Abstract
T box (Tbx) genes are a family of developmental regulators with more than 20 members recently identified in invertebrates and vertebrates. Mutations in Tbx genes have been found to cause several human diseases. Our understanding of functional mechanisms of Tbx products has come mainly from the prototypical T/Brachyury, which is a transcription activator. We previously discovered ET, a Tbx gene expressed in Xenopus embryos. We report here that ET is an ortholog of the human Tbx3 and that ET is a repressor of basal and activated transcription. Functional dissection of the ET protein reveals a novel transcription-repression domain highly conserved among ET, human TBX3, and TBX2. These results reveal a new transcription repressor domain, show the existence of a subfamily of transcription repressors in the Tbx superfamily, and provide a basis for understanding etiology of diseases caused by Tbx3 mutations.
Genes of the T box (Tbx) superfamily play important roles in invertebrate and vertebrate development (1). The first mutation in a Tbx was discovered by Dobrovolskaia-Zavadskaia in 1927 in the mouse Brachyury (T) gene (2–4). Whereas heterozygote mutant mice of Brachyury have short tails, homozygous embryos are defective in mesoderm formation and die early during gestation (5–7). The prototypical mouse T (Brachyury) gene was cloned by Herrmann et al. in 1990 (8). Its orthologs have been found in Xenopus, the zebrafish, and the chicken (9–12). Brachyury is expressed early in embryonic mesoderm in response to mesodermal inducers such as the fibroblast growth factors and activin (9–11, 13–17). Functional studies indicate an important role for Brachyury in mesoderm development (5–7, 9, 18–23).
Since the finding of the mouse T gene and its orthologs, more than 20 Tbx genes have been identified in species ranging from invertebrates such as Drosophila and C. elegans (12, 24–29) to vertebrates including mammals (30–60). All of the Tbx genes whose functions have been studied are essential for development. In Drosophila, the optomotor-blind (omb) gene was discovered for its role in optic-lobe formation (61), and it is now known to play multiple roles in the developing wings (28) and abdominal segments (62). There are at least two more Tbx genes in Drosophila whose functions have not been studied (12, 29). In amphibians, in addition to Brachyury (9), there are at least five other Tbx genes: Eomesodermin (37), Xombi/VegT/Antipodean/Brat (40, 42, 43, 46), ET (44), and Newt Tbox1 (55), and Tbx5 (47). Eomesodermin and Xombi/VegT/Antipodean/Brat have been implicated in mesoderm and endoderm development in Xenopus (37, 40, 42, 43, 46), and Tbx5 is involved in heart development (47), whereas functional roles of Xenopus ET and Newt Tbox1 have not been reported. In chicken embryos, the most striking finding regarding Tbx genes is the differential expression of Tbx5 in the forelimb and Tbx4 in the hindlimb, leading to the hypothesis that Tbx genes are involved in determining limb identities in vertebrate embryos (38, 39, 56, 58, 60). In zebrafish, the no-tail gene, an ortholog of the mouse Brachyury, functions in mesoderm and notochord development (10, 11, 21), and spadetail, another Tbx gene, is involved in the formation of trunk and tail mesoderm (57). In the mouse, there is direct evidence for Tbx involvement in neural development: the Tbx6 gene is normally expressed in the paraxial mesoderm (38), and the somites are transformed into neural tubes in mice lacking Tbx6 (63, 64), indicating that Tbx6 normally prevents neural development in the paraxial mesoderm. In humans, two Tbx genes are involved in human diseases. Mutations in the human Tbx5 gene causes Holt-Oram’s syndrome, with characteristic defects in the limb and the heart (45, 52). Mutations in human Tbx3, on the other hand, cause an autosomal dominant disorder, the ulnar-mammary syndrome (51).
Although it is clear that multiple members of the Tbx superfamily play crucial roles in vertebrate and invertebrate development, our understanding of the molecular mechanisms underlying the function of Tbx proteins is quite limited. Most of our knowledge comes from studies of the prototypical T protein. A sequence of approximately 230 aa, the T domain, was initially found to be conserved between the mouse Brachyury and Drosophila Omb proteins (8, 24) and later found among all Tbx proteins. Biochemical studies show that the T domain is a DNA-binding motif (17). The mouse Brachyury protein can activate the transcription of genes under the control of a DNA-binding site for the T domain (65, 23). There are transcription-activation domains outside the T domain of the mouse and Xenopus Brachyury proteins (65, 23). By contrast, transcriptional regulatory domains have not been studied in any other Tbx proteins.
We have previously isolated cDNAs for the partial sequence of a Xenopus Tbx gene, ET (44). We report here the sequences of the full-length ET protein and full-length human TBX3 and show that they are orthologs of each other. We found that both ET and human TBX3 can repress transcription. A repressor domain is located in the C-terminal region of ET and is conserved in human TBX3 and TBX2. In addition to showing the functional diversity of Tbx proteins, these findings provide a foundation for understand the mechanisms of diseases caused by mutations in Tbx genes.
MATERIALS AND METHODS
Reporter Plasmids.
Luciferase reporter plasmids pJDM1825, pJDM1838, and pJDM1849 were kindly provided by J. Milbrandt (Washington University, St. Louis; ref. 94). In these plasmids, five copies of the Gal4 DNA-binding site (CGG AGT ACT GTC CTC CG) were located upstream of the thymidine kinase promoter, adenovirus major late promoter, and SV40 promoter to drive expression of the luciferase gene. To test activated transcription, a reporter plasmid pL2G2TA-Luc was made by placing two copies of LexA-binding sites, two copies of Gal4-binding sites, and adenovirus E1B minimal TATA promoter (11 bp) upstream of a luciferase gene.
Gal4–ET and LexA–ET Fusion Protein Expression Plasmids.
The expression vector pM1 (95) was used to express chimeric proteins of the DNA-binding domain of Gal4 (residues 1–147) and the full length or truncated ET proteins. To express Gal4–ET fusion proteins for Western analysis, cDNAs expressing ET and its fragments were inserted into expression plasmid pCS2+.
Gal4–hTBX2 and Gal4–hTBX3 [524–674] Fusion Protein Express Plasmids.
hTBX2 was cloned by Campbell et al. (34). hTBX2 and the C-terminal region of hTBX3 were expressed as Gal4 and LexA fusion proteins.
LexA–ET Fusion Protein Expression Plasmids.
LexA DNA-binding domain (residues 1–220; ref. 95) was isolated from pBXL1 (a gift from D. Dean, Washington University) by BamHI and EcoRI digestion and inserted into plasmid pcDNA3 to generate the plasmid pcDNA-LexA. Fragments encoding the full-length ET and its truncated versions were isolated and inserted in-frame to the 3′ end of the DNA-binding domain of LexA in pcDNA3-LexA.
Cell Culture and Luciferase Assays.
293T cells were plated in six-well dishes at 20–30% confluence in DMEM supplemented with 10% FBS. After overnight culture, cells were transfected with 0.5 μg of test plasmid, 0.1 μg of reporter plasmid, and 0.1 μg of LacZ-expressing plasmid cytomegalovirus β-galactosidase and supplemented with pBluescript (Strategene) as a carrier, with a total amount of 2 μg in each well. Transfection was carried out with Lipofectamine (GIBCO/BRL) according to the manufacturer’s instructions. Cells were harvested 48 hr later and washed once with buffer A (100 mM potassium phosphate, pH 7.0). For luciferase assays, cells from each well were lysed with 300 μl of buffer B (buffer A containing 0.5% Triton X-100/1 mM DTT/2 μg/ml aprotinin/0.1 mM PMSF/2 μg/ml leupeptin) by shaking for 5 min at room temperature. Luciferase activity was measured from 100 μl of cell lysate with a luminometer. The internal control β-galactosidase activities were obtained from 1 μl of the cell lysate. Each assay was preformed in duplicate and repeated at least three times.
Western Analysis.
Cells were harvested 48 hr after transfection and lysed with RIPA buffer (50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/0.5% sodium deoxycholate/1.0% Nonidet P-40/0.1% SDS/1 mM DTT/2 μg/ml aprotinin/0.1 mM PMSF/2 μg/ml leupeptin). β-Galactosidase assays were performed to measure transfection efficiency, and standardized amounts of cell extracts were separated by using SDS/PAGE, transferred onto the nitrocellulose membrane, and incubated with a polyclonal antibody against Gal4 DNA-binding domain (Santa Cruz Biotechnology).
Transcription Analysis in Xenopus Embryos.
Capped mRNAs encoding Gal4 fusion proteins of ET and its fragments were made by in vitro transcription. One nanogram of mRNA and 100 pg of reporter plasmid were coinjected into the animal pole of both cells at the two-cell stage of Xenopus embryogenesis. Embryos were harvested from stage 19 to stage 30 and washed twice with buffer A. Individual embryos were then lysed with 200 μl of buffer B. Embryonic lysate (100 μl) was used to measure luciferase activity.
RESULTS
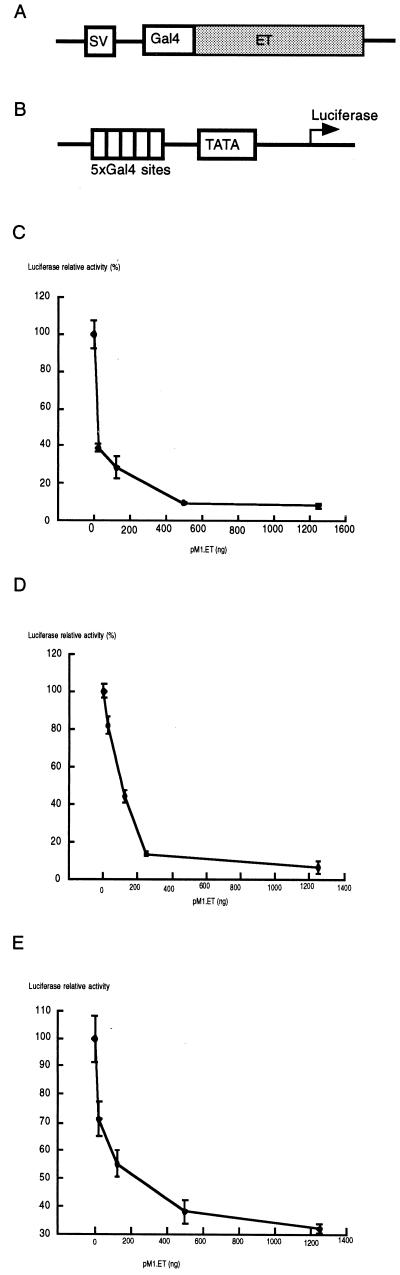
Repression of Basal Transcription by ET. To investigate how ET regulates transcription, we have established a transcription assay with human embryonic kidney (HEK)-derived 293T cells. ET was expressed as a fusion protein with the DNA-binding domain of the yeast transcription factor Gal4 at its N terminus (Fig. 1A). Plasmids for reporting transcription regulation contain five copies of Gal4 DNA-binding sites located upstream of the herpes simplex virus thymidine kinase promoter, the adenovirus major late promoter (AdMLP), or the simian virus (SV)40 promoter, driving the expression of the luciferase gene (Fig. 1B). Gal4–ET-expressing plasmid or control plasmid was cotransfected with a reporter plasmid, cell extracts were prepared 48 hr after transfection, and luciferase activity was measured.
Figure 1.
Repression of basal transcription by ET. (A) A diagram of the plasmid expressing Gal4–ET fusion protein under the SV40 promoter. (B) A diagram of the reporter plasmid with five copies of the Gal4-binding sites upstream of a promoter driving the expression of luciferase. (C) ET can repress the luciferase expression driven by herpes simplex virus thymidine kinase promoter. Reporter plasmid (0.1 μg) was cotransfected with varying amounts (0, 25, 125, 250, 500, or 1,250 ng) of Gal4–ET expression plasmid into 293T cells. The luciferase activity without Gal4–ET was defined as 100%.
We first tested transcription activity of ET by using the reporter driven by the thymidine kinase promoter. ET was found to repress the transcription of the luciferase gene in a dose-dependent manner (Fig. 1C). The luciferase activity was decreased by 60% when 25 ng of ET-expressing plasmid was used, whereas >90% of luciferase activity was reduced with 500 ng of ET-expressing plasmid. ET also repressed transcription driven by the adenovirus major late promoter and SV40 promoters (Fig. 1 D and E). To test whether repression of transcription by ET is cell type-specific, cotransfection experiments were carried out with HeLa and COS-7 cells, and results similar to those obtained from the 293T cells were observed (data not shown). These results indicate that ET is a repressor of basal transcription.
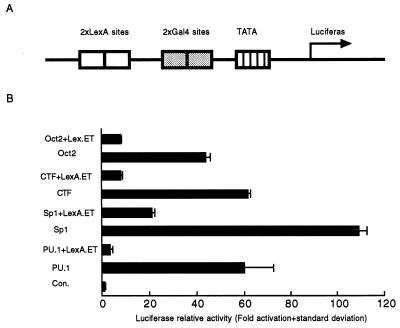
ET Repression of Activated Transcription. Because the expression of eukaryotic genes can be regulated by transcription activators, we tested whether ET protein could affect activated transcription. A fusion protein of ET and the DNA-binding domain of LexA was expressed together with a transactivator fused to the DNA-binding domain of Gal4. A reporter plasmid was constructed to contain two copies of the DNA-binding site for the LexA protein and two copies of the Gal4 DNA-binding site (Fig. 2A). To avoid the possibility that LexA–ET fusion proteins affect transcription by steric hindrance, the LexA-binding sites were located 36 bp upstream of the Gal4-binding sites.
Figure 2.
Repression of activated transcription by ET. (A) A diagram of the reporter plasmid with two copies of LexA binding sites and two copies of Gal4-binding sites upstream of a promoter driving the expression of luciferase. (B) Reporter plasmid (0.1 μg) and LexA–ET expression plasmid (0.5 μg) were cotransfected with or without 50 ng of a plasmid expressing an activator. The reporter plasmid alone with carrier plasmid was cotransfected into 293T cells as control, and the luciferase activity was defined as 1.
When a plasmid expressing the fusion protein of the transactivator domain of Sp1 and Gal4 DNA-binding domain (Gal4-Sp1) was cotransfected with the reporter plasmid, transcription was increased by >100-fold (Fig. 2B) (66). If LexA–ET-expressing plasmid was added, the activation of transcription was reduced by ≈80% (Fig. 2B). When tested for its effect on other activators including CTF, OCT2, and PU.1 (66–68), ET reduced 87% and 82% of transcription activated by CTF and OCT2 (Fig. 2B), respectively, whereas it repressed 95% of transcription activated by PU.1, reducing transcription essentially to the basal level (Fig. 2B). These differences were not caused by different levels of transcription activation by the transactivators, because both CTF and PU.1 activated transcription by ≈60-fold, whereas OCT2 increased transcription by 44-fold. These results indicate that ET represses transcription activated by transactivators and that the repression is stronger for some activators than others.
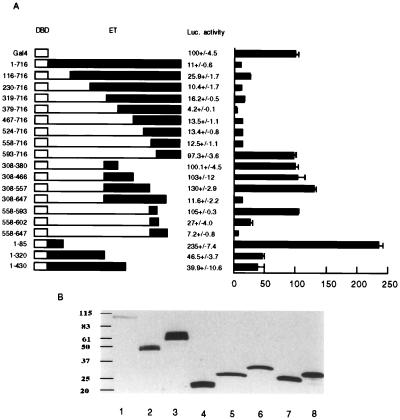
Dissection of Transcription Regulatory Domains in ET. To determine functional domains in ET protein involved in transcription regulation, we made constructs expressing different fragments of ET fused to the DNA-binding domain of Gal4. They were tested for abilities to regulate basal transcription in 293T cells.
The predicted T domain is located from residue 86 to residue 307 in ET. Compared with basal transcription, the full-length ET reduced transcription to ≈11%. An ET fragment without the 115 residues at the N terminus of the full-length ET was still able to reduce transcription to ≈26%. ET fragments without residues 308 to 557 were as effective as the full-length ET in repressing basal transcription (Fig. 3B), indicating that the region between residues 308 and 557 do not contain domains necessary for transcription activation or repression. By contrast, deletions extending to residue 592 rendered ET ineffective in transcription repression (Fig. 3B), indicating that the region of 36 residues between 558 and 592 is essential for transcription repression.
Figure 3.
Dissection of the regulatory domains in the ET protein. (A) Summary of the transcription activities of Gal4–ET fusion constructs. Left, open boxes represent the Gal4 DNA-binding domain (residues 1–147); filled boxes represent sequences of ET protein in the fusion proteins. Center, relative luciferase activities in numbers (mean ± SD); Right, relative luciferase activity as a diagram. (B) Results of Western analysis of the level of ET fusion proteins expressed by cytomegalovirus promoter. Lane1, Gal4–ET; lane 2, Gal4–ET(308–557); lane 3, Gal4–ET(308–647); lane 4, Gal4–ET(558–593); lane 5, Gal4–ET(558–647); lane 6, Gal4–ET(558–716); lane 7, Gal4–ET(1–85); lane 8, Gal4–ET(593–716).
To define the transcription-regulatory domain more precisely, constructs were made to express ET fragments with deletion from both the N and C termini. ET(308–647) was as effective as the full-length in transcription, whereas analysis of further deletions from the C terminus indicated that the region between residues 557 and 647 was required for transcription repression (Fig. 3B).
After defining the region necessary for transcription repression, we determined the minimal region sufficient for transcription repression. We found that ET(558–593) was not sufficient for repression in 293 cells (Fig. 3B). ET(558–602) could repress transcription, although it was not as effective as the full-length ET. ET(558–647), on the other hand, was a strong repressor of basal transcription (Fig. 3B). When ET(558–647) was expressed as a LexA fusion protein and tested for effects on activated transcription, it could repress activated transcription (Fig. 4A). The repression profile of ET(558–647) is quite similar to that of the full-length ET (Fig. 4A).
Figure 4.
Comparison of the repression activities of two domains in ET to that of the full-length ET on activated transcription. (A) Effect of ET(558–647) and full-length ET on transcription activated by PU.1, Sp1, CTF, and Oct2. ET(558–647) is as effective as ET in repressing these activators except Sp1. Relative luciferase activities are shown with the activity in cells transfected with the reporter plasmid alone defined as Fig. 1. (B) Effect of ET(1–85) and full-length ET on transcription activated by PU.1, Sp1, CTF, and Oct2. ET(1–85) can only repress PU.1, but not other activators.
The activity of the N-terminal 85 residues of ET seems to be context-dependent. ET(1–85) alone slightly enhanced basal transcription in 293T cells (Fig. 3A). On the other hand, it reduced the transcription activated by PU.1, but not that by Sp1 or Oct2 (Fig. 4B).
To examine whether the expression levels of ET fusion proteins affected the interpretations of transcription repression, we made another set of constructs expressing ET fusion proteins under the cytomegalovirus promoter. They were individually cotransfected with the reporter plasmid. Results from these experiments on transcription repression were similar to those obtained with ET fusion proteins expressed from SV40 promoter (Fig. 3A), indicating that the repression activities were not dependent on the promoter driving constitutive expression of the ET fusion proteins. To examine the level of ET fusion proteins, we used an anti-Gal4 antibody to determine the expression of fusion proteins by using Western analysis. The potency of transcription repression activities of different fusion proteins was accounted for by their sequence differences to a larger extent than by their relative expression levels. Thus, the minimal domain of ET(558–647) was quite strong in repression because of its sequence characteristics, not just because of its expression level.
Conservation of the Repressor Domain of ET in Its Human Ortholog Tbx3 and in Human Tbx2. We previously reported a partial sequence of ET (44). We have now cloned cDNAs for full-length ET and TBX3. Sequence comparison indicates that ET is an ortholog of human Tbx3 (Fig. 5). ET/TBX3 is also very similar to human TBX2 with a sequence identity of ≈58% between Xenopus ET and human TBX2 and 60% between human TBX2 and TBX3. Among these proteins, the N-terminal region and the T domain are highly conserved. The least conserved region is located between the T domain and the C-terminal repressor domain defined in ET.
Figure 5.
Sequence comparison of ET and human TBX3 and TBX2. Sequences of Xenopus ET and human TBX3 and TBX2 are aligned here. Identical residues are highlighted. The identity of residues is ≈80% between ET and human TBX3, 58% between ET and TBX2, and 60% between human TBX2and TBX3.
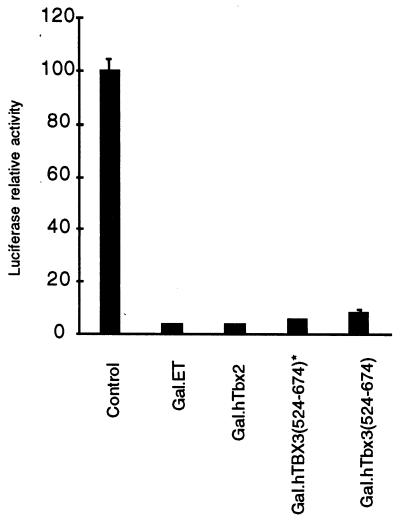
To investigate whether the C-terminal repression domain is functionally conserved in human TBX3, the region from residues 524 to 674 of human TBX3 was fused to the DNA-binding domain of Gal4 and tested for transcription-regulatory activity and was found to be effective in repressing basal transcription (Fig. 6). Similar to that reported recently (69), human TBX2 also repressed transcription in our assays (Fig. 6). These results indicate that the repressor domain defined in ET is conserved in the subfamily of TBX3/ET and TBX2.
Figure 6.
Transcription by the conserved domain in TBX3 and by TBX2. Each test plasmid (0. 1 μg) was transfected into 293T cells. Column 1, 293T cells were cotransfected with the reporter plasmid and carrier DNA, and the luciferase activity is 100 ± 4.8; column 2, cotransfection with Gal4–ET, column 3; cotransfection with Gal4–hTBX2; column 4, cotransfection with Gal4–hTBX3(524–674) carrying a point mutation (Ser-542 → Leu-542) outside the predicted repressor domain; column 5, cotransfection with Gal4–hTBX3(524–674).
Transcription Repression in Xenopus Embryos.
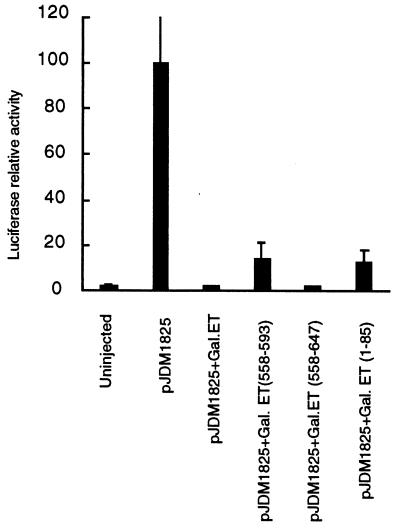
To test the functional significance of ET and its regulatory domains in vivo, we microinjected mRNAs encoding individual ET fusion proteins together with the reporter plasmid into the Xenopus embryos and examined transcription regulation by monitoring luciferase expression. One nanogram of mRNAs for Gal4–ET, Gal4–ET(1–85), Gal4–ET(558–593), or Gal4–ET(558–647) were coinjected with 100 pg of the reporter plasmid with the thymidine kinase promoter into both blastomeres at the two-cell stage of embryogenesis. Individual embryos were collected at stages 20 or 30, and luciferase activity was measured. Full-length ET and ET(558–647) completely repressed transcription of the luciferase gene (Fig. 7). Strong reduction of luciferase expression was also observed in embryos injected with mRNA coding for ET(1–85) and ET(558–593), indicating that both of these small fragments can function as repressors in Xenopus embryos.
Figure 7.
Transcription repression in Xenopus embryos. mRNA encoding Gal4–ET fusion proteins (1 ng) and a reporter plasmid (100 pg) were coinjected into animal pole of both cells at the two-cell stage. Individual embryos were harvested at stage 20. Similar results have been obtained from four experiments. Column 1, uninjected embryos; column 2, injection of reporter plasmid alone, with a relative luciferase activity of 100 ± 28.2; column 3, injection of the reporter plasmid and mRNA encoding Gal4–ET; column 4, injection of the reporter plasmid and mRNA encoding Gal4–ET(558–593); column 5, injection of the reporter plasmid and mRNA encoding Gal4–ET(558–647); column 6, injection of the reporter plasmid and mRNA encoding Gal4–ET(1–85).
DISCUSSION
Results shown here indicate that ET is an ortholog of the human Tbx3 gene, that ET/TBX3 is a transcription repressor, and that there are conserved transcription regulatory domains in the TBX2/TBX3 subfamily of TBX proteins.
The Tbx genes have emerged as a large family of developmental regulators in invertebrates and vertebrates. There seem to be subfamilies in the Tbx genes, although it is not clear when different subfamilies originate in evolution and whether there are functional similarity or relationship among members of the same subfamily. We have previously identified cDNAs for a partial ET protein, mainly around the T domain (44). It was not clear from the partial sequence whether Xenopus ET corresponds to any known mammalian Tbx genes. The sequence of full-length ET protein reported here indicates that ET is an ortholog of the mammalian Tbx3, belonging to a subfamily including the Tbx2, 3, 4, and 5 genes (1). Mutations in a single copy of human TBX3 have recently been found to cause dominant autosomal ulnar-mammary syndrome (51), indicating that the dosage of TBX3 protein is essential for human development and that at least some of the function of TBX3 are not redundant with TBX 2, 4, or 5.
Although the prototypical Brachyury is a transcription activator, we found that ET could repress basal transcription in both human cell lines and in Xenopus embryos. The finding that ET can completely repress transcription in Xenopus embryos indicates that ET is an active repressor. When tested against several transcription activators, ET inhibited transcription activated by these factors, although not to the same extent; PU.1 activated transcription seems to be more susceptible to repression by ET. ET/TBX3 thus joins other molecules as a transcription repressor involved in development. Some of the best studied examples are the products of Drosophila segmentation genes engrailed (70–3), even-skipped (74–77), and kruppel (77–80). In humans, the products of tumor-suppressor genes Rb and WT1 are also transcription repressors (81–93).
Functional dissection of ET protein reveals a strong repressor domain in the region C-terminal to the T domain. The repression activity of this domain is quite similar to the full-length ET in both basal and activated transcription in mammalian cell lines and in Xenopus embryos. It lies between residues 558 and 647. A smaller region within this domain from residues 558 to 593 was not as effective as the full-length or 558–647 in repression transcription in cultured mammalian cells but is a potent repressor of transcription in Xenopus embryos. The activity of 558–593 indicates that the repressor domain does not require alanine-rich repeats for its repression function in Xenopus embryos. The difference of results between mammalian cells and Xenopus embryos could either be caused by species differences or technical differences. The assays in cultured mammalian cells involve introduction of plasmids for the reporter and the repressor at the same time. In Xenopus, although the reporter plasmid and the mRNA for ET fragments were injected at the same time at the two-cell stage, translation of mRNAs into proteins will begin immediately, but transcription from the reporter plasmid will not begin until several hours later, at midblastula transition. Thus. the amount of repressor proteins can be built up before transcription begins from the reporter plasmid. Another possible explanation for differences between mammalian cells and Xenopus embryos lies in the stability of ET protein and its fragments.
The C-terminal repressor domain of ET is conserved in human TBX3 and TBX2. We have shown that the same domain in TBX3 is functionally a repressor, whereas recent studies by others have shown that TBX2 is also a repressor (69). These results suggest that TBX2 and TBX3 constitute a subfamily of transcription repressor in the Tbx superfamily. Although human TBX2 and TBX3 share similarities in sequence, expression pattern, and transcription repression, they are not completely redundant in embryonic development, because mutations in TBX3 cause a haploinsufficient phenotype (51). These findings provide a basis for understanding how ET/TBX3 functions and how mutations in TBX3 can cause functional defects (51). Thus, although truncations including the T domain would result in loss of DNA binding activity, truncations in the C-terminal region could delete the repressor domain, rendering the protein inactive in transcription repression.
The function of the region containing the most N-terminal 85 aa is not clear. In mammalian cells, it behaves as a weak activator for basal transcription but as a repressor for transcription activated by PU.1, but not other activators. In Xenopus embryos, however, it represses basal transcription. It is possible that this domain can regulate transcription in a context-dependent manner, similar to other transcription repressors (76, 78, 79, 87). The significance of the N-terminal domain will be revealed if there are phenotypes associated with mutations in this region in mice or human TBX3 proteins.
So far, two mutations have been found in human Tbx3, which cause the ulnar-mammary syndrome (51). These are truncation mutations; one mutation truncates the TBX3 protein in the region N-terminal to the T domain and the other in the middle of the T domain. Both of these mutations are predicted to eliminate the DNA-binding activity of TBX3. It is interesting that the ulnar-mammary syndrome seems to result from haploinsufficiency of Tbx3; mutations in only one allele can cause the disease (51). Because the dosage of Tbx3 is essential, members of the Tbx2/3/4/5 subfamily of Tbx genes can therefore not be functionally redundant. It would be important to understand the functional mechanisms and significance of other Tbx genes in normal development and in the etiology of human diseases.
Acknowledgments
We are grateful to J. Milbrandt and D. Dean for plasmids; to the National Institutes of Health and National Science Foundation for support (NEI-EY11668 and NSF IBN-9723173 to Y.R. and NIH-DK48796 to C.C.); to the John Merck Fund; and to the Leukemia Society of America for scholar awards (to Y.R. and J.Y.W.).
ABBREVIATIONS
- Tbx
T box
- SV40
simian virus 40
Footnotes
References
- 1.Papaioannou V E, Silver L M. BioEssays. 1998;20:9–19. doi: 10.1002/(SICI)1521-1878(199801)20:1<9::AID-BIES4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Dobrovolskaia-Zavadskaia N. C R Soc Biol. 1927;97:114–116. [Google Scholar]
- 3.Gluecksohn-Waelsch S, Erickson R P. Curr Topics Dev Biol. 1970;5:281–316. doi: 10.1016/s0070-2153(08)60058-7. [DOI] [PubMed] [Google Scholar]
- 4.Bennett D. Cell. 1975;6:441–454. [Google Scholar]
- 5.Chesley P. J Exp Zool. 1935;70:429–459. [Google Scholar]
- 6.Gruneberg H. J Embryol Exp Morphol. 1958;6:424–443. [PubMed] [Google Scholar]
- 7.Herrmann B G, Kispert A. Trends Genet. 1994;10:280–286. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann B G, Labeit S, Poustka A, King T R, Lehrach H. Nature (London) 1990;343:617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 9.Smith J C, Price B M, Green J B, Weigel D, Herrmann B G. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 10.Schulte-Merker S, Ho R K, Herrmann B G, Nusslein-Volhard C. Development (Cambridge, UK) 1992;116:1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- 11.Schulte-Merker S, van Eeden F, Halpern M E, Kimmel C B, Nusslein-Volhard C. Development (Cambridge, UK) 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- 12.Kispert A, Herrmann B G, Leptin M, Reuter R. Genes Dev. 1994;8:2137–2150. doi: 10.1101/gad.8.18.2137. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson D G, Bhatt S, Herrmann B G. Nature (London) 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann B G. Development (Cambridge, UK) 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- 15.Isaacs H V, Pownall M E, Slack J M W. EMBO J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kispert A, Ortner H, Cooke J, Herrmann B G. Dev Biol. 1995;168:406–415. doi: 10.1006/dbio.1995.1090. [DOI] [PubMed] [Google Scholar]
- 17.Kispert A, Koschorz B, Herrmann B G. EMBO J. 1995;14:4763–4772. doi: 10.1002/j.1460-2075.1995.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stott D, Kispert A, Herrmann B G. Genes Dev. 1993;7:197–203. doi: 10.1101/gad.7.2.197. [DOI] [PubMed] [Google Scholar]
- 19.Wilson V, Rashbass P, Beddington R S P. Development (Cambridge, UK) 1993;117:1321–1331. doi: 10.1242/dev.117.4.1321. [DOI] [PubMed] [Google Scholar]
- 20.Wilson V, Manson L, Skarnes W C, Beddington R S P. Development (Cambridge, UK) 1995;121:877–886. doi: 10.1242/dev.121.3.877. [DOI] [PubMed] [Google Scholar]
- 21.Halpern M E, Ho R K, Walker C, Kimmel C B. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- 22.Conlon F L, Wright C V E, Robertson E J. Mech Dev. 1995;49:201–209. doi: 10.1016/0925-4773(94)00318-h. [DOI] [PubMed] [Google Scholar]
- 23.Conlon F L, Sedgwick K, Weston K, Smith J C. Development (Cambridge, UK) 1996;122:2427–2435. doi: 10.1242/dev.122.8.2427. [DOI] [PubMed] [Google Scholar]
- 24.Pflugfelder G O, Roth H, Poeck B. Biochem Biophys Res Commun. 1992;186:918–925. doi: 10.1016/0006-291x(92)90833-7. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik S I, Bollag R J, Silver L M. Genomics. 1995;25:214–219. doi: 10.1016/0888-7543(95)80128-9. [DOI] [PubMed] [Google Scholar]
- 26.Agulnik S I, Garvey N, Hancock S, Ruvinsky I, Chapman D L, Agulnik I, Bollag R, Papaioannou V E, Silver L M. Genetics. 1996;144:249–254. doi: 10.1093/genetics/144.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agulnik S I, Ruvinsky I, Silver L M. Genome. 1997;40:458–464. doi: 10.1139/g97-061. [DOI] [PubMed] [Google Scholar]
- 28.Grimm S, Pflugfelder G O. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- 29.Porsch M, Hofmeyer K, Bausenwein B S, Grimm S, Weber B H, Miassod R, Pflugfelder G O. Gene. 1998;212:237–248. doi: 10.1016/s0378-1119(98)00180-2. [DOI] [PubMed] [Google Scholar]
- 30.Bollag R, Siegfried Z, Cebra-Thomas J A, Garvey N, Davison E M, Silver L M. Nat Genet. 1994;7:383–389. doi: 10.1038/ng0794-383. [DOI] [PubMed] [Google Scholar]
- 31.Bulfone A, Smiga S M, Shimamura K, Peterson A, Puelles L, Rubenstein J L R. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 32.Yasuo H, Satoh N. Dev Growth Differ. 1994;26:9–18. doi: 10.1111/j.1440-169X.1994.00009.x. [DOI] [PubMed] [Google Scholar]
- 33.Holland P W H, Koschorz B, Holland L Z, Herrmann B G. Development (Cambridge, UK) 1995;121:4283–4291. doi: 10.1242/dev.121.12.4283. [DOI] [PubMed] [Google Scholar]
- 34.Campbell C, Goodrich K, Casey G, Beatty B. Genomics. 1995;28:255–260. doi: 10.1006/geno.1995.1139. [DOI] [PubMed] [Google Scholar]
- 35.Law D J, Gebuhr T, Garvey N, Agulnik S I, Silver L M. Mamm Genome. 1995;6:793–797. doi: 10.1007/BF00539006. [DOI] [PubMed] [Google Scholar]
- 36.Law D J, Garvey N, Agulnik S I, Perlroth V, Hahn O M, Rhinehart R E, Gebuhr T C, Silver L M. Mamm Genome. 1998;9:397–399. doi: 10.1007/s003359900780. [DOI] [PubMed] [Google Scholar]
- 37.Ryan K, Garrett N, Mitchell A, Gurdon J B. Cell. 1996;87:989–1000. doi: 10.1016/s0092-8674(00)81794-8. [DOI] [PubMed] [Google Scholar]
- 38.Gibson-Brown J J, Agulnik S I, Chapman D L, Alexiou M, Garvey N, Silver L M, Papaioannou V E. Mech Dev. 1996;56:93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- 39.Gibson-Brown J J, Agulnik S I, Silver L M, Niswander L, Papaioannou V E. Development (Cambridge, UK) 1998;125:2499–2509. doi: 10.1242/dev.125.13.2499. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, King M L. Development (Cambridge, UK) 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Houston D W, King M L, Payne C, Wylie C, Heasman J. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- 42.Lustig K D, Kroll K L, Sun E E, Kirschner M W. Development (Cambridge, UK) 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- 43.Stennard F, Carnac G, Gurdon J B. Development (Cambridge, UK) 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- 44.Li H-S, Tierney C, Wen L, Wu J Y, Rao Y. Development (Cambridge, UK) 1997;124:603–615. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q Y, Newbury-Ecob R A, Terrett J A, Wilson D I, Curtis A R J, Yi C H, Gebuhr T, Bullen P J, Robson S C, Strachan T, et al. Nat Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 46.Horb M E, Thomsen G H. Development (Cambridge, UK) 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- 47.Horb M E, Thomsen G H. Development (Cambridge, UK) 1999;126:1739–1751. doi: 10.1242/dev.126.8.1739. [DOI] [PubMed] [Google Scholar]
- 48.Hug B, Walter V, Grunwald D J. Dev Biol. 1997;183:61–73. doi: 10.1006/dbio.1996.8490. [DOI] [PubMed] [Google Scholar]
- 49.Knezevic V, De Santo R, Mackem S. Development (Cambridge, UK) 1997;124:411–419. doi: 10.1242/dev.124.2.411. [DOI] [PubMed] [Google Scholar]
- 50.Chieffo C, Garvey N, Gong W, Roe B, Zhang G, Silver L, Emanuel B S, Budarf M L. Genomics. 1997;43:267–277. doi: 10.1006/geno.1997.4829. [DOI] [PubMed] [Google Scholar]
- 51.Bamshad M, Lin R C, Law D J, Watkins W S, Krakowiak P A, Moore M E, Franceschini P, Lala R, Holmes L B, Gebuhr T C, et al. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 52.Basson C T, Bachinsky D R, Lin R C, Levi T, Elkins J A, Soults J, Grayzel D, Kroumpouzou E, Trail T A, Leblanc-Straceski J, et al. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 53.Ruvinsky I, Silver L M. Genomics. 1997;40:262–266. doi: 10.1006/geno.1996.4591. [DOI] [PubMed] [Google Scholar]
- 54.Ruvinsky I, Silver L M, Ho R K. Dev Genes Evol. 1998;208:94–99. doi: 10.1007/s004270050158. [DOI] [PubMed] [Google Scholar]
- 55.Simon H-G, Kittappa R, Khan P A, Tsilfidis C, Liversage R A, Oppenheimer S. Development (Cambridge, UK) 1997;124:1355–1366. doi: 10.1242/dev.124.7.1355. [DOI] [PubMed] [Google Scholar]
- 56.Isaac A, Rodriguez-Esteban C, Ryan A, Altabef M, Tsukui T, Patel K, Tickle C, Izpisua Belmonte J C. Development (Cambridge, UK) 1998;125:1867–1875. doi: 10.1242/dev.125.10.1867. [DOI] [PubMed] [Google Scholar]
- 57.Griffin K J P, Amacher S L, Kimmel C B, Kimelman D. Development (Cambridge, UK) 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- 58.Logan M, Simon H-G, Tabin C. Development (Cambridge, UK) 1998;125:2825–2835. doi: 10.1242/dev.125.15.2825. [DOI] [PubMed] [Google Scholar]
- 59.Wattler S, Russ A, Evans M, Nehls M A. Genomics. 1998;48:24–33. doi: 10.1006/geno.1997.5150. [DOI] [PubMed] [Google Scholar]
- 60.Ohuchi H, Takeuchi J, Yoshioka H, Ishimaru Y, Ogura K, Takahasi N, Ogura T, Noji S. Development (Cambridge, UK) 1998;125:51–60. doi: 10.1242/dev.125.1.51. [DOI] [PubMed] [Google Scholar]
- 61.Pflugfelder G O, Roth H, Poeck B, Kerscher S, Schwarz H, Jonschker B, Heisenberg M. Proc Natl Acad Sci USA. 1992;89:1199–1203. doi: 10.1073/pnas.89.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopp A, Duncan I. Development (Cambridge, UK) 1997;124:3715–3726. doi: 10.1242/dev.124.19.3715. [DOI] [PubMed] [Google Scholar]
- 63.Chapman D L, Agulnik I, Hancock S, Silver L M, Papaioannou V E. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- 64.Chapman D L, Papaioannou V E. Nature (London) 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 65.Kispert A, Herrmann B G. EMBO J. 1993;12:3211–3220. doi: 10.1002/j.1460-2075.1993.tb05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seipel K, Georgiev O, Schaffner W. EMBO J. 1992;11:4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagemeier C, Bannister A J, Cook A, Kouzarides T. Proc Natl Acad Sci USA. 1993;90:1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kunzler M, Braus G H, Georgiev O, Seipel K, Schaffner W. EMBO J. 1994;13:641–645. doi: 10.1002/j.1460-2075.1994.tb06302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carreira S, Dexter T J, Yavuzer U, Easty D J, Goding C R. Mol Cell Biol. 1998;18:5099–5108. doi: 10.1128/mcb.18.9.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desplan C, Theis J, O’Farrell P H. Nature (London) 1985;318:630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohkuma Y, Horikoshi M, Roeder R G, Desplan C. Cell. 1990;61:475–484. doi: 10.1016/0092-8674(90)90529-n. [DOI] [PubMed] [Google Scholar]
- 72.Jaynes J B, O’Farrell P H. EMBO J. 1991;10:1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han K, Manley J L. EMBO J. 1993;12:2723–2733. doi: 10.1002/j.1460-2075.1993.tb05934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macdonald P M, Ingham P, Struhl G. Cell. 1986;47:721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- 75.Ingham P W, Baker N E, Martinez-Arias A. Nature (London) 1988;331:73–75. doi: 10.1038/331073a0. [DOI] [PubMed] [Google Scholar]
- 76.Han K, Manley J L. Genes Dev. 1993;7:491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- 77.Johnson A D. Cell. 1995;82:655–658. doi: 10.1016/0092-8674(95)90524-3. [DOI] [PubMed] [Google Scholar]
- 78.Licht J D, Grossel M J, Figge J, Hansen U M. Nature (London) 1990;346:76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- 79.Hanna-Rose W, Licht J D, Hansen U. Mol Cell Biol. 1997;17:4820–4829. doi: 10.1128/mcb.17.8.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanna-Rose W, Licht J D, Hansen U. Mol Cell Biol. 1994;14:4057–4066. doi: 10.1128/mcb.14.6.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weintraub S J, Chow K N, Luo R X, Zhang S H, He S, Dean D C. Nature (London) 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 82.Gessler M, Konig A, Moore J, Qualman S, Arden K, Cavenee W, Bruns G. Genes Chromosomes Cancer. 1993;7:131–136. doi: 10.1002/gcc.2870070304. [DOI] [PubMed] [Google Scholar]
- 83.Gessler M, Poustka A, Cavenee W, Neve R L, Orkin S H, Bruns G A. Nature (London) 1990;343:774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- 84.Call K M, Glaser T, Ito C Y, Buckler A J, Pelletier J, Haber D A, Rose E A, Kral A, Yeger H, Lewis W H, et al. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 85.Haber D A, Buckler A J, Glaser T, Call K M, Pelletier J, Sohn R L, Douglass E C, Housman D E. Cell. 1990;61:1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- 86.Guan L-S, Liu J J, Xu Y-H, Wang Z-Y. Cancer Res. 1998;58:4180–4184. [PubMed] [Google Scholar]
- 87.Madden S L, Cook D M, Morris J F, Gashler A, Sukhatme V K, Rauscher F J., III Science. 1991;253:1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- 88.Pelletier J, Bruening W, Li F P, Haber D A, Glaser T, Housman D E. Nature (London) 1991;353:431–434. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]
- 89.Pelletier J, Bruening W, Kashton C E, Maver S M, Manivel J C, Striegel J E, Habit R, Houston D C, Junien C, Haber R, et al. Cell. 1991;67:437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- 90.Luo R X, Postigo A A, Dean D C. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 91.Chow K N, Starostik P, Dean D C. Mol Cell Biol. 1996;16:7173–7181. doi: 10.1128/mcb.16.12.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chow K N, Dean D C. Mol Cell Biol. 1996;16:4862–4868. doi: 10.1128/mcb.16.9.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Day M L, Foster R G, Day K C, Zhao X, Humphrey P, Swanson P, Postigo A A, Zhang S H, Dean D C. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- 94.Svaren J, Sevetson B R, Apel E D, Zimonjic D B, Popescu N C, Milbrandt J. Mol Cell Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sadowski I, Bell B, Broad P, Hollis M. Gene. 1992;118:137–141. doi: 10.1016/0378-1119(92)90261-m. [DOI] [PubMed] [Google Scholar]