Abstract
Although it is generally accepted that females can gain material benefits by mating with more than one male, the proposal that polyandry provides genetic benefits remains controversial, largely because direct experimental support is lacking. Here, we report the results of a study testing for genetic benefits to polyandry in the pseudoscorpion Cordylochernes scorpioides. In an experiment that controlled for male mating experience and the number of spermatophores accepted by a female, twice-mated females received either one sperm-packet from each of two different males (the “DM” treatment) or two sperm-packets from a single male (the same male or “SM” treatment). Over their lifetime, DM females gave birth to 32% more offspring than did SM females, primarily because of a significantly reduced rate of spontaneous abortion. This result could not be attributed to male infertility nor to lack of sexual receptivity in males paired with previous mates. Spermatophore and sperm numbers did not differ between males presented with a previous mate and males paired with a new female. Because SM and DM females received the same quantity of ejaculate, it was possible to eliminate material benefits as a contributor to the enhanced reproductive success of DM females. The reduction in embryo failure rate achieved by DM females is most consistent with the genetic incompatibility avoidance hypothesis, i.e., that polyandry enables females to exploit postcopulatory mechanisms for reducing the risk and/or cost of fertilization by genetically incompatible sperm. This study, which rigorously controlled for material benefits and excluded inbreeding effects, demonstrates that polyandry provides genetic benefits that significantly enhance female lifetime reproductive success.
The revolution in molecular genetic techniques that has taken place over the past decade has revealed one of nature’s best-kept secrets, namely, that across a wide array of species, females frequently mate with more than one male (1, 2). As DNA evidence of multiple paternity accumulates for organisms as ecologically and phylogenetically divergent as fruit flies (3) and humpback whales (4), it is becoming clear that polyandry is a common female mating strategy, although it is often covert and difficult to detect at the behavioral level. Polyandry as a pervasive feature of natural populations challenges the long-held view of females as the choosy, monogamous sex (5–7) and raises the question of why, considering the potentially high risks and costs involved, females would mate with multiple males (8–9).
As evolutionary biologists increasingly recognize the importance of considering reproduction from the female perspective (10), a variety of hypotheses have been proposed to explain why selection should favor the evolution of polyandry. With the exception of forced copulation, these hypotheses fall into two broad but distinct categories. On one hand, it has been argued that, by mating with more than one male, females may enhance their survivorship and/or fecundity through some form of direct, material benefit. Such benefits include: (i) an adequate sperm supply (11); (ii) additional paternal care or access to male territories (12); (iii) sexually-transmitted antipredator defense chemicals (13); and (iv) male nutrient donations from nuptial gifts (14), spermatophores, or seminal fluid (15–18).
On the other hand, several hypotheses propose that the payoff to polyandry is genetically based. Genetic benefit hypotheses include: (i) the trading-up hypothesis, that females in socially monogamous species engage in extra-pair copulations to compensate for a poor-quality mate (19, 20); (ii) the offspring diversity hypothesis, that the increased offspring diversity resulting from multiple paternity enhances female fitness, either by reducing sibling competition or by serving as a hedge against environmental uncertainty (21–23); (iii) the intrinsic male quality hypothesis, that polyandry enables sperm competition or female choice of sperm to increase the probability that eggs are fertilized by high-quality sperm or sperm from high-quality males (24, 25); (iv) the sexually selected sperm hypothesis, that genetic benefits derive from Fisherian sexual selection, by which the sons of multiply-mated females produce competitively superior sperm or ejaculates (9); (v) the inbreeding avoidance hypothesis, that polyandry diminishes the cost of inbreeding in situations in which females cannot avoid mating with close relatives (26, 27); and (vi) the genetic incompatibility avoidance hypothesis, that mating with several males enables females to exploit postcopulatory mechanisms to minimize the risk and/or cost of fertilization by genetically incompatible sperm (28, 29).
Although it is generally accepted that females can gain material benefits by mating with more than one male (30), the argument that polyandry provides genetic benefits remains controversial (31). This disparity results, at least in part, from the paucity of direct experimental support for genetic benefit hypotheses, compared with the relatively large number of studies that have been interpreted as providing clear evidence of material benefits (32). Indeed, with the exception of one recent study of field crickets (33), the few investigations that provide support for genetic benefit hypotheses have been based strictly on observational evidence. Field investigations of adders (24) and sand lizards (34, 35) have demonstrated a correlation between mate number and female fitness. However, as in the field cricket study (33), the populations investigated were likely to have experienced recent genetic bottlenecks and thus inbreeding effects may have exerted an atypically strong effect on female mating tactics and reproductive success (RS). Although a strong case has been made for genetic benefits in adders and sand lizards (24, 34), potentially confounding effects could not be completely eliminated, because females were not randomly assigned to experimental treatments. A recent survey of the literature (36) indicates that confounding of potential material and genetic benefits is a general weakness of studies of polyandry and that many investigations purporting to show material benefits have not adequately controlled for possible genetic effects.
In this paper, we report the results of a study designed specifically to determine whether polyandry confers genetic benefits in outbred populations of the neotropical pseudoscorpion Cordylochernes scorpioides. Previous research has shown that field-inseminated females produce mixed-paternity broods sired by up to four males (37). Polyandry in this pseudoscorpion is an active strategy in which females recognize and reject previous mates, preferring instead to mate with different males (38). As a consequence of mating with more than one male, C. scorpioides females are able to enhance their RS. Females mated to two or three males in the laboratory were found to produce more offspring in their first brood than females mated to a single male (39). However, because both the number of mates and the amount of ejaculate received by each female varied among mating treatments in this study, it was not possible to determine conclusively whether the benefit to the polyandrous females was genetic or material. We therefore carried out an experiment that investigated the lifetime RS of females mated twice to the same male, compared with that of females mated once to each of two different males. By controlling for the effects of male mating experience and the amount of ejaculate received by females in the two experimental treatments, we were able to demonstrate that the higher lifetime RS achieved by females that mate with more than one male results from genetic, rather than material, benefits in this pseudoscorpion.
MATERIALS AND METHODS
Experiment I, Polyandry and Female Reproductive Success. Pseudoscorpions for this experiment were derived from 45 females and 38 males collected as nymphs in July 1997 from seven large populations inhabiting decaying Ficus spp. and Brosimum sp. trees in the former Canal Zone and the surrounding areas of the Republic of Panama (see ref. 38). After molting to the adult stage in the laboratory, these field-collected, virgin individuals were used in single-male matings, and the progeny were individually reared to provide virgin males (n = 210) and virgin females (n = 272) for the study. A further 32 experimental males were reared to virgin adulthood in the laboratory from nymphs collected in December 1997 from an additional decaying Canal Zone Ficus insipida tree. Field populations of this pseudoscorpion are known from both multilocus (40) and single-locus (41) minisatellite DNA fingerprinting to be outbred and highly genetically variable at the marker loci.
To examine the effect of polyandry on female RS, we carried out an experiment in which females were assigned to one of two treatments: (i) a “same-male” (SM) treatment in which females (n = 138) were each mated to a male and, 2 days after this first mating (mean ± SEM = 46 hr ± 0.5 hr), were given the opportunity to remate with the same male; and (ii) a “different-male” (DM) treatment in which females (n = 134) were each mated to a male (male A) and, 2 days later (46 hr ± 0.5 hr), were given the opportunity to mate with a second male (male B). This 2-day intermating interval design was selected to ensure that females in the SM treatment would not recognize and discriminate against previous mates (38). Using this regime, we mated 89% of the females (n = 242), first to a virgin male and then to a once-mated male. Because of a shortage of virgin males, the remaining 30 females, distributed equally across the treatments, were paired with males that had previously mated twice. To allow for replenishment of sperm supply, these “experienced” males were given a three-week rest period before re-use. To avoid possible confounding effects of inbreeding depression, no matings were carried out between full- or half-siblings. With this exception, males and females were assigned randomly to the experimental treatments.
To equalize male mating experience and refractory period in the SM and DM treatments, replications in the DM treatment were carried out in pairs and, for each pair, males were switched between the two replications for the second mating. For example, male A in replication 1 was used as male B in replication 2 and male A in replication 2 was used as male B in replication 1. Each replication was initiated by placing a virgin female with a male in a 28-mm diameter mating arena. Interactions were videotaped with a Super VHS camcorder for 30 min under red, fiber-optics illumination. Following this first mating opportunity, males and females were returned to their individual vials for 2 days.
The second mating opportunity was carried out and videotaped as above, with females being presented either with the same male or with a different male, depending on the experimental treatment. The videotape of each mating was then transcribed to determine the number of spermatophores deposited and the number of sperm-packets successfully transferred by the male to the female. In this pseudoscorpion, the combination of external spermatophore deposition and diagnostic sperm-uptake behavior by females provides a unique, noninvasive window on mating event characteristics, such as the number of spermatophores accepted and rejected by a female (for details of sperm transfer behavior, see ref. 38). Because the numbers of sperm-packets accepted by females in their second mating were binomially (0 or 1 packet) rather than normally distributed, the maximum likelihood option in the SAS categorical data modeling procedure, catmod (42), was used in the statistical analyses of female sexual receptivity.
The effect of mating treatment on female lifetime RS was quantified by closely monitoring the reproductive status of each female until she died. Females were maintained individually in 9-dram (32-ml), transparent plastic vials containing naturally produced Ficus frass, and were fed 8 to 10 late-instar Tribolium confusum larvae per week. Female reproductive status was checked every 3 to 4 days, increasing to once per day during the later stages of brood gestation until the nymphs were born. Pseudoscorpions are viviparous, the embryos being nourished with ovarian secretions as they develop within a translucent, external brood sac overlying the mother’s genital aperture (43). After becoming gravid, C. scorpioides females use frass and silken threads to construct a brood nest. Embryos require approximately 14 days to complete development, and after birth, the nymphs remain in the nest for 2 to 3 days until they are fully sclerotized (39). Because females generally construct their brood nests on the walls of their vials and embryonic development within the brood sac is readily visible with the naked eye, it was possible to monitor females and embryos with minimal disturbance. After nymphs had emerged from a female’s brood sac and shortly before they had completed sclerotization, the brood nest was carefully removed from the vial and the nymphs were counted. This process was repeated for each brood produced by each female. Female pseudoscorpions possess sperm storage organs and can produce multiple broods from a single insemination.
Because size is known to influence nymph production in this pseudoscorpion (39), we included female size as a covariate in all of our statistical analyses of female RS. Soon after the final nymphal molt to the adult stage and before mating, each female was held flat under a glass slide with the right pedipalp fully extended, and a digital image was recorded with a high-magnification (approximately ×30) video camera. The National Institutes of Health computer program image (version 1.58) was then used to compute linear measurements of six pedipalp and cephalothorax traits that are fixed in size at the terminal molt to the adult stage: total chela length, chela hand depth, tibia depth, femur depth, cephalothorax length, and cephalothorax width (see ref. 37). Using principal component analysis of these six, loge-transformed, morphological traits, we calculated a composite measure of size (principal component 1) for each female (see ref. 37).
To assess whether failure of females to successfully produce offspring could be attributed to male inability to produce sperm, we obtained a postexperimental estimate of the number of sperm transferred by a subset of the experimental males (n = 20). This was carried out by placing each male with a non-experimental female under a stereomicroscope (×20 magnification) and interrupting the mating immediately after spermatophore deposition. The sperm-packet was collected by adhesion to a dissecting needle and was broken up in 10 μl of distilled water to disperse the encysted sperm. A hemocytometer was used to count sperm, which are visible without staining, under a compound microscope at ×200 magnification (see ref. 44).
Experiment II, Sperm Production by Males Mated to New Females vs. Previous Mates.
A second experiment was conducted to ascertain whether female type (previous mate or new female) influences the number of sperm males allocate to their spermatophores. Virgin individuals for this experiment (n = 42 males and n = 62 females) were obtained from the same source population as in experiment I, and were randomly assigned to two mating treatments: (i) a same-female (SF) treatment, in which males (n = 22) were each mated to a female and, 2 days after this first mating (46 hr ± 0.5 hr), were given the opportunity to remate with the same female; and (ii) a different-female (DF) treatment, in which males (n = 20) were each mated to a female (female A) and, 2 days later (46 hr ± 0.5 hr), were given the opportunity to mate with a second female (female B). As in experiment I, to equalize male mating experience and refractory period in the SF and DF treatments, replications in the DF treatment were carried out in pairs; for each pair, males were switched between the two replications for the second mating. In both mating treatments, the male’s first mating was allowed to proceed uninterrupted for 30 min. In the second matings, males and females were observed under a stereomicroscope (×20 magnification) and the mating was interrupted immediately after deposition of the first spermatophore. The sperm-packet was collected and the sperm were counted, as described above.
RESULTS
Experiment I, Female and Male Sexual Receptivity to New vs. Previous Mates.
Female sexual receptivity and the production of complete spermatophores by males could not be unambiguously scored (see ref. 38) for 28 of the initial 272 females, and these females were excluded from further analysis. Of the remaining females, nearly all (240 of 244, or 98%) accepted a single sperm-packet in their first mating. Four females (1 DM and 3 SM) accepted two sperm-packets. Female sexual receptivity at second mating did not differ between experimental treatments. In both the SM and DM treatments, 64% of females accepted a single sperm-packet from the male (DM treatment: 78 of 122 females; SM treatment, 78 of 122 females). The remaining 88 females (DM, n = 44; SM, n = 44) accepted no sperm-packets during their second mating. These individuals constituted a third category of females, i.e., females that accepted a total of only one sperm-packet across their two mating opportunities; these females are henceforth termed “one sperm-packet” (OS) females. Males in their second mating showed no evidence of distinguishing between previous mates and new females: female type had no effect on the number of spermatophores males attempted to transfer [mean number of spermatophores produced ± SEM: SM = 1.67 ± 0.05, DM = 1.64 ± 0.05 (SAS CATMOD: χ2 = 1.11, P = 0.77)].
The Effect of Polyandry on Female Lifetime RS. A few (8 of 244) females died within a few days of mating and were excluded from subsequent analyses. A total of 34,770 nymphs were collected from the remaining 72 DM females, 77 SM females, and 87 OS females over the course of this experiment. It should be noted that OS females did not constitute a third treatment; they were placed in this category because of their sexual unreceptivity at second mating. The decision of OS females to accept only a single sperm-packet could, in theory, be linked to their RS, if, for example, OS females were in better condition than SM females. Such a difference would mask the reproductive consequences of additional material benefits gained through the acceptance of two sperm-packets. This explanation seems unlikely, however, because there was no difference between OS and SM females in either size or longevity (see below). Nonetheless, because the OS females did not represent a true (randomized) experimental treatment, they were not included in the main analyses. To provide a heuristic assessment of the effect of the number (one or two) of sperm-packets accepted by a female on her RS, we carried out a separate analysis that compared the lifetime RS of females in the DM, SM, and OS categories (see All-Female Analysis below).
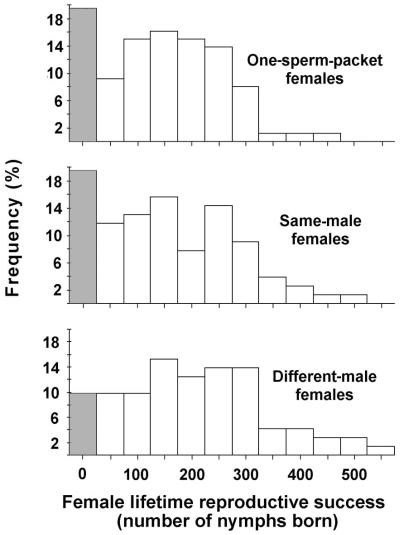
Females accepting one sperm-packet from each of two different males achieved 32% higher lifetime RS than females accepting two sperm-packets from a single male (mean number of nymphs born ± SEM: DM = 181 ± 15, SM = 137 ± 13; see Fig. 1). An analysis of covariance (ANCOVA) indicated that this treatment effect was highly significant (F1,148 = 7.71, P = 0.006). After fitting the ANCOVA model to the data, the residuals were found to be approximately, but not exactly, normally distributed (Shapiro–Wilk test: W = 0.965). A nonparametic Kruskal–Wallis test was therefore carried out on the residuals of a regression of nymph number on female cephalothorax length. The treatment effect remained highly significant (χ2 = 7.23, P = 0.007). Cephalothorax length was used as the covariate in these analyses because this trait exhibited the highest correlation with female lifetime RS (r = 0.268, P < 0.0001). The correlation coefficients for the other traits were as follows: total chela length, r = 0.143; chela hand depth, r = 0.186; tibia depth, r = 0.100; femur depth, r = 0.138; cephalothorax width, r = 0.077; composite size measure (principal component 1), r = 0.166.
Figure 1.
Frequency distribution of the number of nymphs born over the lifetime of females in the three mating categories. In each histogram, the stippled bar represents the proportion of females producing no offspring.
The higher RS of DM females was found to result primarily from a significantly lower rate of complete brood failure for DM females compared with that suffered by SM females. Whereas there was no significant between-treatment difference in the number of females that did not become gravid (4 of 72 DM females vs. 8 of 77 SM females, Fisher’s exact test, P > 0.10), for those females that produced at least one brood, the mean percentage of broods that spontaneously aborted per female was 24% ± 3% for DM females (n = 68) compared with 36% ± 4% for SM females (n = 69).
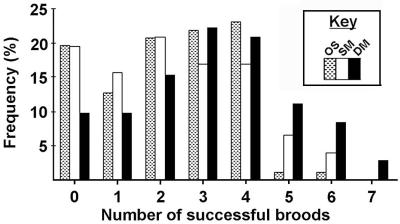
This difference was highly significant; the Kruskal–Wallis test on residuals of a regression of the percentage of aborted broods on female cephalothorax length yielded χ2 = 7.57, P = 0.006. As a consequence, the mean number of successful broods (broods carried to term) was significantly higher for DM females than for SM females (DM = 3.15 ± 0.21, SM = 2.31 ± 0.20); the Kruskal–Wallis test on residuals of a regression of number of successful broods on female cephalothorax length yielded χ2 = 11.37 (P = 0.001) (Fig. 2). Although DM females produced more successful broods, the mean number of nymphs born per successful brood did not differ between treatments (DM = 53.97 ± 2.17, SM = 56.02 ± 2.25); the Kruskal–Wallis test on residuals of a regression of nymph number per successful brood on female cephalothorax length yielded χ2 = 0.002 (P = 0.962).
Figure 2.
Frequency distribution of the number of successful broods produced over the lifetime of females in the three mating categories.
All-Female Analysis.
Females in the OS category achieved a mean lifetime RS of 129 ± 11 nymphs born, a value which was 94% of that for SM females and 71% of that for DM females. In an ANCOVA carried out on nymph production by females in the DM (two sperm-packets, two mates), SM (two sperm-packets, one mate) and OS (one sperm-packet, one mate) categories, lifetime RS was unaffected by the number of sperm-packets accepted by a female but was strongly influenced by the number of mates (overall treatment effect: F2,232 = 4.82, P = 0.009). The contrast based on the hypothesis that female RS was influenced by sperm-packet number and not by mate number (i.e., RSDM = RSSM > RSOS) was not significant (t = 1.39, P = 0.165), whereas the contrast positing that female RS was influenced by mate number and not by sperm-packet number (i.e., RSDM > RSOS = RSSM) was highly significant (t = 3.10, P = 0.002).
Female Size and Survivorship.
Females in the three mating categories did not differ significantly in size for any of the six measured traits (ANOVA, P > 0.05). In addition, neither an ANOVA nor a product-limit survival estimate method revealed any significant effect of mating category on female survivorship (F2,224 = 0.833, P = 0.436; χ2 = 1.511, P = 0.470). The mean age at death (measured in weeks) for the three categories was: DM = 27.27 ± 0.89, SM = 26.01 ± 0.84, and OS female = 25.81 ± 0.81).
Females Achieving Zero RS:
The Result of Male Infertility? Sperm counting provided no evidence that females achieving zero RS, i.e., females that never became gravid or females that aborted all broods initiated, did so as a result of mating with males that produced abnormally low numbers of sperm. All males produced spermatophores containing sperm. The sperm counts of males (n = 11) mated to a subset of these “failed” OS and SM females did not differ significantly from those of males (n = 9) mated to “successful” OS and SM females achieving normal brood production (failed = 2,529 ± 377, successful = 2,259 ± 339; t = 0.521, P = 0.610).
Experiment II, Sperm Production by Males Mated to New Females vs. Previous Mates.
All of the 42 males used in this experiment produced spermatophores containing sperm (sperm count range, 1,111 to 4,867). We found no evidence that males transfer fewer sperm to previous mates than to new females (DF = 2,353 ± 161, SF = 2,340 ± 195; t = 0.048, P = 0.962).
DISCUSSION
This study provides evidence that, by mating with more than one male, C. scorpioides females significantly enhance their RS through genetic benefits that decrease the probability of embryo failure. Females that accepted one sperm-packet from each of two males gave birth to 32% more offspring over their lifetime than did females that accepted two sperm-packets from a single male. This result is attributable largely to the significantly lower rate of complete brood failure experienced by the polyandrous DM females, rather than to any between-treatment difference in either the rate of failure to ever become gravid or the number of nymphs born per successful brood. The lower RS of females mated to a single male could not be explained by the inability of some males to produce sperm or by a lack of sexual receptivity on the part of males paired with previous mates. All males examined produced sperm-packets containing sperm, and the mates of females with zero RS were found to have normal sperm counts. At second mating, whether or not a male had previously transferred sperm to a female influenced neither his willingness to produce a spermatophore nor the number of sperm contained in the sperm-packet.
Females in the DM and SM treatments received the same number of sperm-packets in an experimental design that controlled for male mating experience and refractory period. It is therefore extremely unlikely that the lower rate of spontaneous abortion suffered by DM females could have resulted from between-treatment differences in the quantity of any material benefits received from males. Consistent with this interpretation is the fact that females receiving two sperm-packets from a single male (SM females) did not achieve significantly higher RS than females receiving only one sperm-packet (OS females). Our results thus indicate that genetic rather than material benefits to polyandry are responsible for the higher RS of those females that were mated to two males.
What form of genetic benefits do C. scorpioides females gain by mating with more than one male? Two genetic benefit hypotheses can be readily eliminated: (i) the sexually selected sperm hypothesis (9), because it predicts an advantage to polyandry that is not manifested until male offspring attain sexual maturity, and (ii) the inbreeding avoidance hypothesis (26, 27), because all experimental pseudoscorpions were first-generation, laboratory-reared, outbred offspring of individuals from large, genetically diverse, field populations.
In addition, the offspring diversity hypothesis (21–23) cannot adequately explain why polyandrous C. scorpioides females spontaneously aborted fewer broods than monandrous females under uniform laboratory conditions. The trading-up hypothesis (19, 20) can also be rejected as an explanation for our results. As Yasui (30) has pointed out, trading-up is actually a mate-choice hypothesis, because it presents polyandry as the incidental outcome of limitations to choice of initial mate on the part of essentially monandrous females. In C. scorpioides, polyandry has been shown to be an active strategy, with no evidence that females choose mates based on phenotypic indicators of inherent male genetic quality (38). Instead, a strategy of short-term discrimination against previous mates and accumulation of sperm from more than one male appear to be the driving forces shaping female mating decisions in this pseudoscorpion (38). Given the experimental design of the study, the trading-up hypothesis could not explain our results, because females were assigned males randomly, and the intermating interval was selected to ensure that females would not recognize previous mates.
On the basis of the data reported here, we cannot reject the intrinsic male quality hypothesis (24, 25) as a possible explanation of our findings. As in an earlier study of polyandry in this pseudoscorpion (39), females that received sperm from more than one male achieved higher RS, primarily as a consequence of a significantly lower rate of embryo failure than that suffered by females mated to a single male. Embryonic inviability leading to spontaneous abortion could have stemmed from fertilization by sperm carrying intrinsically “inferior” haploid genomes, incapable of successfully supporting embryonic development. However, this explanation is unlikely. If poor genetic quality of males and/or sperm were the underlying cause of embryo failure, the reproductive consequences of mating with a particular male should be consistent across females. As has been demonstrated elsewhere, however, this is not the case in C. scorpioides. In a study in which 67 males were each mated to two females, the numbers of nymphs born to each male’s two mates were found to be completely uncorrelated. The absence of a consistent effect of intrinsic male quality was most apparent in the 29 cases of complete brood failure. Broods of both females failed for only two of the 67 males. However, for 25 of the remaining males, mating resulted in complete brood failure in one female but successful nymph production in the other (39).
Given the absence of consistent effects of male genotype on female RS in C. scorpioides, the results of our study are most concordant with the genetic incompatibility avoidance hypothesis (28, 29). This hypothesis posits that embryo failure resulting from intraspecific genetic incompatibility between maternal and paternal genomes is a significant factor influencing the evolution of female mating strategies (28, 29). By accumulating sperm from several males, females that engage in polyandry can enhance their RS by exploiting postcopulatory mechanisms that reduce the risk and/or cost of fertilization by genetically incompatible sperm. Three postcopulatory mechanisms could account for the reduced incidence of spontaneous abortion in the DM treatment of this study. Sperm competition and/or female choice of sperm may have acted as barriers to fertilization by sperm carrying haploid genomes incompatible with either the nuclear or cytoplasmic genome of the female. In addition, the rate of complete brood failure may have been lower in this treatment, because maternal resources in this viviparous species were reallocated from inviable to viable embryos in mixed-paternity broods of the DM females.
The potential for postcopulatory processes to provide a defense against genetic incompatibility is likely to be influenced, of course, by the pattern of sperm precedence exhibited by the species in question. In C. scorpioides, DNA profiling has shown that, when a female is mated to two males with a 24-hr intermating interval, the second male sires essentially all the offspring (the proportion of offspring sired by the second male to mate, P2, = 0.98). Last-male sperm precedence was reduced significantly when the intermating interval was increased to 48 hr (P2 = 0.75; ref. 44). This reduction in P2 value was the consequence of complete last-male sperm precedence occurring in approximately half the replications but an absence of any mating order effects (i.e., mixed paternity) in the remaining replications. Interestingly, if this pattern of last-male sperm precedence is taken into account, the number of cases in which DM females failed to achieve any RS (7 of 72 females) is highly concordant with the number that would be predicted by the genetic incompatibility avoidance hypothesis (8.38 of 72 females; χ2 = 0.257, P > 0.05).
The expected proportion of females producing no offspring in the DM treatment can be calculated as follows. The probability of a randomly chosen C. scorpioides male being genetically incompatible [P(IC)] with a female is estimated as the proportion of SM females that failed to give birth to any offspring (15 of 77 = 0.1948). Similarly, the probability of genetic compatibility [P(C)] is 62/77, or 0.8052. In the DM treatment, a female received sperm from one of four possible male A/male B pairs: (i) male A and male B both genetically compatible with the female (C, C), a pair expected to occur with a probability of P(C) × P(C) = 0.6483; (ii) a compatible male A and an incompatible male B (C, IC), a permutation with a probability of P(C) × P(IC) = 0.1569; (iii) an incompatible male A and a compatible male B (IC, C), again with a probability of 0.1569, and (iv) an incompatible male A and an incompatible male B (IC, IC), a permutation that occurs with a probability of P(IC) × P(IC) = 0.0379.
Based on patterns of sperm precedence in this pseudoscorpion (see above), the (C, IC) male pair should result in mating order constraints overriding postcopulatory incompatibility avoidance in approximately 50% of cases. In the (IC, C) case, because the compatible male mates second, any mating order constraints will not contribute to fertilization by incompatible sperm. Given these considerations, the expected level of zero RS in the DM treatment is 0.1569/2 + 0.0379 = 0.1164, compared with the observed value of 0.0972. This is probably a conservative estimate of the capacity of postcopulatory mechanisms to enhance female RS in natural populations of C. scorpioides, because two lines of evidence suggest that mating order effects are not important in this pseudoscorpion in nature. First, last-male sperm precedence breaks down completely when females are mated to three males (44). Second, females naturally inseminated in the field generally produce mixed-paternity broods with no evidence of strong mating order effects (37).
The results of this study demonstrate that C. scorpioides females derive genetic benefits from mating with more than one male, and that these benefits most likely take the form of genetic incompatibility avoidance. We suggest that incompatibility avoidance may also explain, at least partially, the advantages of polyandry in a number of other species. Studies of grasshoppers (45), crickets (46), and house sparrows (47) have found that females mated to single males more frequently fail to produce any offspring or lay eggs that never hatch. These may be unfertilized eggs laid by sperm-depleted females (48). Alternatively, hatching failure may be the result of fertilization by genetically incompatible sperm and subsequent embryo failure. For example, Birkhead and colleagues (49) found that 80% of house sparrow eggs that failed to hatch had been fertilized. Indeed, a survey of the literature on female choice of sperm indicates that the best documented examples of this form of postcopulatory sexual selection involve genetic incompatibility avoidance (50).
Many authors have suggested that it is material benefits rather than genetic benefits that provide the greatest selective advantage in the evolution of polyandry (7, 14, 30, 32). Certainly there are numerous examples of mating systems, particularly in Orthoptera and Lepidoptera, in which males make material contributions that enhance the RS and/or survival of their mates. Nonetheless, most studies that have investigated material benefits have not adequately controlled for potential genetic benefits. A recent review of the literature on 23 species in which females benefit from mating with multiple males illustrates this point (36). Of 15 studies in which the authors concluded that females acquire material benefits from polyandry, only two considered genetic benefits as a possible factor influencing their results (51, 52). Neither of these studies properly controlled for genetic benefits by maintaining the number of mates or the number of matings constant across treatments. In only two of the 15 studies could benefits to multiple mating be attributed exclusively to material donations (15, 53).
For eight of the 23 species, the authors concluded that females gain genetic benefits from mating with multiple males. Three of these studies also incorporated correlative methods to consider potential material benefits (24, 34, 39). However, as with studies concluding genetic benefits to polyandry based on field observations (19, 22, 27, 47), the findings were correlational and were not based on a controlled experiment with a randomized design. In only one study (33) was a randomized, experimental design used to factor out possible material benefits and thereby demonstrate genetic benefits to polyandry. However, the generality of this finding remains unclear. As the authors pointed out, their experiment was carried out on individuals from a population of Gryllus bimaculatus (crickets) maintained in the laboratory for approximately 30 years. The possibility that the results were influenced by a high level of inbreeding could not therefore be excluded (33). The study reported here has factored out potential material benefits and demonstrated, in the absence of inbreeding effects, that polyandry is a mating strategy that provides females with genetic benefits that significantly enhance their lifetime RS.
Acknowledgments
We thank the Instituto Nacionale de Recursos Naturales Renovables for permission to collect pseudoscorpions in the Republic of Panama. We are grateful for the assistance of the Smithsonian Tropical Research Institute, for logistical support from Lisa Meffert and Gerard Wellington, and for comments by Ed Bryant and Merrill Hiscock on an earlier version of the manuscript. The research was supported by the University of Houston Coastal Center, the National Geographic Society, and grants from the National Science Foundation to J.A.Z (IBN-9603735) and to D.W.Z. (IBN-9514245).
ABBREVIATIONS
- DF
different female
- DM
different male
- OS
one sperm-packet (female)
- RS
reproductive success
- SF
same female
- SM
same male
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Eberhard W G. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton Univ. Press; 1996. [Google Scholar]
- 2.Birkhead T R, Møller A P, editors. Sperm Competition. New York: Academic; 1998. [Google Scholar]
- 3.Imhof M, Harr B, Brem G, Schlotterer C. Mol Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Clapham P J, Palsboll P J. Proc R Soc Lond Ser B. 1997;264:95–98. [Google Scholar]
- 5.Williams G C. Adaptation and Natural Selection. Princeton: Princeton Univ. Press; 1966. [Google Scholar]
- 6.Trivers R L. In: Sexual Selection and the Descent of Man 1871–1971. Campbell B, editor. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- 7.Maynard Smith J. Trends Ecol Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. [DOI] [PubMed] [Google Scholar]
- 8.Chapman T, Liddle L F, Kalb J M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 9.Keller L, Reeve H K. Adv Stud Behav. 1995;24:291–315. [Google Scholar]
- 10.Gowaty P A, editor. Feminism and Evolutionary Biology. New York: Chapman and Hall; 1997. [Google Scholar]
- 11.Ridley M. Biol Rev. 1988;63:509–549. [Google Scholar]
- 12.Davies N B. Dunnock Behavior and Social Evolution. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 13.González A, Rossini C, Eisner M, Eisner T. Proc Natl Acad Sci USA. 1999;96:5571–5574. doi: 10.1073/pnas.96.10.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard Univ. Press; 1983. [Google Scholar]
- 15.Gwynne D T. Nature (London) 1984;307:361–363. [Google Scholar]
- 16.Vahed K. Biol Rev Camb Philos Soc. 1998;73:43–78. [Google Scholar]
- 17.LaMunyon C W, Eisner T. Proc Natl Acad Sci USA. 1993;90:4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitnick S, Spicer G S, Markow T. Evolution. 1997;51:833–845. doi: 10.1111/j.1558-5646.1997.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 19.Kempenaers B, Verheyen G R, van der Broeck M, Burke T, van Broeckhoven C, Dhondt A A. Nature (London) 1992;357:494–496. [Google Scholar]
- 20.Hasselquist D, Bensch S, von Schantz T. Nature (London) 1996;381:229–232. [Google Scholar]
- 21.Loman J, Madsen T, Håkansson T. Oikos. 1988;52:69–72. [Google Scholar]
- 22.Watson P J. Anim Behav. 1991;41:343–360. [Google Scholar]
- 23.Ridley M. Am Nat. 1993;142:893–910. [Google Scholar]
- 24.Madsen T, Shine R, Loman J, Håkansson T. Nature (London) 1992;355:440–441. [Google Scholar]
- 25.Birkhead T R, Møller A P, Sutherland W J. J Theor Biol. 1993;161:51–60. [Google Scholar]
- 26.Brooker M G, Rowley I, Adams M, Baverstock P R. Behav Ecol Sociobiol. 1990;26:191–199. [Google Scholar]
- 27.Stockley P, Searle J B, MacDonald D W, Jones C S. Proc R Soc Lond Ser B. 1993;254:173–179. doi: 10.1098/rspb.1993.0143. [DOI] [PubMed] [Google Scholar]
- 28.Zeh J A, Zeh D W. Proc R Soc Lond Ser B. 1996;263:1711–1717. [Google Scholar]
- 29.Zeh J A, Zeh D W. Proc R Soc Lond Ser B. 1997;264:69–75. [Google Scholar]
- 30.Yasui Y. Trends Ecol Evol. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- 31.Stockley P. Proc R Soc Lond Ser B. 1997;264:1497–1500. doi: 10.1098/rspb.1997.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker G A. Nature (London) 1992;355:395–396. [Google Scholar]
- 33.Tregenza T, Wedell N. Evolution. 1998;52:1726–1730. doi: 10.1111/j.1558-5646.1998.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 34.Olsson M, Madsen T, Shine R, Gullberg A, Tegelström H. Nature (London) 1994;372:230. [Google Scholar]
- 35.Olsson M, Shine R, Madsen T. Nature (London) 1996;383:585. [Google Scholar]
- 36.Newcomer S D. M.S. thesis. Houston, TX: University of Houston; 1998. [Google Scholar]
- 37.Zeh D W, Zeh J A, Bermingham E. Proc R Soc Lond Ser B. 1997;264:119–125. [Google Scholar]
- 38.Zeh J A, Newcomer S D, Zeh D W. Proc Natl Acad Sci USA. 1998;95:13732–13736. doi: 10.1073/pnas.95.23.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeh J A. Behav Ecol Sociobiol. 1997;40:111–118. [Google Scholar]
- 40.Zeh D W, Zeh J A, Coffroth M A, Bermingham E. Heredity. 1992;69:201–208. [Google Scholar]
- 41.Zeh D W, Zeh J A, May C A. Mol Ecol. 1994;3:517–522. doi: 10.1111/j.1365-294x.1994.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 42.SAS/STAT User’s Guide (1988) (SAS Institute, Cary, NC), Release 6.03.
- 43.Weygoldt P. The Biology of Pseudoscorpions. Cambridge, MA: Harvard Univ. Press; 1969. [Google Scholar]
- 44.Zeh J A, Zeh D W. Proc R Soc Lond Ser B. 1994;257:287–292. [Google Scholar]
- 45.Butlin R K, Woodhatch C W, Hewitt G M. Evolution. 1987;41:221–225. doi: 10.1111/j.1558-5646.1987.tb05785.x. [DOI] [PubMed] [Google Scholar]
- 46.Sakaluk S K, Cade W H. Can J Zool. 1980;58:404–411. [Google Scholar]
- 47.Wetton J H, Parkin D T. Proc R Soc Lond Ser B. 1991;261:55–63. [Google Scholar]
- 48.Wedell N. Evolution. 1993;47:1203–1212. doi: 10.1111/j.1558-5646.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 49.Birkhead T R, Veiga J P, Fletcher F. J Avian Biol. 1995;26:343–345. [Google Scholar]
- 50.Birkhead T R. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 51.Arnqvist G. Anim Behav. 1989;38:749–756. [Google Scholar]
- 52.LaMunyon C W. Ecol Entomol. 1997;22:69–73. [Google Scholar]
- 53.Turner M E, Anderson W W. Evolution. 1983;37:714–723. doi: 10.1111/j.1558-5646.1983.tb05593.x. [DOI] [PubMed] [Google Scholar]