Abstract
MHC class I molecules usually present peptides derived from endogenous antigens that are bound in the endoplasmic reticulum. Loading of exogenous antigens on class I molecules, e.g., in cross-priming, sometimes occurs, but the intracellular location where interaction between the antigenic fragment and class I takes place is unclear. Here we show that measles virus F protein can be presented by class I in transporters associated with antigen processing-independent, NH4Cl-sensitive manner, suggesting that class I molecules are able to interact and bind antigen in acidic compartments, like class II molecules. Studies on intracellular transport of green fluorescent protein-tagged class I molecules in living cells confirmed that a small fraction of class I molecules indeed enters classical MHC class II compartments (MIICs) and is transported in MIICs back to the plasma membrane. Fractionation studies show that class I complexes in MIICs contain peptides. The pH in MIIC (around 5.0) is such that efficient peptide exchange can occur. We thus present evidence for a pathway for class I loading that is shared with class II molecules.
MHC molecules display antigenic peptides on the cell surface for surveillance by T lymphocytes. MHC class I molecules present peptides to CD8+ cytotoxic T cells, whereas MHC class II molecules present peptides to CD4+ Th cells. The current dogma is that antigens from the extracellular fluid enter the exogenous processing pathway by endocytosis and are partially degraded in acidic endosomal or lysosomal structures to yield peptides that bind MHC class II molecules. This type of processing is inhibited by reagents that prevent endosomal acidification (chloroquine, NH4Cl) (1). In the endogenous processing pathway intracellular proteins are degraded in the cytosol by the proteasome complex, generating peptides that are transported from the cytoplasm into the lumen of the endoplasmic reticulum (ER) by the transporters associated with antigen processing (TAP), where they bind to nascent MHC class I heavy chain-β2-microglobulin (β2m) heterodimers. Fully assembled class I/peptide complexes exit the ER and are transported through the Golgi to the cell surface by the constitutive secretory route. This processing pathway can be blocked by proteasome inhibitors or Brefeldin A (BFA), an inhibitor of anterograde ER-Golgi transport, but not by lysosomotropic agents. Thus, in general endogenous antigens are presented by MHC class I molecules, and exogenous antigens are displayed at the cell surface by MHC class II molecules. However accumulating evidence has shown that this dichotomy in presentation of antigen from endogenous and exogenous origin is not absolute. It was demonstrated that cytotoxic T lymphocyte (CTL) responses can be primed in vitro and in vivo with exogenous antigen (reviewed in refs. 2 and 3). At least two fundamentally different pathways for presentation of exogenous antigens by MHC class I molecules in vitro have been described: one involving access of exogenous antigen to the classical MHC class I loading pathway (TAP dependent and BFA sensitive) and another involving unconventional post-Golgi loading of MHC class I molecules (TAP independent and BFA resistant). In the latter pathway the antigen presumably is processed in an acidic endosomal or lysosomal compartment (chloroquine and leupeptin sensitive). How peptides generated by endosomal/lysosomal degradation are loaded onto MHC class I molecules is unknown. The antigenic peptides either can be regurgitated followed by binding to peptide-receptive cell surface MHC class I or they can be captured by endocytosed MHC class I molecules, which then recycle back to the cell surface as proposed by Schirmbeck and coworkers (4). Indeed endocytosis and recycling of MHC class I molecules has been suggested (reviewed in refs. 2 and 3).
We show that class I molecules can present epitopes of the measles virus (MV) F protein in a TAP-independent and NH4Cl-sensitive manner. We have tagged class I HLA-A2 molecules with the green fluorescent protein (GFP) to study intracellular transport in living cells. Our results show that a fraction of internalized cell surface class I molecules intersect the class II presentation pathway. Upon arrival in acidic MHC class II compartments (MIICs) these MHC class I complexes can release their peptides, and together with MHC class II molecules, they are transported to the cell surface. In transit or at the cell surface, these recycling MHC class I molecules can bind new peptides for presentation to cytotoxic T cells.
MATERIALS AND METHODS
Cell Lines and Culture Conditions.
The Epstein–Barr virus (EBV)-transformed B lymphoblastoid cell lines WH, VDK, and JP (5) and T2 HLA-B*2705 were used as antigen-presenting cells (APCs). To obtain high expression of HLA-B27 in the human TAP-deficient T2 cell line, a genomic clone of HLA-B*2705 (6) was cloned into the BamHI site of the pREP9 expression vector and introduced into TAP-deficient T2 cells (T2 HLA-B*2705). T2 HLA-B*2705 cells showed stable high cell surface expression of HLA-B*2705 molecules by FACS analysis (7). MV-infected or peptide-pulsed APCs were used as target cells in a 51Cr-release assay or after fixation with paraformaldehyde as stimulator cells in a proliferative T cell assay as described (5). The CD8+ T cell clone WH-F40 is HLA-B*2705 restricted, MV-F protein (epitope sequence RRYPDAVYL) specific (5). JP III.8 is a CD8+, class I-restricted MV-F protein-specific T cell clone (5). To investigate the effect of NH4Cl on MHC class I restricted presentation of the MV-F protein, APCs were infected with MV in the presence of 20 mM NH4Cl for 1 h, washed twice, and further cultured in RPMI 1640, 10% FCS, and 20 mM NH4Cl overnight, then cells were fixed with paraformaldehyde. The human melanoma cell line Mel JuSo (8) was transfected with HLA-A2-GFP. The HLA-A2 cDNA was kindly obtained from T. Boon, Ludwig Institute for Cancer Research, Brussels. The stop codon of HLA-A2 was eliminated and a 3′ BamHI site was introduced by PCR using oligo 5′ CGGCGGATCCCACTTTACAAGCTGTGAGAG and T3 primer. The 1,096-bp PCR product was subcloned into EcoRI/BamHI-digested pEGFP-N3 (CLONTECH). Subsequently, the 893-bp PCRed EcoRI/BpuAI fragment from pEGFP-N3 was exchanged with the original 893-bp EcoRI/BpuAI fragment from pBKS-HLA-A2. Positive clones were selected by FACS and maintained in Iscove’s medium supplemented with 8% FCS and 0.5 mg/ml G418.
Biochemical Analysis.
Mel JuSo HLA-A2-GFP cells stimulated for 48 h with 200 units/ml of human recombinant IFN-γ (Boehringer Ingelheim, Ingelheim, Germany) were labeled for 15 min with 150 μCi of 35S Met/Cys and chased for 0, 15, 30, 60, 120, and 240 min followed by lysis in 1% digitonin lysis buffer (50 mM Tris⋅HCl pH 7.4/10 mM MgCl2/150 mM NaCl) and split into three equal aliquots. Aliquots 1 and 2 were incubated for 1 h at 0°C and 37°C, respectively. MHC class I complexes were isolated from equal amounts of trichloroacetic acid-precipitable radioactivity by W6/32 (9). Aliquot 3 was kept at 4°C and immunoprecipitated with anti-TAP2 serum (10). Subsequently, aliquots 1 and 3 were immunoprecipitated with anti-heavy chain αHC (11) and anti-human β2m BBM.1 (12) antibodies, respectively. All samples were analyzed by 10% SDS/PAGE.
Analysis by Confocal Laser Scanning Microscopy.
Routinely, cells were cultured for 2 days in the presence of 200 units/ml of human recombinant IFN-γ before analysis. Immunofluorescence with anti-human MHC class II HLA-DR serum (13) and anti-CD63 antibodies (14) was performed as described (8). When indicated, cells were cultured for 4 h in the presence of 10 μg/ml BFA (Sigma) or for 30 min in the presence of 100 μM chloroquine. Alternatively, cells were incubated for 1 min in the presence of 300 nM LysoTracker Red DND99 (Molecular Probes) before analysis. Confocal analyses of fixed and living cells were performed as described (8). Trails of moving vesicles were visualized by making images every second during a 1-min window and subsequently superimposing all 60 images.
Electron Microscopy.
Mel JuSo HLA-A2-GFP cells stimulated for 48 h with 200 units/ml of human recombinant IFN-γ were fixed and processed for electron microscopy as described (8). Sections were stained with a mouse mAb against CD63 (mAb 435) and a rabbit polyclonal anti-MHC class I antibody. Sections were examined with a CM10 electron microscope (Philips Electronic Instruments, Eindhoven, The Netherlands).
pH-Dependent MHC Class I Dissociation Assay.
Mel JuSo HLA-A2-GFP cells stimulated for 72 h with 200 units/ml of human recombinant IFN-γ were lysed in 1 ml of 1% NP-40 lysis buffer (50 mM NaAc/HAc/10 mM MgCl2/150 mM NaCl) pH 4.0, 4.5, 5.0, 5.5, 6.0, or 8.0 ± 0.15 for 30 min on ice. Each lysate was split into four equal aliquots. No peptide was added to aliquots 1 and 4; for aliquot 2, 0.1 mg/ml HPV16 E7-derived HLA-A2-binding peptide (15) and for aliquot 3, 0.1 mg/ml HPV16 E7-derived peptide + 0.1 M Tris⋅HCl pH 7.4 was added. Tris-HCl (pH 7.4 suffices to neutralize the NaAc/Hac buffers). All samples were incubated for 1 h on ice and subsequently aliquots 2, 3, and 4 were incubated for 2 h at 37°C while aliquot 1 remained on ice. For the dissociation time-course experiment each lysate was split into four equal aliquots. All samples were incubated for 1 h on ice and subsequently aliquots 2, 3, and 4 were incubated for 1, 2, and 4 h at 37°C, respectively, while aliquot 1 remained on ice. After the various incubations, 1 ml of 1% NP-40 lysis buffer with 50 mM Tris⋅HCl, pH 7.4 was added to all aliquots, and MHC class I complexes were isolated with the W6/32 antibody. The samples were analyzed by Western blotting after separation by 10% SDS/PAGE using anti-class I heavy chain antibodies.
Subcellular Fractionation by Density Gradient Electrophoresis (DGE).
Mel JuSo HLA-A2-GFP cells stimulated for 48 h with 200 units/ml of human recombinant IFN-γ were incubated for 30 min with 1 mg/ml of horseradish peroxidase (HRP) at 37°C. Organelles were prepared and incubated with 0.1 mg/ml of Triticum vulgaris wheat germ agglutinin, which allows further separation of plasma membranes and ER, as described (16). Fractions of 250 μl were collected, and HRP and β-hexosaminidase activities, to identify the endosomal and lysosomal fractions, were determined as described (8). Every five fractions were pooled such that the five lysosomal peak fractions were pooled together. The pooled fractions were lysed in 2.5 ml of 1% NP-40 lysis buffer, and each pooled fraction was split in two: one half was incubated for 2 h on ice (A) and the other half was incubated for 2 h at 37°C (B). MHC class I molecules subsequently were isolated by using W6/32. The lysates subsequently were immunoprecipitated with anti-MHC class I heavy chain serum, followed by isolation of MHC class II complexes with Tü36 (17). All samples were analyzed by Western blotting after separation by 10% SDS/PAGE using anti-class I heavy chain serum, HCA2 and HC10 (18, 19) mAbs, and anti-human MHC class II α chain serum (α-α) (20).
RESULTS
TAP-Independent and NH4Cl-Sensitive Presentation of MV-F Protein by MHC Class I Molecules.
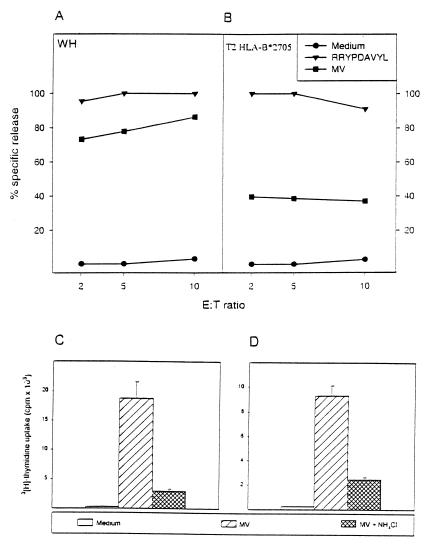
A TAP-deficient T2 HLA-B*2705 transfectant with high cell surface expression of HLA-B27 in FACS analysis (data not shown), when pulsed with peptide is killed with equal efficiency (>90% specific lysis; Fig. 1B) as the autologous (WH) targets by the CTL clone WH-F40 (Fig. 1A). Surprisingly, when infected with MV, T2 HLA-B*2705 cells presented MV-F-derived peptides in a TAP-independent fashion (Fig. 1B). If MV-F is not loaded onto class I molecules in a TAP-dependent manner, loading in endo/lysosomes onto recycling MHC class I molecules would be an alternative. If so, the degradation and presentation of MV-F by class I molecules should be sensitive to lysosomotropic agents like NH4Cl. To test this, autologous (JP) and HLA-B*2705 matched EBV-transformed B lymphoblastoid cell lines were infected with MV in the presence of NH4Cl, fixed, and used to stimulate JP III.8 and WH-F40 T cell clones in proliferative T cell assays. Because NH4Cl prevents killing by CTLs, proliferation was used as a read-out. MV infection of cells occurs at neutral pH (21, 22) and thus is not affected by lysosomotrophic agents like NH4Cl, which was confirmed by equal levels of surface-expressed MV-F protein in cells infected and cultured in the presence or absence of NH4Cl as measured by FACS (not shown). Surprisingly, NH4Cl treatment profoundly inhibited the response of both T cell clones (Fig. 1 C and D), indicating the involvement of acidic compartments in the presentation of MV-F by class I molecules. At the dose NH4Cl used (20 mM), no effects were seen on: (i) the viability of the antigen-presenting cell, (ii) the level of MHC class I expression after infection as measured by FACS analysis, and (iii) total DNA and protein synthesis as measured by 3[H]Tdr and l-[4,5-3H]leucine incorporation, respectively (data not shown), indicating that normal cellular processes, including the classical MHC class I loading pathway, were not affected.
Figure 1.
TAP-independent and NH4Cl-sensitive presentation of the MV-F protein to MHC class I-restricted T cell clones. Autologous EBV- transformed B lymphoblastoid cell lines (EBV B-LCL) WH (A) and the T2 HLA-B*2705 transfectant (B) were infected either with MV at a multiplicity of infection of 3.0, sham-infected, or pulsed with 1 μM peptide (RRYPDAVYL) for 24 h, as indicated. Cells were used as targets in a 4-h 51Cr release assay with the MV-F-specific CTL clone WH-F40 at effector-to-target (E:T) cell ratios of 2, 5, and 10. Results are expressed as the mean percentages of specific target cell lysis of triplicate cultures. Both peptide pulsed targets were killed with equal efficiency. TAP-deficient T2 HLA-B*2705 transfectant was efficiently lysed. To determine whether class I-restricted presentation is affected by lysosomotropic agents, EBV B-LCL JP (C) and WH (D) were infected with MV in the presence or absence of 20 mM NH4Cl. Fixed cells were used as stimulator cells for JP III.8 (C) and WH-F40 T cell clones (D) in a proliferative T cell assay. cpm ± SD of triplicate cultures are shown. Treatment of stimulator cells during MV infection with NH4Cl inhibits their capacity to present MV-F protein-derived peptide in a MHC class I-restricted fashion.
GFP Tagging Does Not Affect TAP Association, Assembly, and Intracellular Transport of HLA-A2.
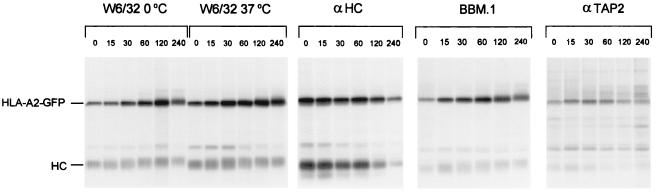
To follow intracellular trafficking of MHC class I molecules, the HLA-A2 heavy chain was tagged with GFP. Human melanoma Mel JuSo cells, which express all molecules necessary for efficient MHC class I and class II antigen presentation (8), were stably transfected with the HLA-A2-GFP heavy chain. Proper assembly of the GFP-tagged HLA-A2 heavy chain with endogenous β2m and peptide, and subsequent transport of this complex to the cell surface were verified biochemically in a pulse–chase experiment in which class I molecules were immunoprecipitated with W6/32, αHC, BBM.1, and αTAP2 antibodies and analyzed by SDS/PAGE (Fig. 2). The GFP-tagged class I complex migrates at 68 kDa, whereas endogenous class I runs at 43 kDa by SDS/PAGE. HLA-A2-GFP forms a complex with endogenous β2m and peptide as is visualized by the disappearance of the free heavy chains and the appearance of HLA-A2-GFP-containing complexes that can be recovered by W6/32 and BBM.1, conformation-specific antibodies that recognize only heavy chain-β2m complexes (Fig. 2, lanes αHC, W6/32 0°C, and BBM.1). These complexes have stably bound peptide as 37°C-resistant HLA-A2-GFP complexes can be retrieved by the conformation-specific antibody W6/32 (Fig. 2, lanes W6/32 37°C). Temperature stability as a result of peptide binding is a well-documented and sensitive assay for antigenic peptide loading of MHC class I molecules (23). Before being loaded with peptide, empty HLA-A2 complexes associate with the peptide transporter complex TAP, an interaction that likely facilitates peptide loading of a large portion of the empty class I molecule (10, 24). Like endogenous MHC class I complexes, HLA-A2-GFP complexes can be retrieved with anti-TAP antibodies showing that the GFP moiety does not interfere with TAP binding (Fig. 2, lanes αTAP2). Transport of MHC class I complexes along the secretory pathway is reflected by maturation of their carbohydrates and by an increased molecular weight. Fig. 2 shows that HLA-A2-GFP complexes are transported (lanes W6/32 0°C, W6/32 37°C, and BBM.1), although HLA-A2(-GFP) is somewhat slower than the endogenous class I molecules (HLA-A1, -B8). This finding is in line with the allele-specific differences observed before (11). Thus, GFP-tagged HLA-A2 behaves like endogenous class I molecules with respect to TAP association, assembly, peptide loading, and intracellular transport, indicating that attachment of GFP to the cytosolic tail of class I heavy chain does not affect the processes occurring on the luminal side of the molecule.
Figure 2.
GFP tagging does not affect assembly and transport of HLA-A2. To determine whether cytoplasmic tagging of the HLA-A2 heavy chain with GFP affects its maturation and transport, a pulse–chase experiment was performed. Mel JuSo stably transfected with HLA-A2-GFP was biosynthetically labeled for 15 min followed by culture for the times indicated. Cells were lysed, and the lysate was split into three equal aliquots. Aliquots 1 and 2 were incubated for 1 h at 0°C and 37°C, respectively and MHC class I complexes were immunoprecipitated with the conformation-specific antibody W6/32. Subsequently, aliquots 1 and 3 were immunoprecipitated with anti-heavy chain and anti-human β2m antibodies, respectively. Aliquot 3 was kept at 4°C and immunoprecipitated with anti-TAP2 serum. All samples were analyzed by 10% SDS/PAGE. The position of endogenous class I (HC) and GFP-tagged HLA-A2 (HLA-A2-GFP) are indicated. HLA-A2-GFP heavy chains associate with β2m (lanes W6/32 0°C, αHC, and BBM.1) to form complexes with stably bound peptides (lanes W6/32 37°C). In addition, HLA-A2-GFP-containing MHC class I complexes still are able to bind transiently to the TAP transporter (lanes αTAP2). HLA-A2-GFP containing complexes behave like endogenous complexes with respect to maturation, assembly, and TAP dissociation.
Some HLA-A2-GFP-Containing Vesicles Have Similar Features as MIICs.
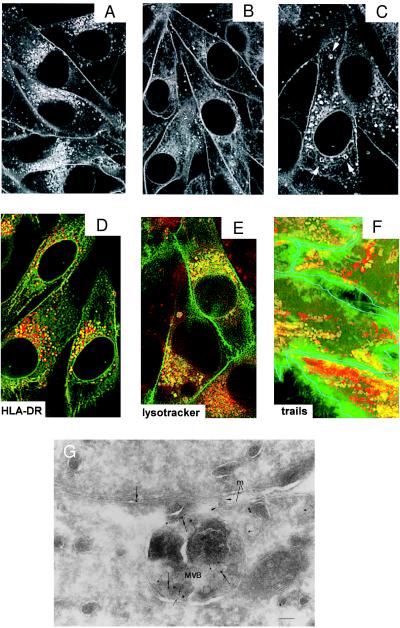
The intracellular distribution of HLA-A2-GFP molecules was determined by confocal laser scanning microscopy. In stably transfected Mel JuSo cells HLA-A2-GFP localizes at the cell surface and in the ER. IFN-γ treatment results in enhanced cell surface staining and appearance of fluorescent perinuclear and peripheral vesicles (Fig. 3A). Most of these vesicles are derived from the constitutive secretory pathway (the Golgi and trans-Golgi network) as incubation of living Mel JuSo HLA-A2-GFP with BFA (Fig. 3B) or cycloheximide (data not shown) for 4 h leads to a strong decrease in the amount of vesicles. However, a subset of the vesicles is resistant to BFA treatment. To determine the nature of these HLA-A2-GFP-containing vesicles that are not directly derived from the biosynthetic pathway, the cells were fixed and stained with antibodies against markers for lysosomal(-like) structures such as CD63 and MHC class II. Some of the HLA-A2-GFP containing vesicles are HLA-DR (Fig. 3D) and CD63 (data not shown) positive. To analyze whether HLA-A2-GFP-containing vesicles are acidic, cells were treated with the weak base chloroquine. Chloroquine raises the pH and causes swelling of the acidic compartments (25). Indeed, in chloroquine-treated cells a few HLA-A2-GFP vesicles are swollen (Fig. 3C). Additional proof that some HLA-A2-GFP vesicles are acidic was obtained by incubating living cells with LysoTracker Red, a membrane-diffusable fluorochrome that accumulates in acidic compartments (Fig. 3E). In summary, these results indicate that a fraction of the HLA-A2-GFP molecules reside in lysosomal (-like) MIICs. Real-time confocal imaging on living cells incubated with LysoTracker Red reveals that, in addition to moving acidic vesicles that do not contain HLA-A2-GFP (Fig. 3F, red trails), a fraction of HLA-A2-GFP-containing vesicles are acidic and move in a stop-and-go fashion (Fig. 3F, yellow trails) similar to HLA-DRβ-GFP-containing MIICs (8). Fusion of MHC class I-containing MIICs with the plasma membrane was visualized by immuno electron microscopy on the transfectants stimulated with IFN-γ. To verify that a fusing structure is derived from MIIC and not from the plasma membrane, we labeled the sections with anti-CD63 (CD63 is an MIIC marker that is not at the plasma membrane) (Fig. 3G, small gold) and anti-MHC class I antibodies (Fig. 3G, large gold). Fig. 3G shows a trapped fusion event of a multivesicular body containing class I and CD63 with the plasma membrane, similar to what we have described for class II molecules (8). Thus, a fraction of the HLA-A2-GFP-containing vesicles are acidic, contain MHC class II and CD63 molecules, move like the MHC class II-containing vesicles, and eventually fuse with the plasma membrane, showing a full cycle of class I that uses the class II pathway for reappearance at the cell surface.
Figure 3.
HLA-A2-GFP-containing endosomal vesicles have similar features as MIICs. Confocal analysis of the intracellular distribution of HLA-A2-GFP in living Mel JuSo HLA-A2-GFP stimulated for 48 h with 200 units/ml of IFN-γ and cultured at 37°C (A) without any further treatment. Fluorescence is observed at the cell surface and in perinuclear and peripheral vesicles. (B) Transfectants after additional treatment with 10 μg/ml BFA for 4 h resulting in a strong decrease of the amount of fluorescent perinuclear vesicles and (C) after additional treatment with 100 μM chloroquine for 30 min. Chloroquine causes swelling of a fraction of the HLA-A2-GFP-containing vesicles (indicated by arrows). (D) Merged image of double labeling of fixed Mel JuSo HLA-A2-GFP (green) stained with anti-HLA-DR antibodies visualized with Texas Red-conjugated secondary antibodies. Colocalization is seen in yellow because of the combination of the green and red signal showing that a fraction of the HLA-A2-GFP-containing vesicles is positive for MHC class II molecules. (E) Merged image of double labeling of living Mel JuSo HLA-A2-GFP cells incubated with LysoTracker Red to label acidic compartments showing that a fraction of the HLA-A2-GFP-containing vesicles is acidic. (F) Trail analysis of moving vesicles in living Mel JuSo HLA-A2-GFP cells incubated with LysoTracker Red. Images of the moving vesicles were made every sec for 1 min and all 60 images were superimposed. Green trails are created by vesicles that contain only HLA-A2-GFP, yellow trails are created by vesicles that contain HLA-A2-GFP and LysoTracker Red, and red trails are created by vesicles that contain only LysoTracker Red. For clarity, the cell surface of the different cells is outlined in blue. A fraction of HLA-A2-GFP vesicles are acidic and move as MIIC vesicles. (G) Fusion of a class I-containing multivesicular body (MVB) with the plasma membrane. Mel JuSo HLA-A2-GFP stimulated for 48 h with 200 units/ml of IFN-γ were fixed, and cryosections were labeled with anti-CD63 (5 nm gold; small arrows) and anti-MHC class I heavy chain (10 nm gold; large arrows) antibodies. A MVB trapped in the process of fusion with the plasma membrane (m) and containing both class I and CD63 is shown. Note the continuity of the limiting membrane of the MVB with the plasma membrane (arrowheads) and that the lumen is open to the intercellular space between two cells. (Bar: 100 nm.)
Formation of Empty HLA-A2-GFP Complexes at pH 5.0.
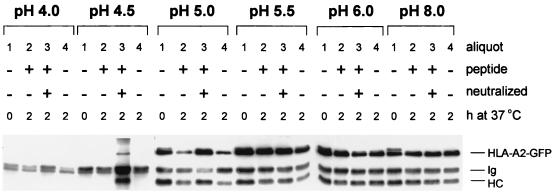
To assay the effect of acidification on the integrity of MHC class I complexes, Mel JuSo HLA-A2-GFP cells were lysed in buffers of different pH. After a 30-min incubation on ice, either no peptide or an HLA-A2 binding peptide was added to lysates with or without neutralizing the lysis buffer. To discriminate between MHC class I complexes that were empty and MHC class I complexes that had stably bound peptide, the lysates were incubated at 37°C for 2 h or maintained at 0°C followed by immunoprecipitation with the conformation-specific antibody W6/32. Fig. 4 shows that at pH ranging from 8 to 5.5 MHC class I molecules with stably bound peptides can be retrieved under every condition assayed. At pH 5.0 the peptide dissociates from the MHC class I complex as visualized by the decrease in temperature stability resulting in the retrieval of less MHC class I complexes by W6/32 after incubation at 37°C (Fig. 4, compare lanes 1 and 4 of pH 5.0). Addition of peptide only does not increase the amount of stable MHC class I complexes at pH 5.0 (Fig. 4, lane 2, pH 5.0) unless the acidic pH is neutralized by the addition of Tris buffer (Fig. 4, lane 3, pH 5.0). Incubation of MHC class I complexes at a pH lower than 5.0 results in their complete dissociation because W6/32 precipitable material cannot be detected anymore (Fig. 4, lanes 1, 2, and 4 of pH 4.0 and 4.5). Note that a fraction of class I molecules is stable at 37°C at pH 5.0, which may indicate that they contain “acid-resistant” peptide or that the dissociation has not gone to completion. To distinguish between these possibilities, class I molecules were incubated at pH 5.0 or 5.5 for various times, followed by a temperature-stability assay. Release of peptide at pH 5.0 is a kinetic process, indicating that there is not a pool of MHC class I complexes that remains stable at pH 5.0 (data not shown). Prolonged incubation at pH 5.5 does not result in any release of peptide (data not shown). In summary, MHC class I complexes can retain their integrity until pH 5.5, but at pH 5.0 the MHC class I complex first releases its peptide and at pH lower than 5.0 the heavy chain-β2m heterodimer dissociates completely. These results suggest that at pH 5.0, which corresponds to the pH of late endosomal/early lysosomal compartments like MIIC, peptide-receptive “empty” MHC class I complexes can be generated.
Figure 4.
pH-dependent peptide release and MHC class I dissociation. Mel JuSo HLA-A2-GFP cells were stimulated for 72 h with 200 units/ml of IFN-γ lysed in 1% NP-40 lysis buffer at pH 4.0, 4.5, 5.0, 5.5, 6.0, or 8.0, and each lysate was split into four equal aliquots. No peptide was added to aliquots 1 and 4. HPV16 E7-derived HLA-A2-binding peptide was added to aliquot 2, and HPV16 E7-derived HLA-A2-binding peptide + Tris⋅HCl, pH 7.4 was added to aliquot 3. All aliquots were incubated for 1 h on ice, and subsequently aliquots 2, 3, and 4 were incubated for 2 h at 37°C, while aliquot 1 remained on ice. Then 1 ml of NP-40 lysis buffer, pH 7.4 was added to each aliquot, and MHC class I complexes were immunoprecipitated with the conformation-specific antibody W6/32 without prior preclearing. The samples were analyzed by Western blotting after separation by 10% SDS/PAGE using anti-heavy chain antibodies. The position of endogenous class I (HC), GFP-tagged HLA-A2 (HLA-A2-GFP), and antibody heavy chain (Ig) is indicated. At a pH lower than 5.0 MHC class I complexes dissociate completely. At pH 5.0 peptide is dissociating from MHC class I complexes as is visualized by the decrease in temperature stable complexes that can be retrieved by W6/32.
Detection of HLA-A2-GFP-Peptide Complexes in MIIC.
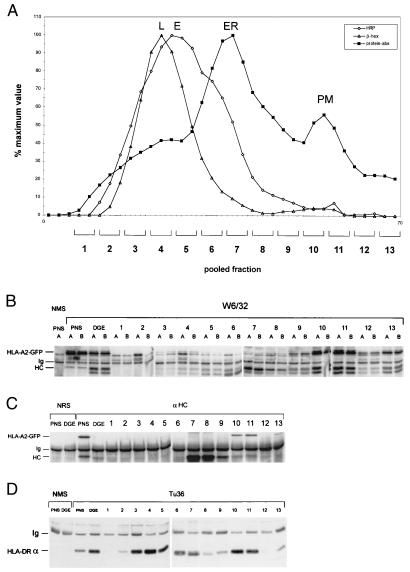
Because we were able to detect stable and peptide-receptive MHC class I complexes at lysosomal pH in vitro, as well as a fraction of HLA-A2-GFP in class II-containing lysosomes, we investigated whether HLA-A2-GFP in lysosomal class II compartments was complexed to peptide, free of peptide but assembled with β2m or present as free heavy chains. In confocal and electron microscopy analysis, the GFP labeling would have been observed in all three cases because it visualizes the heavy chain. Therefore, we performed subcellular fractionation of Mel JuSo HLA-A2-GFP cells by DGE using a protocol that separates class II containing early lysosomes (MIIC) from endosomes, ER, and plasma membrane (16). Cells stimulated for 48 h with IFN-γ were cultured in the presence of the fluid phase endocytosis marker HRP for 30 min to label the endosomes. After fractionation, HRP and β-hexosaminidase activities were measured to determine the position of endosomes and lysosomes in the DGE fractions (Fig. 5A). Every five fractions were pooled such that the β-hexosaminidase activity peak fractions were in one pool. Na− H+ ATPase has positioned the plasma membrane in fractions equivalent to pooled fractions 10 and 11 and the ER in fractions equivalent to pooled fraction 7 (16). The pooled fractions were lysed and split in two equal halves. One half was incubated on ice (Fig. 5B, lanes A) and the other half was incubated at 37°C (Fig. 5B, lanes B) for 2 h to determine the amount of stable versus unstable MHC class I complexes by immunoprecipitation with W6/32. Fig. 5B shows that most of the endogenous and HLA-A2-GFP complexes can be found at the plasma membrane (pooled fractions 10 and 11), but that a small amount of endogenous and HLA-A2-GFP complexes also can be detected in the lysosomal fractions (pooled fractions 3 and 4). These complexes have stably bound peptides because the same amount of W6/32 reactive material can be precipitated at 4°C and 37°C (Fig. 5B, compare lanes A and B). Sequential immunoprecipitation of lysates A with αHC recovered only HLA-A2-GFP and endogenous heavy chains at the plasma membrane and ER (mainly endogenous), but not in the pooled lysosomal fraction (Fig. 5C). This lack of free MHC class I heavy chains in lysosomes is probably caused by rapid degradation of free heavy chains after complete dissociation of MHC class I complexes at acidic pH. Because a fraction of class I resides in class II-containing compartments (see Fig. 3D), lysates A were further precipitated with the class II-specific mAb Tü36. Fig. 5D shows the presence of MHC class II complexes at the plasma membrane and in the pooled lysosomal fraction, where the small pool of temperature stable class I also was detected. Note the relative difference in distribution of class I and class II complexes over lysosomal and plasma membrane fractions.
Figure 5.
HLA-A2-GFP complexes can be detected in MIICs after subcellular fractionation by DGE in the presence of the lectin wheat germ agglutinin. (A) Postnuclear supernatant of Mel JuSo HLA-A2-GFP stimulated for 48 h with 200 units/ml of IFN-γ and cultured for 30 min with HRP was fractionated by DGE. Total protein concentration (squares) and enzymatic activity of HRP (diamonds) and β-hexosaminidase (triangles) were determined in alternate fractions. The peak positions of the lysosomes (L), late endosomes (E), the ER, and plasma membrane (PM) are indicated; early endosomes migrate around fraction 6. Every five fractions were pooled such that the lysosomal fractions (peak β-hexosaminidase activity) were in one pool. (B) The pooled fractions were lysed and split in two: one half was incubated on ice for 2 h (lanes A) and the other half was incubated at 37°C for 2 h (lanes B). MHC class I complexes were immunoprecipitated with W6/32 and visualized by Western blotting after separation by 10% SDS/PAGE using anti-heavy chain antibodies. Endogenous and HLA-A2-GFP complexes are present mainly at the plasma membrane (fractions 10 and 11), but a small amount of endogenous and HLA-A2-GFP complexes with stably bound peptide can be found in the lysosomes (fractions 3 and 4). (C) Sequential immunoprecipitation of the DGE fractions with anti-heavy chain antibodies. MHC class I heavy chains were immunoprecipitated with αHC and analyzed by Western blotting after separation by 10% SDS/PAGE using the anti-heavy chain mAbs HCA2 and HC10. HLA-A2-GFP and endogenous heavy chains can be retrieved from plasma membrane (fractions 10 and 11) and ER (fraction 7), but not from lysosomes and early and late endosomes (fractions 3–6). (D) Sequential immunoprecipitation of the DGE fractions with anti-MHC class II complex antibodies. MHC class II complexes were immunoprecipitated with Tü36 and analyzed by Western blotting after separation by 10% SDS/PAGE using anti-MHC class II α chain serum. MHC class II complexes are present at the plasma membrane (fractions 10 and 11), in the ER (fraction 7), and in MIIC (fraction 3 and 4) where class I complexes reside as well (B).
DISCUSSION
The strict segregation of the MHC class I and class II loading pathways has been challenged by recent reports indicating that MHC class I molecules can acquire peptides derived from exogenous antigens (reviewed in refs. 2, 3, and 26). Exogenous antigens are internalized and degraded in endocytic compartments by proteases that are active at low pH. The question of where and how such peptides generated by endosomal/lysosomal degradation are loaded onto class I molecules is still a matter of debate. Here we show that stable class I-peptide complexes are able to arrive in endocytic compartments, also known as MIICs, which usually are designated for loading MHC class II molecules.
By tagging the HLA-A2 heavy chain with GFP, we have been able to show that a small fraction of these class I molecules can be found in acidic compartments that contain CD63 and MHC class II molecules. Unlike class II molecules, the HLA-A2-GFP molecules in these compartments are not directly derived from the biosynthetic pathway because peripheral fluorescent vesicles are still detectable after incubation with BFA for 4 h. This finding suggests that these MHC class I molecules are routed from the cell surface into the endocytic pathway where they intersect the MHC class II intracellular pathway. These MHC class I molecules thus are exposed to the acidic environment of the endosomes. We show that at pH 5.0 MHC class I complexes release their peptides, which implies that, at this pH, peptide-receptive MHC class I molecules are generated to which some peptides may bind with high affinity (27). Therefore, we conclude that reloading of these peptide-receptive MHC class I molecules in endo/lysosomal compartments and/or upon reappearance at the plasma membrane can occur. This assumption is supported by the finding that stable MHC class I complexes can be detected in MIICs by DGE fractionation. At a pH lower than 5.0, β2m dissociates from the MHC class I heavy chain. The resulting free heavy chain is probably rapidly degraded as we failed to detect it in our fractionation studies, whereas MHC class I-peptide complexes present in MIIC vesicles can be recycled to the plasma membrane.
TAP-deficient cells expressing high cell surface levels of HLA-B27 can present a MV-F protein peptide in a TAP-independent manner. This phenomenon is not observed in TAP-deficient cells with lower cell surface expression of HLA-B27 (5). This finding suggests that high cell surface expression probably allows sufficient amounts of HLA-B27 to cycle to and survive in endosomal compartments, where binding of the MV epitope occurs, followed by presentation at the cell surface. In addition, we observed that class I presentation of MV-F protein epitopes by several B cell lines was severely inhibited by NH4Cl. Recently, the importance of presentation of exogenous antigens by MHC class I for the initiation of CTL responses to viral infections has been shown (26). The authors proposed a TAP-dependent exogenous MHC class I pathway. Our results support an alternative model in which cell surface MHC class I molecules are endocytosed and arrive in MHC class II compartments. In these compartments MHC class I molecules release their peptide and exogenous peptides can be bound here or after reappearance at the cell surface. Interestingly, presentation of hsp73-associated peptides is TAP independent and NH4Cl sensitive (28). Uptake of hsp73-associated peptides in the recycling MHC class I pathway we have visualized appears to be a plausible mechanism.
During cross-priming antigenic material from immunogenic cells is transferred to professional antigen-presenting cells of the host, thereby inducing CTL reactivity. The mechanism by which the antigenic fragments arrive in the class I pathway is largely unclear, but uptake by recycling MHC class I molecules would be an efficient mechanism. Cells with a high plasma membrane turnover, as may be expected for dendritic cells and macrophages upon apoptotic body uptake, consequently will cointernalize many surface class I molecules (29). Regulation of the pH of the MIICs by activation of professional antigen-presenting cells after uptake of antigen (as described for B cells; ref. 30) could make this mechanism even more efficient. Conversely, disturbance of low pH by lysosomotrophic agents like NH4Cl or chloroquine could prevent peptide release from MHC class I complexes in lysosomes, resulting in a lack of peptide-receptive MHC class I complexes in these compartments. It is possible that Mycobacterium uses a similar strategy to survive intracellularly by preventing acidification of phagosomes (31). Therefore, the fraction of recycling MHC class I complexes that present exogenous antigens may be highly sensitive to factors influencing endosomal pH because peptide exchange, as we have shown, can occur efficiently only at appropriate low pH.
Acknowledgments
This paper is dedicated to the memory of Dr. Ab Tulp, who passed away in January 1999. We thank Eric Nooteboom for FACsorting the cells and Lauran Oomen and Martien Poelen for expert technical assistance. This research was supported by a Pioneer grant from the Dutch Society of Scientific Research.
ABBREVIATIONS
- ER
endoplasmic reticulum
- TAP
transporters associated with antigen processing
- β2m
β2-microglobulin
- BFA
Brefeldin A
- CTL
cytotoxic T lymphocyte
- GFP
green fluorescent protein
- MIIC
MHC class II compartment
- EBV
Epstein–Barr virus
- MV
measles virus
- DGE
density gradient electrophoresis
- HRP
horseradish peroxidase
References
- 1.Ziegler H K, Unanue E R. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock K L. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 3.Jondal M, Schirmbeck R, Reimann J. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 4.Schirmbeck R, Melber K, Reimann J. Eur J Immunol. 1995;25:1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 5.Van Binnendijk R S, van Baalen C A, Poelen M C M, de Vries P, Boes J, Cerundolo V, Osterhaus A D M E, Uytdehaag F G C M. J Exp Med. 1992;176:119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss E H, Kuon W, Dorner C, Lang M, Riethmuller G. Immunobiology. 1985;170:367–380. doi: 10.1016/S0171-2985(85)80061-9. [DOI] [PubMed] [Google Scholar]
- 7.Ellis S A, Taylor C, McMichael A. Hum Immunol. 1982;5:49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- 8.Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J. J Cell Biol. 1996;135:611–622. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnstable C J, Bodmer W F, Brown G, Galfre B B, Milstein C, Williams A F, Ziegler A. Cell. 1977;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 10.Neisig A, Wubbolts R, Zang X, Melief C, Neefjes J. J Immunol. 1996;156:3196–3206. [PubMed] [Google Scholar]
- 11.Neefjes J J, Ploegh H L. Eur J Immunol. 1988;18:801–810. doi: 10.1002/eji.1830180522. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky F M, Bodmer W F, Parham P. Eur J Immunol. 1979;9:536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- 13.Neefjes J J, Hensen E J, de Kroon T I P, Ploegh H L. Immunogenetics. 1986;23:341–347. doi: 10.1007/BF00398799. [DOI] [PubMed] [Google Scholar]
- 14.Vennegoor C, Calafat J, Hageman P, van Buitenen F, Janssen H, Kolk A, Rumke P. Int J Cancer. 1985;35:287–295. doi: 10.1002/ijc.2910350302. [DOI] [PubMed] [Google Scholar]
- 15.Ressing M E, Sette A, Brandt R M, Ruppert J, Wentworth P A, Hartman M, Oseroff C, Grey H M, Melief C J, Kast W M. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 16.Tulp A, Verwoerd D, Neefjes J. Electrophoresis. 1999;20:438–444. doi: 10.1002/(SICI)1522-2683(19990301)20:3<438::AID-ELPS438>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Shaw S, Ziegler A, DeMars R. Hum Immunol. 1985;12:191–211. doi: 10.1016/0198-8859(85)90336-2. [DOI] [PubMed] [Google Scholar]
- 18.Stam N J, Spits H, Ploegh H L. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 19.Stam N J, Vroom T M, Peters P J, Pastoors E B, Ploegh H L. Int Immunol. 1990;2:113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 20.Neefjes J J, Stollorz V, Peters P J, Geuze H J, Ploegh H L. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 21.Lamb R A, Kolakofsky D. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott–Raven; 1996. pp. 1177–1204. [Google Scholar]
- 22.Griffin D E, Bellini W J. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott–Raven; 1996. pp. 1267–1312. [Google Scholar]
- 23.Schumacher T N M, Heemels M-T, Neefjes J J, Kast W M, Melief C J, Ploegh H L. Cell. 1990;62:563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- 24.Ortmann B, Androlewicz M J, Cresswell P. Nature (London) 1994;368:864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- 25.Ohkuma S, Poole B. J Cell Biol. 1981;90:656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigal L J, Crotty S, Andino R, Rock K L. Nature (London) 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 27.Stryhn A, Pedersen L Ø, Romme T, Olsen A C, Nissen M H, Thorpe C J, Buus S. J Immunol. 1996;156:4191–4197. [PubMed] [Google Scholar]
- 28.Schirmbeck R, Reimann J. Eur J Immunol. 1994;24:1478–1486. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta J D, Watkins S, Slayter H, Yunis E J. J Immunol. 1988;141:2577–2580. [PubMed] [Google Scholar]
- 30.Siemasko K, Eisfelder B J, Williamson E, Kabak S, Clark M R. J Immunol. 1998;160:5203–5208. [PubMed] [Google Scholar]
- 31.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]