Abstract
Saccharomyces cerevisiae cells are exquisitely sensitive to altered dosage of the spindle pole body duplication gene, NDC1. We show that the NDC1 locus is haploinsufficient because diploid yeast cells cannot survive with a single chromosomal copy of the NDC1 gene. Diploid cells with a single copy of NDC1 can survive by gaining an extra copy of the NDC1-containing chromosome. NDC1 haploinsufficiency is a dominant loss-of-function phenotype that leads to aneuploidy. Furthermore, we report that overexpression of NDC1 leads to spindle pole body duplication defects indistinguishable from those observed in ndc1–1 mutant cells. Cells overexpressing NDC1 arrest with monopolar spindles and exhibit increase-in-ploidy phenotypes. Thus, both increased and decreased NDC1 dosage can lead to aneuploidy. The striking sensitivity of yeast cells to changes in NDC1 gene dosage suggests a model for the behavior of some tumor suppressor genes and oncogenes in which loss-of-function mutations and overexpression, respectively, lead to increased genetic instability.
Many different events can lead to aneuploidy, including multipolar mitosis, monopolar mitosis, and nondisjunction of chromosomes during mitosis. Until recently, aneuploidy has been considered a consequence, rather than a cause, of cellular transformation. However, recent studies of chemically transformed cells (1), colorectal cancer cells (2, 3), and papillary renal carcinomas (4) suggest that aneuploidy can cause cancer.
Centrosomes play a critical role in the maintenance of genomic integrity. A study of centrosome morphology in human breast tumor cells reveals a compelling correlation between aberrant centrosome structure and aneuploidy in advanced tumors (5). A direct link between abnormal centrosome number, aneuploidy, and cellular transformation was revealed by studies of aurora2/STK15/BTAK, a centrosome-associated protein kinase (6, 7). It is amplified in many human tumor cells, and overexpression of aurora2 in nontumor cells leads to increased centrosome number, aneuploidy, and tumorigenesis (6, 7). This suggests that an oncogene may induce cellular transformation by perturbing centrosome function.
In the budding yeast S. cerevisiae, the spindle pole body (SPB) functions as the centrosome-equivalent organelle. In early G1, yeast cells contain a single SPB that must be duplicated precisely for chromosome segregation to occur properly. Mutations that disrupt SPB duplication lead to aneuploidy and polyploidy because of monopolar mitosis (reviewed in ref. 8).
The NDC1 (nuclear division cycle) gene (9) is required for a late step in SPB duplication. Although SPB duplication is initiated in ndc1–1 strains at the nonpermissive temperature, the newly synthesized SPB is not inserted into the nuclear envelope (10). All of the chromosomes remain associated with the preexisting, functional SPB in these cells. In response to their monopolar spindles, ndc1–1 cells arrest in mitosis because they activate the mitotic spindle assembly checkpoint (11). Eventually, the cells break through this mitotic arrest and all of their chromosomes segregate with the single, functional SPB (9). As a result, one cell doubles in ploidy and the other cell lacks chromosomal DNA. NDC1 encodes an essential 74-kDa protein with six to seven predicted transmembrane domains (10). Ndc1p is a shared component of SPBs and nuclear pore complexes (NPCs) (12).
We report that the NDC1 gene establishes a connection between gene dosage, SPB duplication, and genetic stability. Yeast cells are sensitive to both increased and decreased NDC1 dosage, often resulting in aneuploidy and polyploidy. This study has led to insights concerning the mechanisms by which yeast cells can exhibit aneuploidy and polyploidy and has also demonstrated how specific types of aneuploidy can allow cells to survive under conditions that are normally lethal.
MATERIALS AND METHODS
Yeast Strains and Media. Yeast strains are listed in Table 1 and were constructed by using standard techniques (13). The ndc1Δ∷HIS3 allele was constructed by using a one-step gene-replacement technique (14). The ndc1Δ∷KANMX allele was constructed by using a two-step gene-replacement technique (15) to replace the entire NDC1 ORF with the KANMX gene (16). Yeast strains in which the NDC1 locus contained two linked genetic markers, TRP1 and KANMX, were constructed by transformation of a wild-type yeast strain with a TRP1 integrative plasmid containing the ndc1Δ∷KANMX allele (pRS304-ndc1Δ∷KANMX, see paragraph on plasmids). The yeast strain containing GAL-NDC1–3xpk at the LEU2 locus [HC14–10c(1235); see Table 1] was constructed by transformation of a wild-type yeast strain with a LEU2 integrative vector containing GAL-NDC1–3xpk (pRS305-GAL-NDC1–3xpk; see paragraph on plasmids). The yeast strain that contained both GAL-NDC1–3xpk and SPC42-GFP was constructed by crossing yeast strain HC14–10c(1235) to yeast strain IAY18 (a kind gift from I. Adams and J. Kilmartin, MRC Laboratory of Molecular Biology, Cambridge, UK) that contains spc42Δ∷LEU2 and TRP1∷SPC42-GFP(3x) (17).
Table 1.
Yeast strains
| Yeast strain | Genotype |
|---|---|
| HC14-10c/5d | MATa/MATα ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 adeΔ426/ade2Δ426 ade3Δ/ade3Δ |
| HC14-10c/HC5-8c(799) | MATaMATα NDC1/ndc1-1 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC7-31c(1134)/HC14-5d | MATa/MATα ndc1Δ∷HIS3/NDC1 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC7-31c(1134)/HC5-8c | MATa/MATα ndc1Δ∷HIS3/ndc1-1 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC14-10c/5d(1197) | MATa/MATα NDC1/ndc1∷ΔKANMX ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC14-10c(1405)/HC29-6b | MATa/MATα NDC1∷TRP1∷ndc1Δ∷KANMX/ndc1Δ∷HIS3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC14-10c(1404)/HC29-6b | MATa/MATα NDC1∷TRP1∷ndc1Δ∷KANMX/ndc1Δ∷HIS3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC14-10c(1405)/HC29-8a | MATa/MATα NDC1∷TRP1∷ndc1Δ∷KANMX/ndc1Δ∷HIS3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| HC-14c(1404)/HC29-8a | MATa/MATα NDC1∷TRP1∷ndc1Δ∷KANMX/ndc1Δ∷HIS3 ura3-52/ura3-52 his3Δ200/his3Δ200 trp1Δ63/TRP1 leu2Δ1/leu2Δ1 LYS2/lys2-801 ade2Δ426/ade2Δ426 ade3Δ/ade3Δ (pALR10-NDC1) |
| WX257-2d | MATa ura3-52 his3Δ200 leu2-3,112 |
| WX257-4c | MATα ura3-52 his3Δ200 leu2-3,112 |
| HC14-10c | MATa ura3-52 his3Δ200 trp1Δ63 leu2Δ1 ade2Δ426 ade3Δ |
| HC14-5d | MATα ura3-52 his3Δ200 leu2Δ1 lys2-801 ade2Δ426 ade3Δ |
| HC14-10c(1235) | MATa leu2Δ1∷GAL-NDC1-3xpk∷LEU2 ura3-52 his3Δ200 trp1Δ63 ade2Δ426 ade3Δ |
| HC44-2a | MATa leu2Δ1∷GAL-NDC1-3xpk∷LEU2 TRP1∷SPC42-GFP(3x) ura3-52 his3Δ200 lys2-801 ade2Δ426 ade3Δ |
All yeast strains were grown as described, in YPD (1% yeast extract/2% bactopeptone/2% glucose), YPR (1% yeast extract/2% bactopeptone/3% raffinose), or synthetic medium supplemented with the appropriate amino acids and 2% glucose. For galactose induction, we used YPR containing 2% galactose. Yeast plates containing 5-fluoroorotic acid (5-FOA) or geneticin (G418) were prepared as in refs. 16 and 18. Cells were arrested in G1 by using α-factor (Macromolecular Resources, Fort Collins, CO) at a concentration of 11 μg/ml.
Diploid strains that are heterozygous for the ndc1 null allele and contain a plasmid-borne copy of NDC1 with the URA3 marker [HC14–10c(1405)/HC29–6b, HC14–10c(1404)/HC29–6b, HC14–10c(1405)/HC29–8a, and HC14–10c(1404)/HC29–8a; see Table 1] were used to determine the frequency of survivors of NDC1 haploinsufficiency. Various dilutions of overnight cultures were plated to 5-FOA-containing plates to isolate haploinsufficiency survivors and to YPD control plates. The number of cells plated on the 5-FOA-containing plates was normalized by using the YPD control plates, and a frequency of survival was determined. Similar results were obtained in two independent experiments by using all four strains.
Plasmids.
DNA was manipulated by using standard techniques as described in ref. 19. A subclone of the NDC1 ORF was made by cloning a 2.8-kb AgeI-SacI NDC1 fragment into the XbaI/SmaI restriction sites of the pRS315 vector (20). The pALR10-NDC1 plasmid is described in ref. 12. The 2-μm LEU2 vector containing NDC1 (pRS425-NDC1) was constructed by cloning a XhoI-SpeI NDC1 fragment into XhoI/SpeI-digested pRS425 (21). The pRS304-ndc1Δ∷KANMX plasmid is a derivative of pRS315-NDC1 wherein the NDC1 ORF has been replaced with the KANMX gene (16). The 2-μm URA3 vectors containing either NDC1 or NDC1–3xmyc (pRS426-NDC1 and pRS426-NDC1–3xmyc, respectively) were made by cloning a XhoI-SpeI fragment containing either NDC1 or NDC1–3xmyc, respectively, into XhoI/SpeI-digested pRS426 (21). The pRS305-GAL-NDC1–3xpk plasmid was made by using a modified pRS305 vector (20) that contains the GAL1–10 promoter [derived from the pBM272 vector (22)] cloned into the EcoRI and BamHI sites. A BamHI site was introduced at the start of the NDC1 ORF, and a BamHI-SpeI fragment was cloned into the BamHI and SpeI sites in this modified vector. The 3xpk epitope tag (23) was introduced as a XbaI fragment into an AvrII linker engineered at the 3′ end of NDC1.
Cytological Techniques.
Indirect immunofluorescence microscopy was carried out as described in ref. 12, except the cells were fixed for 45 min and were not extracted with methanol/acetone. The primary antibody was YOL1/34 (Accurate Chemicals; diluted 1:150 in blocker), and the secondary antibody was Texas Red-conjugated donkey anti-rat IgGs (The Jackson Laboratory; diluted 1:75 in blocker). Deconvolution microscopy was carried out as in ref. 12. Immunoelectron microscopy analysis was carried out as in ref. 12. Thin sections were labeled with an anti-pk mouse monoclonal antiserum (a kind gift from I. Hagan, University of Manchester, Manchester, UK) diluted 1:1 and goat anti-mouse 15-nm colloidal gold conjugate (Ted Pella, Redding, CA) diluted 1:20. Flow cytometry was carried out as in ref. 24 by using propidium iodide to stain DNA (Sigma). Stained cells were analyzed by using a Becton-Dickinson FACScan flow cytometer and the cell quest software package for data analysis.
RESULTS
The NDC1 Locus Is Haploinsufficient.
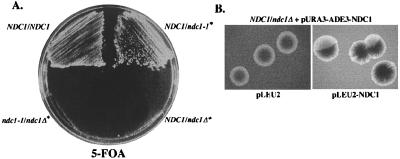
Early studies of the S. cerevisiae NDC1 gene suggested that this locus might be haploinsufficient. It was difficult to construct a diploid strain that was heterozygous for a disruption allele of NDC1. We analyzed this phenotype and found that the NDC1 locus is indeed haploinsufficient. We tested whether diploid strains heterozygous for a ndc1 null allele require a plasmid-borne copy of NDC1 for viability. The NDC1plasmid also contained the URA3 and ADE3 markers, allowing plasmid dependency to be monitored by a failure to grow in the presence of 5-FOA because of the URA3 marker (18) and by a failure to sector because of the ADE3 marker (25). Using both criteria, diploid yeast strains could not survive with a single chromosomal copy of NDC1 (Fig. 1). This plasmid dependency was due to a specific requirement for NDC1, because the 5-FOA sensitive phenotype (data not shown) and the nonsectoring phenotype (Fig. 1B) were both suppressed by introducing a second plasmid-borne copy of NDC1. This also suggests that NDC1 haploinsufficiency is due to lowered NDC1 expression, rather than a perturbation of chromatin structure at the NDC1 locus.
Figure 1.
The NDC1 locus is haploinsufficient. (A) Diploid yeast strains that were either NDC1/NDC1 (HC14–10c/5d; see Table 1), NDC1/ndc1–1 [HC14–10c/HC5–8c(799)], NDC1/ndc1Δ [HC7–31c(1134)/HC14–5d], or ndc1–1/ndc1Δ [HC7–31c(1134)/HC5–8c] were streaked to 5-FOA-containing plates to test whether they are able to lose a plasmid-borne copy of NDC1 (pURA3-ADE3-NDC1; pALR10-NDC1) at 30°C. Asterisks indicate strains that initially contained the plasmid-borne copy of NDC1. (B) A NDC1/ndc1Δ yeast strain [HC14–10c/5d(1197); see Table 1] containing pALR10-NDC1 (pURA3-ADE3-NDC1) was transformed with either a LEU2 vector (pLEU2; pRS425) or with the LEU2 vector containing NDC1 (pLEU2-NDC1; pRS425-NDC1), and the resulting strains were used in a plasmid-sectoring assay. Solid, red colonies indicate a requirement for the plasmid-borne copy of NDC1, whereas sectoring colonies indicate that the cells can lose the NDC1-containing plasmid.
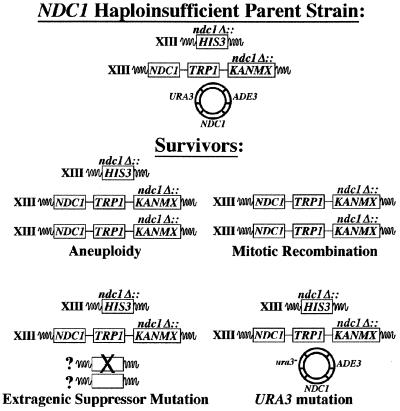
Characterization of NDC1 Haploinsufficiency Survival Mechanisms. After extended growth in the presence of 5-FOA to induce loss of the NDC1-containing plasmid, strains heterozygous for the ndc1 null allele produced “survivor” colonies. The frequency of survival was approximately 1 in 500 cells plated (see Materials and Methods). These yeast strains contain a complete deletion of the NDC1 ORF that is replaced with HIS3, so simple reversion is not the mechanism of generating survivors. We reasoned that the cells could use a number of mechanisms to survive, including aneuploidy, mitotic recombination, an extragenic suppressor mutation, or mutation of the URA3 marker on the plasmid (Fig. 2 and see below).
Figure 2.
NDC1 haploinsufficiency survival mechanisms. Schematic of the mechanisms by which diploid strains that are heterozygous for the ndc1 null allele survive in the absence of a plasmid-borne copy of NDC1. The starting yeast strain (Haploinsufficient Parent Yeast Strain) has both copies of chromosome XIII, where NDC1 resides, marked with specific genes corresponding to the ndc1 null-containing chromosome (HIS3) or the NDC1-containing chromosome (TRP1 and KANMX). This yeast strain also contains a plasmid-borne copy of NDC1 with the URA3 and ADE3 markers.
We constructed diploid strains that were heterozygous for the ndc1 null allele in which both copies of chromosome XIII contained specific genetic markers linked to the NDC1 loci. The NDC1 allele was marked by using both TRP1 and KANMX (G418 resistance), and the ndc1 null allele was marked by using HIS3 (Fig. 2). These strains containing a plasmid-borne copy of NDC1 were streaked to 5-FOA-containing plates to select cells that lost the plasmid-borne URA3 gene. We selected five individual survivors and examined their meiotic products (n = 20 tetrads). The segregation of the NDC1-linked genetic markers, ploidy of survivors and spore clones, and PCR confirmation of markers all were used to determine the possible mechanism(s) used for surviving the NDC1 haploinsufficient phenotype.
The survivors used all of the suggested mechanisms, individually or in combinations, to overcome the NDC1 haploinsufficient phenotype. Three survivors were aneuploid, because a number of their spore clones contained both the NDC1 chromosome and the ndc1 null chromosome. One of the survivors had undergone mitotic recombination to replace the ndc1 null allele with the NDC1 allele. Another survivor appeared to have acquired a suppressor mutation that, in addition to its ability to suppress the NDC1 haploinsufficient phenotype, also functioned as a weak bypass suppressor of the ndc1 null allele; some slow-growing spore clones derived from this survivor did not contain the NDC1 gene, and flow cytometry showed that they exhibited a variety of DNA contents (data not shown). This survivor also exhibited aneuploidy for the NDC1-containing chromosome. Finally, we identified survivors that mutated the URA3 marker carried on the NDC1-containing plasmid. None of the survivors corresponded to diploid cells that were heterozygous for the ndc1 null allele. Furthermore, heterogeneity in the ploidy of spore clones indicated that these cells continue to display genetic instability as they are cultured (data not shown).
Moderate Constitutive Overexpression of NDC1 Causes Haploid Cells to Increase in Ploidy.
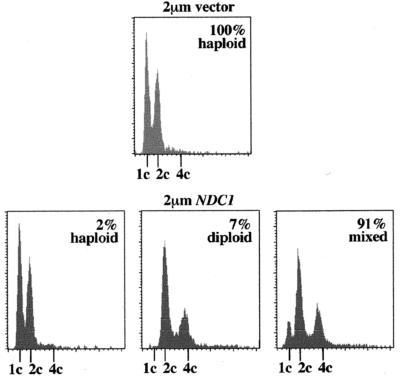
NDC1 was moderately overexpressed (5 to 10 times) in cells by transforming them with a 2-μm plasmid containing either NDC1 or NDC1 tagged with three repeats of the myc epitope. When haploid cells were transformed with the 2-μm vector alone, all of the transformants exhibited a stable haploid DNA content as determined by flow cytometry (n = 33) (Fig. 3). However, when haploid cells were transformed with the 2-μm vector containing NDC1, only 2% of the transformants exhibited a typical haploid DNA content (n = 112); 91% of the transformants showed a mixed ploidy population of typical haploid and diploid DNA peaks, and 7% exhibited a diploid DNA content (Fig. 3). Although increased NDC1 dosage caused cells to increase in ploidy, it did not appear to affect viability.
Figure 3.
Mild overexpression of NDC1 causes haploid cells to increase in ploidy. Haploid yeast strains (WX257–2d, WX257–4c, HC14–10c, and HC14–5d; see Table 1) were transformed with either a 2-μm URA3 vector (2-μm vector; pRS426) or with the 2-μm URA3 vector containing NDC1 (2-μm NDC1; pRS426-NDC1). After transformation, individual colonies (n = 33 for vector alone; n = 112 for 2-μm NDC1) were selected and inoculated into Ura− broth overnight, and samples were prepared for flow cytometry the following day. Shown here are examples of flow cytometric profiles for haploid strains transformed with either the 2-μm vector or with the 2-μm vector containing NDC1.
Induced NDC1 Overexpression in Synchronized Cells Leads to Monopolar Spindle Phenotypes. To study overexpression phenotypes further, we used a haploid strain containing NDC1 with three repeats of the pk epitope at its COOH terminus expressed under the control of the GAL1-inducible promoter (GAL-NDC1–3xpk) integrated at the LEU2 chromosomal locus. This tagged allele of NDC1 is fully functional when expressed by using the endogenous NDC1 promoter (data not shown). This yeast strain still contains the endogenous chromosomal copy of NDC1. NDC1–3xpk was overexpressed in synchronized cells to examine phenotypes in the first SPB duplication cycle. Cells were synchronized in G1 by using α-factor (see Materials and Methods) before the NDC1 execution point (10). Cells were held at the arrest for 1.5 hr to induce expression of NDC1–3xpk and then were released into the cell cycle to determine the effect of excess Ndc1p on SPB duplication.
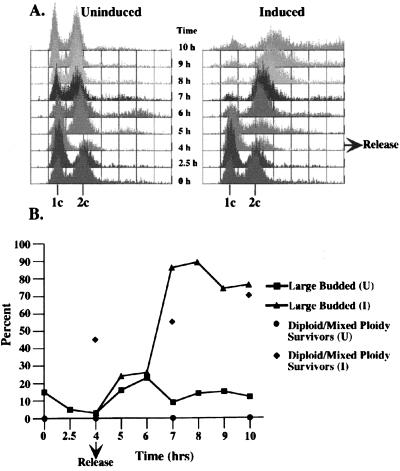
Cells from the uninduced culture released synchronously from the α-factor arrest and eventually became asynchronous (Fig. 4A Left). Cells overexpressing NDC1–3xpk released synchronously from the α-factor arrest, but then arrested with a G2 DNA content in the first cell cycle (Fig. 4A Right). The arrested cells were large-budded (Fig. 4B), contained a single region of unsegregated nuclear DNA, and exhibited abnormal microtubule staining, all characteristic of cells containing monopolar spindles. Cells overexpressing NDC1–3xpk did not exhibit a dramatic decrease in viability, but a large percentage of the surviving cells exhibited increased ploidy (Fig. 4B). Immunoblot analysis showed that Ndc1p-3xpk was highly overexpressed (20 to 30 times) in these cells (data not shown).
Figure 4.
Overexpression of NDC1–3xpk causes cells to arrest and increase in ploidy. Asynchronous cultures of yeast cells containing GAL-NDC1–3xpk integrated at the LEU2 chromosomal locus [HC14–10c(1235); see Table 1] were arrested in G1 by using α-factor for 2.5 hr. The culture then was split; one half was resuspended in medium containing α-factor with glucose (Uninduced), and the other half was resuspended in medium containing α-factor with galactose (Induced) for an additional 1.5 hr to induce the expression of NDC1–3xpk in the induced culture. The cells then were rinsed and resuspended in either glucose-containing (Uninduced) or galactose-containing (Induced) medium to release them from the α-factor block. Samples were taken for flow cytometry, budding index analysis, and viability every hour for 5 hr. (A) Flow cytometric profiles of uninduced and induced cultures. (B) A graph showing the percentages of large-budded cells and higher ploidy surviving cells, either diploid or mixed ploidy, at selected time points.
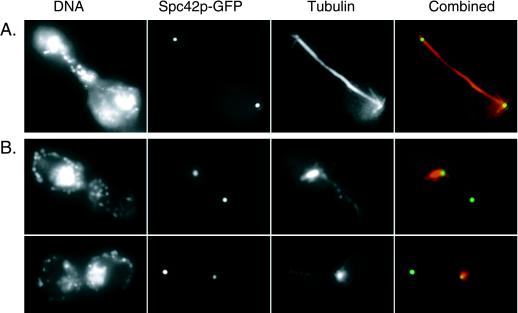
We examined microtubule staining and the localization of an SPB component, Spc42p tagged with green fluorescent protein (Spc42p-GFP) (17), in cells overexpressing NDC1–3xpk. Four hours after release from the α-factor block, the uninduced control cells were asynchronous, and large-budded cells typically contained a long mitotic spindle with separated DNA (Fig. 5A). Cells overexpressing NDC1–3xpk, however, exhibited a G2 arrest, and 83% of the cells were large-budded. Of these large-budded cells, 89% (n = 90) contained unsegregated nuclear DNA and 81% contained two spots of Spc42p-GFP signal. Only one spot of Spc42p-GFP signal was associated with the nuclear DNA, and it nucleated an abnormal array of microtubules (Fig. 5B); the second spot of Spc42p-GFP signal was not associated with the nuclear DNA, but a small amount of microtubule staining emanated from it.
Figure 5.
Cells overexpressing NDC1–3xpk exhibit aberrant microtubule staining and Spc42p-GFP localization. Indirect immunofluorescence microscopy was used to examine microtubules, and SPBs were visualized by the autofluorescence of Spc42p-GFP in cells overexpressing NDC1–3xpk. This experiment was carried out as described in Fig. 4 by using a yeast strain that contains GAL-NDC1–3xpk integrated at the LEU2 chromosomal locus, as well as a chromosomally integrated SPC42-GFP allele (HC44–2a; see Table 1). Shown here are representative examples of tubulin and Spc42p-GFP localization at 4 hr after release from the α-factor arrest. Tubulin was labeled indirectly by using Texas Red. Shown are both tubulin (red) and Spc42p-GFP (green) signals (overlap is yellow). DNA, tubulin, and Spc42p-GFP localization are shown in a large-budded, uninduced cell (A) and in large-budded cells from the induced culture (B).
The phenotypes associated with NDC1 overexpression are strikingly similar to those observed previously for the ndc1–1 allele (9, 10). We used electron microscopy to examine SPB morphology in cells overexpressing NDC1–3xpk 1 hr after release from the α-factor block and found that these cells contain monopolar spindles and defective SPBs similar to what has been reported for ndc1–1 cells at the nonpermissive temperature (10) (data not shown).
Ndc1p Is Mislocalized When Overexpressed.
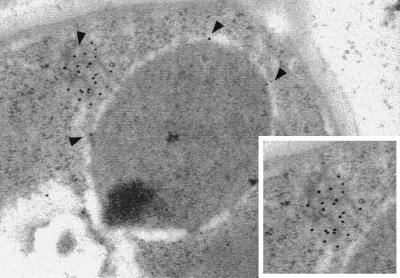
Normally, Ndc1p exhibits punctate nuclear peripheral staining, corresponding to its localization to both NPCs and SPBs (12). We examined the localization of overexpressed Ndc1p-3xpk by using indirect immunofluorescence microscopy and found that Ndc1p-3xpk was mislocalized; it formed large spots in the cytoplasm (data not shown). We carried out immunoelectron microscopy analysis to examine this localization more closely. The uninduced control cells showed little or no immunogold labeling (data not shown). Cells overexpressing NDC1–3xpk showed large cytoplasmic membranous structures filled with immunogold labeling, corresponding to overexpressed Ndc1p-3xpk; these structures often were associated with the nuclear envelope (Fig. 6).
Figure 6.
NDC1p-3xpk is mislocalized when overexpressed. NDC1–3xpk was overexpressed by galactose induction, as described in Fig. 4. Immunoelectron microscopy analysis (see Materials and Methods) was used to examine the localization of overexpressed Ndc1p-3xpk. Shown is a cell at 40 min after release from the α-factor arrest in the presence of galactose. Arrows indicate membranous cytoplasmic structures and NPCs that display immunogold labeling, corresponding to Ndc1p-3xpk. Inset shows a closer view of the immunogold-labeled structure.
DISCUSSION
Yeast cells are strikingly sensitive to altered NDC1 gene dosage. The NDC1 locus is haploinsufficient, meaning that diploid cells cannot survive with a single chromosomal copy of NDC1. It is remarkable that yeast cells are so sensitive to a 2-fold change in NDC1 gene dosage. This phenotype of haploinsufficiency resulting in lethality has been observed for only one other yeast gene, MLC1, which encodes a myosin light chain (26) and has no functional relationship to NDC1. Chromosome XIII, where NDC1 resides, also contains the TUB1 and TUB3 genes, both of which encode α-tubulin. Interestingly, diploid strains with a single copy of TUB1 exhibit aneuploidy for chromosome XIII (27). It is possible that changes in α-tubulin dosage also may contribute to NDC1 haploinsufficiency survival.
We determined the mechanisms by which rare haploinsufficiency “survivors” arose. None of the surviving cells corresponded to typical diploid yeast strains that were heterozygous for the ndc1 null allele; most exhibited dramatic changes in their chromosomal composition. We observed four mechanisms for surviving NDC1 haploinsufficiency, including (i) aneuploidy for chromosome XIII, where NDC1 resides, (ii) mitotic recombination to replace the ndc1 null allele with a wild-type copy of NDC1, (iii) acquisition of an extragenic suppressor mutation, and (iv) mutation of the NDC1-containing plasmid, allowing it to be maintained even under negative selection. Additionally, we found that the survivors that exhibited aneuploidy were not genetically stable. Most survivors had effectively restored normal NDC1 gene dosage. It is likely that the NDC1 haploinsufficient phenotype is due, at least in part, to a defect in SPB duplication. It is interesting to note that a SPB duplication defect actually may allow more cells to overcome the NDC1 haploinsufficient phenotype; it would contribute to the ability of these cells to exhibit aneuploidy, one of the main mechanisms by which they survive.
We also found that yeast cells are sensitive to increased NDC1 gene dosage. When NDC1 was moderately overexpressed, haploid cells exhibited increased ploidy. We tested whether the increase-in-ploidy phenotype was due to a SPB duplication defect. The overexpression of NDC1–3xpk in synchronized cells resulted in a SPB duplication defect that was strikingly similar to that observed in ndc1–1 mutant yeast strains (9, 10). This phenomena of overexpression mimicking loss-of-function phenotypes is not unprecedented for a gene involved in SPB duplication; the overexpression of KAR1 leads to SPB duplication defects that are similar to kar1 loss-of-function alleles (28).
It is possible that a precise distribution of Ndc1p among SPBs and NPCs is critical for its function and, ultimately, for cell viability. Changes in NDC1 gene dosage might alter this distribution, leading to the phenotypes we observed. The depletion of Ndc1p in diploid strains that are heterozygous for the ndc1 null allele may cause them to fail in SPB duplication because of an insufficient pool of Ndc1p at the SPB. Similarly, the overexpression of Ndc1p may lead to failed SPB duplication by depleting Ndc1p or a key cofactor from the SPB. A function for Ndc1p at NPCs is not known at this time, and we did not observe any obvious NPC-related phenotypes in cells with altered NDC1 gene dosage (H.J.C. and M.W., unpublished observation). Our results suggest that the SPB-related function of NDC1 is more sensitive to changes in NDC1 gene dosage.
The striking sensitivity of yeast cells to changes in NDC1 gene dosage suggests models for the behavior of genes involved in cellular transformation. The phenotypes associated with NDC1 overexpression and NDC1 haploinsufficiency mimic the behavior of oncogenes and tumor suppressor genes, respectively. Oncogenes typically encode positive regulators of cell cycle progression in which either gain-of-function mutations or overexpression of the wild-type gene leads to cellular transformation (29). The increase-in-ploidy phenotypes associated with NDC1 overexpression could serve as a model for the behavior of an oncogene that normally functions to maintain centrosome fidelity, such as the aurora2/STK15/BTAK protein kinase (6, 7). The overexpression of this type of oncogene could lead to defects in chromosome segregation, resulting in aneuploidy or polyploidy, which are early genetic events in some cancers.
Contrasting with oncogenes, tumor suppressor genes generally encode negative regulators of cell cycle progression whose function is eliminated during cellular transformation (30). The behavior of these genes is the basis for Knudson’s “two-hit” model for cellular transformation, in which the first hit is a mutation in the tumor suppressor gene and the second hit is a mutation in the remaining wild-type allele of this gene, leading to a loss of heterozygosity (31). Partial loss-of-function alleles of NDC1, such as the ndc1–1 mutation, would adhere to this model. The NDC1 haploinsufficient phenotype could serve as an example of a tumor suppressor gene in which complete loss-of-function mutations do not adhere to the traditional two-hit model. The NDC1 model of cellular transformation would be that a complete loss-of-function mutation in a haploinsufficient gene immediately would give rise to aneuploid surviving cells that had restored the normal gene dosage of the remaining wild-type allele. These cells may also behave similarly to the survivors of NDC1 haploinsufficiency and exhibit genetic instability in later cell cycles, possibly even eliminating the mutation that induced the aneuploidy. This model may be used to reinterpret the behavior of some genes involved in cancer, such as p53.
Recently, it was shown that mice that are heterozygous for a null allele of the p53 gene exhibit higher rates of tumor formation, even though they still maintain a single wild-type copy of the p53 gene, suggesting that decreased p53 gene dosage can lead to tumorigenesis (32). Perhaps p53 is haploinsufficient for some activity such that heterozygous null mice have higher levels of genetic instability. If mutations in p53 or other genes behave similarly to the complete loss-of-function alleles of NDC1, they would display a potent and dominant aneuploidy-generating phenotype that could lead to cellular transformation.
Acknowledgments
We thank I. Adams, J. Kilmartin, and I. Hagan for reagents, A. Castillo and S. McBratney for reading this manuscript, S. Jones, F. Luca, and E. Steiner for discussions, and D. Botstein for guidance in the early stages of this work. Support was obtained from the American Cancer Society (RPG-96–101-03-CSM to M.W), the Pew Scholars Program (P0020SC to M.W.), and a National Institutes of Health training grant (GM-07135 to H.J.C).
ABBREVIATIONS
- SPB
spindle pole body
- NDC
nuclear division cycle
- NPC
nuclear pore complex
- 5-FOA
5-fluoroorotic acid
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Li R, Yerganian G, Duesberg P, Kraemer A, Willer A, Rausch C, Hehlmann R. Proc Natl Acad Sci USA. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 3.Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K, Markowitz S D, Kinzler K W, Vogelstein B. Nature (London) 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 4.Zhuang Z, Park W S, Pack S, Schmidt L, Vortmeyer A O, Pak E, Pham T, Weil R J, Candidus S, Lubensky I A, et al. Nat Genet. 1998;20:66–69. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]
- 5.Lingle W L, Lutz W H, Ingle J N, Maihle N J, Salisbury J L. Proc Natl Acad Sci USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, Kuang J, Zhong L, Kuo W L, Gray J W, Sahin A, Brinkley B R, Sen S. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff J R, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chial, H. J. & Winey, M. (1999) Biol. Cell, in press. [PubMed]
- 9.Thomas J H, Botstein D. Cell. 1986;44:65–76. doi: 10.1016/0092-8674(86)90485-x. [DOI] [PubMed] [Google Scholar]
- 10.Winey M, Hoyt M A, Chan C, Goetsch L, Botstein D, Byers B. J Cell Biol. 1993;122:743–751. doi: 10.1083/jcb.122.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwick K G, Weiss E, Luca F C, Winey M, Murray A W. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- 12.Chial H J, Rout M P, Giddings T H, Winey M. J Cell Biol. 1998;143:1789–1800. doi: 10.1083/jcb.143.7.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman F G, Fink G R, Hicks J B. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. p. 186. [Google Scholar]
- 14.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothstein R. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 16.Wach A, Brachat A, Pohlmann R, Philippsen P. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 17.Schutz A R, Winey M. Mol Biol Cell. 1998;9:759–774. doi: 10.1091/mbc.9.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeke J D, Trueheart J, Natsoulis G, Fink G R. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel J D, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 20.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 22.Johnston M, Davis R W. Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randall R E, Young D F, Goswami K K, Russell W C. J Gen Virol. 1987;68:2769–2780. doi: 10.1099/0022-1317-68-11-2769. [DOI] [PubMed] [Google Scholar]
- 24.Hutter K J, Eipel H E. J Gen Microbiol. 1979;113:369–375. doi: 10.1099/00221287-113-2-369. [DOI] [PubMed] [Google Scholar]
- 25.Bender A, Pringle J R. Mol Cell Biol. 1991;11:1295–1305. doi: 10.1128/mcb.11.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens R C, Davis T N. J Cell Biol. 1998;142:711–722. doi: 10.1083/jcb.142.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schatz P J, Solomon F, Botstein D. Mol Cell Biol. 1986;6:3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose M D, Fink G R. Cell. 1987;48:1047–1060. doi: 10.1016/0092-8674(87)90712-4. [DOI] [PubMed] [Google Scholar]
- 29.Hunter T. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg R A. Science. 1991;254:1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- 31.Knudson A G., Jr Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatachalam S, Shi Y P, Jones S N, Vogel H, Bradley A, Pinkel D, Donehower L A. EMBO J. 1998;17:4657–4667. doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]