostate (6) or small-cell lung cancer (7). Germ-line mutations of PTEN are the cause of Cowden disease, an autosomal-dominant hamartoma syndrome with increased risk for development of tumors in a variety of tissues (8).
The PTEN gene contains 9 exons and encodes a 403-aa protein that displays high homology in its N-terminal region to dual-specificity protein phosphatases and also to tensin, a cytoskeleton protein (1, 2, 9). A recent study demonstrated that PTEN acts as a phospholipid phosphatase dephosphorylating the position D3 of the phosphatidylinositol 3,4,5-trisphosphate (PIP3), which is the direct product of the phosphatidylinositol 3-OH kinase (PI-3 kinase) (10). Subsequent studies have confirmed this finding and have shown that cells lacking wild-type PTEN from PTEN-deficient mice (11), from gliomas (12), or from patients with Cowden disease (13) have elevated levels of PIP3. As a result, the activity of protein kinase B (PKB/Akt) was also elevated in these cells, indicating that PTEN exerts its tumor-suppressor function by negatively regulating the antiapoptotic PI-3 kinase-PKB-signaling pathway.
Biological evidence that PTEN acts as a tumor suppressor came from studies indicating that wild-type PTEN suppresses the proliferation (14) and the tumor growth (15) of PTEN-deficient glioblastoma cells. In contrast, mutant phosphatase-inactive PTEN failed to suppress cell growth.
The analysis of mutations from tumor specimens or cell lines derived from tumors emphasized the importance of the phosphatase domain for the tumor-suppressor function of PTEN. Indeed, a large proportion of these mutations maps to the region encoding the phosphatase domain. However, there are many mutations and deletions occurring distal to the phosphatase domain in the C-terminal region of PTEN. By a survey of the literature we observed that these mutations cluster in hot spots in exons 7 and 8, most of them resulting in premature stop codons deleting the C terminus of the protein (1, 4, 16–23). To investigate the function of the C-terminal region of PTEN, we engineered a set of mutations frequently arising in tumors. By using an efficient retroviral transfection system we expressed these mutants in PTEN-deficient glioblastoma cells that were tested further for anchorage-independent growth. We showed that the C-terminal mutants inactivated the tumor-suppressor function of PTEN. The stability and phosphatase activity of these mutants also were affected. Because the C-terminal-inactivating mutations occurred in β-strands that are predicted with high probability in this region, conservation of the structure appears to be critical for the regulation of the tumor-suppressor function of PTEN.
MATERIALS AND METHODS
Plasmid Construction.
PTEN (403 aa) was amplified by PCR from a placenta library (CLONTECH), and the entire ORF was sequenced to confirm the correct sequence. A phosphatase-inactive PTEN mutant (PTEN-PI) was produced by PCR with mutated overlapping primers by introducing the H123-to-Y inactivating mutation. Three other point mutants were constructed similarly by deleting the T319 (PTEN-T319Δ) or changing L345-to-Q (PTEN-L345Q) and T348-to-I (PTEN-T348I). C-terminal deletion mutants ending at codons 254, 319, 342, 351, 363, 379, 385, 398, and 401 (PTEN-254, PTEN-319, PTEN-342, PTEN-351, PTEN-363, PTEN-379, PTEN-385, PTEN-398, and PTEN-401, respectively) were produced by inserting stop codons at the mentioned positions. Wild-type PTEN and the mutants were cloned in the pFLAG-CMV-2 vector (Kodak) in-frame with the N-terminal FLAG tag, in the pCX retroviral vector with an N-terminal Myc tag and in pGEX-6P-1 (Pharmacia) to be expressed as glutathione S-transferase-fusion proteins.
Cells, Transfections, Proliferation, and Soft Agar Colony Assay. U-87 MG glioblastoma cell line (American Type Culture Collection), COS-7, 293T, and Bosc23 cells were grown in growth medium (DMEM with 10% FCS).
Transient transfections were performed with 2 μg of DNA and 6 μl of FuGENE (Boehringer Mannheim) in 6-cm dishes as indicated by the manufacturer. Stable transfections were obtained by drug selection after retroviral infection of glioblastoma cells. Bosc23 cells were cotransfected with the pCL-Ampho plasmid (Imgenex, San Diego) containing retroviral gag, pol, and amphotropic gp70 env and the various PTEN constructs in the pCX retroviral vector that also contains the resistance gene for blasticidin. After 48 h, the filtered supernatants containing defective amphotropic retrovirus and 8 μg/ml polybrene (Sigma) were added on U-87 MG glioblastoma cells plated on 6-cm dishes. Forty-eight hours postinfection, the cells were grown in selection medium containing 10 μg/ml blasticidin (ICN) for approximately 5 days until cells stably expressing PTEN were obtained. The cells proliferating in the 6-cm dishes after complete selection were trypsinized and counted by using a hemocytometer.
For the soft agar colony assay, 3 ml of 0.4% top agar Noble (Difco) in growth medium containing 5 × 104 cells stably expressing the various PTEN constructs was added onto 6-cm plates prelayered with 5 ml of 0.5% bottom bacto-agar (Difco) in growth medium. Plates were incubated at 37°C and 5% CO2 for 2–3 weeks, until colonies developed.
Antibodies, Immunoprecipitation, and Western Blotting.
The following antibodies were obtained: M2 anti-FLAG (Kodak), anti-Myc (Invitrogen), anti-phosphoS473 Akt/PKB, and anti-Akt/PKB (New England Biolabs). Cell lysis, immunoprecipitation, and immunoblotting were performed as described previously (24).
RNA Extraction, Reverse Transcription, and PCR.
mRNA was extracted from 107 U-87 MG cells stably transfected with the various PTEN constructs by using the QuickPrep mRNA purification kit (Amersham Pharmacia). The concentration of mRNA was measured by spectrophotometry. Reverse transcription was performed on 140 ng of mRNA by using the SuperScript preamplification system for first-strand cDNA synthesis (GIBCO/BRL) with the oligo(dT) primer. One-tenth of the cDNA was used for 30 cycles of PCR amplification with a sense primer corresponding to the Myc tag and a PTEN-specific antisense primer corresponding to the PTEN nucleotides 764–783. Control PCR amplifications were performed with the same primers directly on extracted RNA (extraction control) and on the cDNA with actin-specific primers (template standardization control). RNA and DNA negative controls also were included for the reverse transcription and PCRs.
Pulse–Chase Assay.
COS-7 cells were transfected with wild-type and mutant PTEN cDNAs and, 24 h later, were trypsinized and evenly distributed in 6-cm tissue culture dishes for the pulse–chase assay. After 24 h of incubation, the cells were washed twice and incubated for 15 min at 37°C in DMEM medium lacking methionine and cysteine (GIBCO/BRL) supplemented with 10% dialyzed FCS. Cells were radiolabeled for 30 min at 37°C in the above medium containing 350 μCi/ml [35S]methionine/cysteine (NEN), washed once in warm PBS to remove the radioactive amino acids, and incubated in chase medium (DMEM supplemented with 10% FCS, 0.15 mg/ml nonradioactive methionine, and 0.24 mg/ml nonradioactive cysteine). After various periods of chase, cell lysates were prepared on ice and the FLAG-tagged PTEN proteins were immunoprecipitated with 1 μg of M2 antibody.
Phosphatase Assay.
The phosphatase reactions were performed in 50 μl of assay buffer containing 100 mM Tris⋅HCl pH 8, 10 mM DTT, and 200 μM water-soluble diC8-PIP3 (Echelon, Salt Lake City). The reactions contained either 2.5 μg glutathione S-transferase-PTEN fusion proteins or PTEN proteins immunoprecipitated on protein A/G-agarose beads (Santa Cruz Laboratories) from transfected cells. After immunoprecipitation the beads were washed twice in a low-stringency buffer containing 20 mM Hepes (pH 7.7), 50 mM NaCl, 0.1 mM EDTA and 2.5 mM MgCl2 and once in phosphatase assay buffer lacking PIP3. The reactions were incubated for 40 min at 37°C and transferred to a 96-well plate. The release of phosphate from the substrate was measured in a colorimetric assay by using the Biomol Green Reagent (Biomol) in accordance with the instructions of the manufacturer. The absorbance at 650 nm was recorded in an ELISA plate reader. A standard curve was performed in each assay, and the amount of free phosphate was calculated from the standard curve line-fit data.
Secondary Structure Prediction.
The secondary structure of PTEN C-terminal region was analyzed by various secondary structure prediction programs: predictprotein from European Molecular Biology Laboratory, Heidelberg (25), secondary structure programs from Baylor College of Medicine, and network protein sequence from Pole Bio-informatique Lyonnais.
RESULTS
C-Terminal Mutations Inactivate the Tumor-Suppressor Function of PTEN. In primary tumors and tumor cell lines many PTEN mutations are detected in the phosphatase domain situated in the N terminus of the protein. Little is known about a subset of mutations that occur at a distance from the phosphatase domain in exons 7 and 8. To study the impact of these mutations on PTEN tumor-suppressor function, we introduced in the PTEN cDNA three of the most commonly detected mutations in the exon 8 as well as three sporadically detected mutations in exon 9 (Fig. 1). A first mutation introduces a stop codon at position 319 (PTEN-319) and is the most frequent site mutation found in a variety of primary tumors of different origins (17–22). A second mutation is an in-frame 3-nt deletion removing only the codon specifying T319 (PTEN-T319Δ), identified also in some tumors (1, 18). A third category of mutations determines frameshifts resulting in stop codons at positions 342 or 343 (1, 4, 16, 17, 19, 21, 23). In this case we introduced directly a stop codon in position 342 (PTEN-342) that eliminates the sequence coded by the last exon of PTEN. The three mutations in exon 9 were L345 to Q (4), T348 to I (21), and a nonsense mutation in position 385 (26). For comparison, we used PTEN-PI, with the inactivating mutation H123-to-Y identified in a tumor (27).
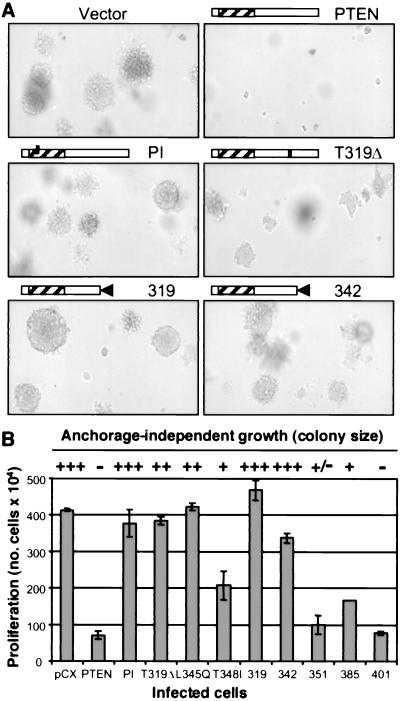
Figure 1.
(A) Failure of PTEN C-terminal mutants to suppress the anchorage-independent growth of U87-MG cells. Retrovirus-infected cells stably expressing PTEN or the indicated mutants (the names of the mutants are abbreviated by the mutation only) were allowed to form colonies in soft agar. The photographs were taken with an Axiovert 135 microscope (Zeiss) at ×10 magnification. (B) The tumor growth of U87-MG cells was assessed as in A, and the size of colonies was scaled from no growth (−) to maximum growth that is similar to vector-transfected cells (+++). The proliferation represents the number of retrovirus-infected U87-MG cells surviving after the completion of the drug selection. These experiments were repeated at least three times with similar results.
The wild-type and various mutants of PTEN were cloned in the pCX retroviral vector and expressed in the U87 MG glioblastoma cell line that is deficient for PTEN. The anchorage-independent growth of these cells, which reflects their transformed phenotype, was evaluated by the ability to form colonies in soft agar (Fig. 1A). Whereas wild-type PTEN efficiently suppressed the formation of colonies of U87 MG cells in soft agar, all the mutants allowed the development of colonies. The size of the colonies varied for the different constructs. Cells expressing the phosphatase-inactive PTEN-PI and the truncated mutants PTEN-319 and PTEN-342 displayed large colonies comparable to those formed by the control U87 MG cells transfected with the vector alone, indicating that these mutants completely abolished the tumor-suppressor function of PTEN. The size of the colonies from cells expressing the point mutants PTEN-T319Δ and PTEN-L345Q was intermediate, and that of PTEN-T348I or the truncated variant PTEN-385 was even smaller than that of control cells, suggesting that these mutations conferred only a partial inactivation of the tumor-suppressor function of PTEN. The size of the colonies generally was correlated to the proliferation rate of cells expressing the various mutants (Fig. 1B), indicating that the tumor-suppressor effect of PTEN is due mainly to the arrest of proliferation.
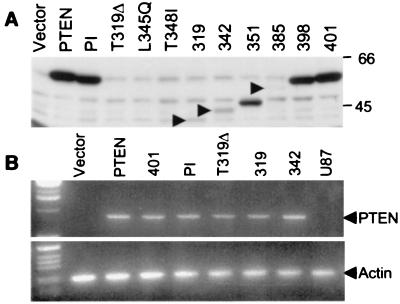
The PTEN C-Terminal Mutants Are Expressed at Low Levels Because of Rapid Degradation. Analysis of U87 MG cells revealed that the C-terminal mutants had lower expression levels compared with wild-type or phosphatase-inactive PTEN (Fig. 2A). This difference was not caused by a modification of the transcription rate because mRNAs were produced in similar amounts (Fig. 2B). The low expression level, confirmed also by immunocytochemistry, was not due to modified transfection efficiency or to cell death because cells cotransfected with PTEN and β-galactosidase expressed similar amounts of β-galactosidase while expressing low levels of the PTEN C-terminal mutants (not shown).
Figure 2.
Reduced protein stability of PTEN C-terminal mutants. (A) Proteins (50 μg) from lysates of stably transfected U87-MG (U87) cells were resolved by SDS/PAGE and analyzed by immunoblotting with anti-Myc antibodies recognizing Myc-tagged PTEN and mutants. Arrowheads indicate the position of low-molecular-weight variants of PTEN. The point mutants of PTEN have low expression levels and are not evident in total lysates, but they can be demonstrated by immunoprecipitation (not shown). (B) Analysis of extracted mRNAs from the same cells by reverse transcription and PCR with primers specific for Myc-tagged PTEN (Upper) or actin (Lower).
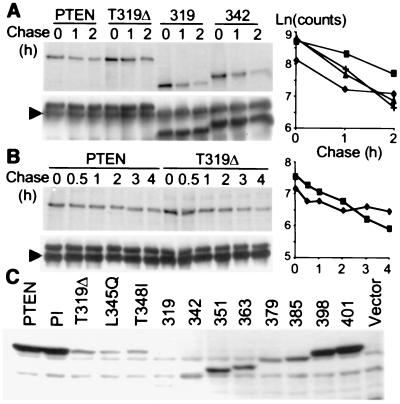
To determine the basis for the low expression levels of the mutants, a pulse–chase analysis of protein turnover was carried out in COS-7 cells transiently expressing PTEN and three mutants (Fig. 3 A and B). The deletion mutants PTEN-319 and PTEN-342 that completely inactivated the tumor-suppressor function of PTEN had a significantly higher degradation rate compared with wild-type PTEN (Fig. 3A). A longer chase period was necessary to demonstrate a higher degradation rate for the point mutant PTEN-T319Δ (Fig. 3B). The linear-regression analysis of the degradation profiles showed that the rate of degradation, represented by the slope of the curve, was highest for PTEN-319 and PTEN-342, intermediate for PTEN-T319Δ, and low for PTEN. These degradation profiles rather than the expression level correlated very well with the size of the colonies in soft agar.
Figure 3.
Rapid degradation of PTEN C-terminal mutants (A and B). Pulse–chase assays were performed in COS-7 cells transfected with FLAG-tagged PTEN and mutants. Cells were pulse–radiolabeled for 30 min and chased for the time periods indicated, up to 2 h in A or up to 4 h in B. Proteins were immunoprecipitated from lysates with the M2 antibody, and the filter first was exposed (Upper) and then immunoblotted with the M2 antibody to monitor the amount of immunoprecipitated proteins (Lower). The arrowhead indicates the Ig heavy chain. The densitometric analysis using a Molecular Imager System (Bio-Rad) is shown in the graphs (♦, ■, +, and ▴ represent PTEN, PTEN-T319Δ, PTEN-319, and PTEN-342, respectively). (C) The expression level of the PTEN C-terminal mutants is shown in total lysates (50 μg of protein) from transiently transfected 293T cells analyzed by immunoblotting with the M2 antibody.
Protein degradation in the cell follows first-order (exponential) kinetics, and the square of correlation coefficient (r2) shows how close the experimental values fit to the exponential model. The degradation profiles of the three PTEN mutants presented high coefficients (r2 > 0.99), showing that they decreased exponentially in time. In contrast, the PTEN degradation profile was unusual, showing a relatively poor fit to an exponential curve (r2 < 0.8). After an exponential decrease, a constant amount of labeled PTEN protein persisted in the cells, suggesting sequestration of the newly formed protein in a cell compartment or complex that would prevent its degradation.
PTEN Contains Two PEST Sequences and a PDZ Motif in the C Terminus. To investigate the mechanism of degradation of PTEN, we searched the coding sequence of PTEN for degradation motifs and detected two putative PEST sequences situated between amino acids 350–375 and 379–396 of the C terminus. The probability that these represent true PEST sequences was very high because their scores were +20.49 and +19.41, which ranked them well above PEST sequences from other proteins, e.g., Fos PEST sequence has a score of +10.1 (28). The role of PEST sequences would be to target proteins with short intracellular half-life for proteolytic degradation, and their deletion usually determines an increased expression level of the protein. In PTEN, deletions affecting both or only one of the PEST sequences in the constructs PTEN-351, PTEN-363, PTEN-379, and PTEN-385 resulted in lower expression levels of the proteins (Fig. 3C). The last 3 aa of PTEN, T-K-V, form the consensus (T-X-V-COOH) for binding PDZ domains. Because in the previous constructs the terminal PDZ-binding motif also was deleted, two mutants removing only this motif, PTEN-401 and PTEN-398, also were expressed (Fig. 3C). For these, the protein level was similar to the full-length protein, indicating that this motif did not affect the expression level of PTEN. Taken together, these results showed that truncations of the PEST sequences decreased the expression of PTEN, most likely by impairing the folding of the protein.
To assess the importance of these motifs for the tumor-suppressor function of PTEN, the mutants lacking both PEST sequences or the terminal PDZ-binding motif (PTEN-351 and PTEN-401, respectively) were expressed in U87-MG cells and were found to induce effects comparable to wild-type PTEN on cell growth (Fig. 1). These data showed that the PEST and PDZ-binding motifs are not essential for the tumor-suppressor function.
The C-Terminal Point Mutations Destabilize the Predicted Secondary Structure. The decreased expression level of the C-terminal mutants could have resulted from misfolding of the protein. To test this hypothesis we searched and found sequences in which elements of secondary structure were predicted with high probability by all the programs used (Fig. 4). Most interestingly, these sequences corresponded to the regions where most of the natural mutations were detected. In particular, the point deletion of T319 in exon 8 and the missense mutations in exon 9, L345 to Q and T348 to I, mapped to regions predicted to form β-strands. By running the prediction programs with these mutations in the sequence, the probability value of the β-strand formation was modified (Fig. 4), indicating that these mutations destabilized the predicted secondary structure of the protein.
Figure 4.
The PTEN C-terminal mutants map to predicted β-strands. The predicted secondary structures (SS), β-strand (b and double-headed arrows) and loop (L), are shown for PTEN and the variants, with point deletion (Δ) in position 319 or point mutations in positions 345 and 348. The changed amino acids and β-strands are shown in bold. The numbers indicate the probability, scaled from 0 to 9, for assigning β-strands (pr:b) by the program predictprotein from European Molecular Biology Laboratory, Heidelberg.
The degree of destabilization of the β-strands rather than the expression level of the mutants correlated to the cell growth phenotype (shown in Fig. 1). The T348I mutation that preserves a modified β-strand still suppressed cell growth, although not as efficiently as wild-type PTEN. The mutations T319Δ and L345Q, which have the most destabilizing effect on the β-strands, reversed efficiently the tumor-suppressor phenotype of PTEN.
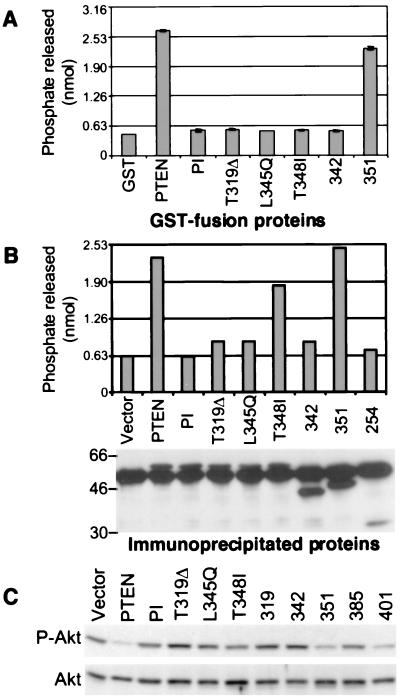
The C-Terminal Mutations Affect the Phosphatase Activity of PTEN. Because all the C-terminal mutations affected the stability of the protein, we analyzed whether they also had an effect on the phosphatase activity of the protein. Surprisingly, similar to the phosphatase-inactive mutant, the C-terminal mutants abolished the phosphatase activity in a phosphatase assay by using purified glutathione S-transferase-fusion proteins and PIP3 as substrate (Fig. 5A). This suggested that the conformational change induced by these mutations is most likely deleterious for the folding of the phosphatase domain. It also suggested that the loss of phosphatase activity is responsible for the phenotype induced by these mutants. In support of this is the finding that the removal of the PEST sequences in PTEN-351 affected only slightly the enzymatic activity. However, the point mutant PTEN-T348I showed no phosphatase activity but affected only mildly the tumor-suppressor function of the protein (compare Figs. 1 and 5A). We hypothesized that this mutant adopts a functional conformation in the cells probably because of interactions with unknown cell components that are not present in the assay using purified proteins. By performing a phosphatase assay with proteins immunoprecipitated from cells (Fig. 5B), we could show that the PTEN-T348I mutant had an intermediate phosphatase activity that correlated well with the tumor-suppressor phenotype. We also showed that a truncated mutant PTEN-254, containing only the N-terminal domain of PTEN, does not have phosphatase activity (Fig. 5B).
Figure 5.
The phosphatase activity of the PTEN C-terminal mutants. Shown is a phosphatase assay using 2.5 μg glutathione S-transferase-fusion proteins (A) or immunoprecipitated proteins from lysates of 293T cells transfected with FLAG-tagged PTEN and mutants (B). (B Lower) The amount of the immunoprecipitated proteins that, at the end of the phosphatase reaction, were resolved on SDS/PAGE and analyzed by immunoblotting with M2 antibody. The amount of free phosphate released in the reaction was measured in a colorimetric assay and compared with a standard curve. (C) PKB/Akt phosphorylation in U87-MG cells stably expressing PTEN and the indicated mutants. Proteins (50 μg) from total lysates were analyzed by immunoblotting with anti-PKB/Akt antibodies recognizing total levels of PKB/Akt (Akt) or only the phosphorylated form (P-Akt). These experiments demonstrated repeatedly similar results.
It has been shown that inactivating mutations in the phosphatase domain impair the ability of PTEN to down-regulate the activation of PKB/Akt (11–13). We found that the C-terminal mutants that affected the phosphatase activity of PTEN also failed to inactivate PKB/Akt (Fig. 5C). This effect correlated well to the degree of phosphatase inactivation and to the growth phenotype.
DISCUSSION
The tumor-suppressor function of PTEN has been associated with its ability to dephosphorylate phosphoinositides at position D3 of the inositol ring and to antagonize the PI 3-kinase-PKB/Akt antiapoptotic pathway (10, 11). Mutations occurring in tumors map frequently to the phosphatase region of PTEN situated in the N-terminal part of the molecule. We show here that the C-terminal region of PTEN is also essential for the tumor-suppressor function of PTEN. The mutations detected in tumors in this region cluster mainly in exons 7 and 8, and they result usually in premature termination of the ORF. In some reports, these mutations constitute up to 50% of the mutations detected in tumor specimens (4, 19, 23). By expressing PTEN variants with the most frequently reported mutations in exons 8 and 9 in U87-MG glioblastoma cells, we observed that the tumor growth and proliferation of these cells is no longer suppressed. Two of these variants are truncated proteins at amino acids 319 and 342, and, in both cases, the tumor-suppressing ability of PTEN is completely abolished (see Fig. 1). Predictably, the upstream truncations occurring in exon 7 would lead to a similar phenotype.
In a first step to identify the mechanism responsible for the inactivation of the tumor-suppressor function, we observed that, relative to wild-type PTEN, all the C-terminal mutants had decreased protein expression levels because of a more rapid degradation. Rapid degradation is a regulatory mechanism for the inactivation of the tumor-suppressor function that also was described for p53 (reviewed in ref. 29). p53 tumor suppressor is polyubiquitinated and degraded rapidly by the proteasome (30). For PTEN, we could not detect by immunoprecipitation in transfected COS-7 cells ubiquitinated forms of the wild-type or mutant proteins (not shown), suggesting a ubiquitin-independent degradation. Although PTEN contains two PEST sequences, their role in targeting the protein for degradation is not clear. PTEN has a relatively long half-life (more than 2 h), and the deletion of the PEST sequences actually decreased the expression level of the protein in cells. A possibility could be that this truncation affected the structure or the chemical properties of the molecule, leading to its destabilization. It is remarkable that the majority of protein phosphatases contain PEST sequences (31). Similar to PTEN, it also is not known whether their role is to target these proteins for degradation because the half-lives of some investigated phosphatases are also relatively long (32, 33).
Another motif present in the C-terminal region of PTEN is a PDZ-binding motif. Although this motif may have a role in the regulation of PTEN, its deletion did not have a detectable influence on the tumor-suppressor activity of PTEN. It is possible that PTEN is recruited by other mechanisms to the cell membrane, but an alternative explanation could be that the protein lacking the PDZ motif reaches the membrane because of overexpression in the stably transfected U87-MG cells.
The deletion of both PEST and PDZ-binding sequences did not have a significant effect on the tumor-suppressor function of PTEN, but a truncation of only nine residues upstream completely inactivated it (see mutants PTEN-351and PTEN-342 in Fig. 1). We also observed that most of the mutations arising in tumors map within a 50-aa region immediately upstream the PEST sequences. The mechanism responsible for the inactivation of the tumor-suppressor function of PTEN became clear when variants with minor sequence modifications were investigated. Two point mutants (positions 345 and 348) and a single residue deletion mutant (position 319) displayed reduced expression levels in cells and reversed to different extents the tumor-suppressor activity of PTEN. Amino acid 319 is in the +4 position from a tyrosine that could represent a putative phosphorylation site (1). By using antiphosphotyrosine antibodies, we could not detect tyrosine phosphorylation of PTEN or PTEN-PI in cells stimulated with epidermal growth factor (not shown), suggesting that the phenotypic change does not relate to changes in tyrosine phosphorylation. Because proteins with defects in folding become unstable (34), we suspected that these three mutations interfere with the structure of PTEN. We found that all 3 aa are located in two predicted β-strands. Moreover, the growth phenotype of the mutants correlates with the degree of disruption of these β-strands. The presence of the last β-strand in the variant deleted at position 351 (PTEN-351) and its elimination in the shorter variant, PTEN-342, would explain the difference in phenotype between them. The actual configuration of predicted secondary structure elements in the context of the whole molecule also depends on the interactions with other structural elements (35). To determine the exact folding of these amino acid stretches in their environment and the conformational changes induced by the mutations detected in tumors, it is necessary to perform three-dimensional analysis. By such analysis, mutations from tumors affecting the folding of another tumor-suppressor protein, p16(INK4) (36), were mapped to regions conferring stability to the protein (37).
Although the destabilization of the C-terminal structure could explain the decreased expression level, it could not explain why mutants with similar expression levels had different effects on the phenotype (compare PTEN-L345Q with PTEN-T348I in Fig. 1). The answer came from the examination of the phosphatase activity that was affected differentially by these mutants. These data indicated that mutations in the C-terminal region inactivate the tumor-suppressor function of PTEN by affecting its intrinsic phosphatase activity most likely as a result of conformational changes. This effect is reflected also by the persistence of PKB/Akt activation in cells expressing these mutants. Although the boundaries of the PTEN phosphatase domain have been established by analogy to other protein phosphatases (27), we show here that the presence of a correct folding in the C terminus is essential for the phosphatase activity. Apparently, the effect of the C-terminal mutations on the phosphatase activity can be modulated in cellular context, as shown for the mutant PTEN-T348I (see Fig. 5), suggesting another important regulatory role for the C-terminal region. We are investigating at present which cellular component is necessary for the recovery of the enzymatic activity because this approach could have therapeutic implications for correcting PTEN misfolding.
In conclusion, the C-terminal region of PTEN contains predicted secondary structure elements that are essential for the tumor-suppressor function of the protein. PTEN variants with disruptions of these structures are degraded rapidly, have impaired enzymatic activity, and allow tumor growth of the cells. The effects on the stability and enzymatic function as well as the perfect conservation in mammals of the C-terminal region call for an important complementary study, which is to determine the three-dimensional organization of the whole protein.
Acknowledgments
We thank J. Kuriyan and M. Rechsteiner for helpful discussions. This work was supported by National Institutes of Health Grant CA44356. M.M.G. was supported by fellowships from the Medical Research Council of Canada and the National Cancer Institute (CA09673).
ABBREVIATIONS
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PI-3 kinase
phosphatidylinositol 3-OH kinase
- PKB/Akt
protein kinase B (Akt indicating the cellular counterpart of the AKT8 virus oncoprotein)
References
- 1.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 3.Rasheed B K, Stenzel T T, McLendon R E, Parsons R, Friedman A H, Friedman H S, Bigner D D, Bigner S H. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- 4.Wang S I, Puc J, Li J, Bruce J N, Cairns P, Sidransky D, Parsons R. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- 5.Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 6.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman J G, Jen J, Isaacs W B, Bova G S, Sidransky D. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 7.Yokomizo A, Tindall D J, Drabkin H, Gemmill R, Franklin W, Yang P, Sugio K, Smith D I, Liu W. Oncogene. 1998;17:475–479. doi: 10.1038/sj.onc.1201956. [DOI] [PubMed] [Google Scholar]
- 8.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 9.Li D M, Sun H. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 10.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 11.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 12.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 13.Myers M P, Pass I, Batty I H, Van der Kaay J, Stolarov J P, Hemmings B A, Wigler M H, Downes C P, Tonks N K. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnari F B, Lin H, Huang H S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheney I W, Johnson D E, Vaillancourt M T, Avanzini J, Morimoto A, Demers G W, Wills K N, Shabram P W, Bolen J B, Tavtigian S V, et al. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 16.Bostrom J, Cobbers J M, Wolter M, Tabatabai G, Weber R G, Lichter P, Collins V P, Reifenberger G. Cancer Res. 1998;58:29–33. [PubMed] [Google Scholar]
- 17.Chiariello E, Roz L, Albarosa R, Magnani I, Finocchiaro G. Oncogene. 1998;16:541–545. doi: 10.1038/sj.onc.1201689. [DOI] [PubMed] [Google Scholar]
- 18.Duerr E M, Rollbrocker B, Hayashi Y, Peters N, Meyer-Puttlitz B, Louis D N, Schramm J, Wiestler O D, Parsons R, Eng C, et al. Oncogene. 1998;16:2259–2264. doi: 10.1038/sj.onc.1201756. [DOI] [PubMed] [Google Scholar]
- 19.Kurose K, Bando K, Fukino K, Sugisaki Y, Araki T, Emi M. Jpn J Cancer Res. 1998;89:842–848. doi: 10.1111/j.1349-7006.1998.tb00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine R L, Cargile C B, Blazes M S, van Rees B, Kurman R J, Ellenson L H. Cancer Res. 1998;58:3254–3258. [PubMed] [Google Scholar]
- 21.Maxwell G L, Risinger J I, Gumbs C, Shaw H, Bentley R C, Barrett J C, Berchuck A, Futreal P A. Cancer Res. 1998;58:2500–2503. [PubMed] [Google Scholar]
- 22.Obata K, Morland S J, Watson R H, Hitchcock A, Chenevix-Trench G, Thomas E J, Campbell I G. Cancer Res. 1998;58:2095–2097. [PubMed] [Google Scholar]
- 23.Risinger J I, Hayes A K, Berchuck A, Barrett J C. Cancer Res. 1997;57:4736–4738. [PubMed] [Google Scholar]
- 24.Georgescu M M, Kirsch K H, Shishido T, Zong C, Hanafusa H. Mol Cell Biol. 1999;19:1171–1181. doi: 10.1128/mcb.19.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rost B, Sander C. Proc Natl Acad Sci USA. 1993;90:7558–7562. doi: 10.1073/pnas.90.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhei E, Kang L, Bogomolniy F, Federici M G, Borgen P I, Boyd J. Cancer Res. 1997;57:3657–3659. [PubMed] [Google Scholar]
- 27.Myers M P, Stolarov J P, Eng C, Li J, Wang S I, Wigler M H, Parsons R, Tonks N K. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers S, Wells R, Rechsteiner M. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 29.Kubbutat M H, Vousden K H. Mol Med Today. 1998;4:250–256. doi: 10.1016/s1357-4310(98)01260-x. [DOI] [PubMed] [Google Scholar]
- 30.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 31.Gomes A V, Barnes J A. Biochem Mol Biol Int. 1997;41:65–73. doi: 10.1080/15216549700201071. [DOI] [PubMed] [Google Scholar]
- 32.Flores E, Roy G, Patel D, Shaw A, Thomas M L. Mol Cell Biol. 1994;14:4938–4946. doi: 10.1128/mcb.14.7.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charest A, Wagner J, Shen S H, Tremblay M L. Biochem J. 1995;308:425–432. doi: 10.1042/bj3080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg D P. Annu Rev Biophys Biophys Chem. 1988;17:481–507. doi: 10.1146/annurev.bb.17.060188.002405. [DOI] [PubMed] [Google Scholar]
- 35.Zhong L, Johnson W C., Jr Proc Natl Acad Sci USA. 1992;89:4462–4465. doi: 10.1073/pnas.89.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Peng Z. J Biol Chem. 1996;271:28734–28737. [PubMed] [Google Scholar]
- 37.Venkataramani R, Swaminathan K, Marmorstein R. Nat Struct Biol. 1998;5:74–81. doi: 10.1038/nsb0198-74. [DOI] [PubMed] [Google Scholar]