Abstract
Effective host T lymphocyte sensitization to malignant cells depends on successful antigen presentation. In this study, we examined the capacity of malignant myeloid progenitor cells of patients in the chronic phase of chronic myelogenous leukemia (CML) to acquire characteristics of activated dendritic cells (DCs) after intracellular calcium mobilization, thereby bypassing a need for third-party antigen-presenting cells. Treatment of purified CD33+ CML cells from 15 patients with calcium ionophore (CI) consistently resulted in de novo expression of the costimulatory molecules CD80 (B7.1) and CD86 (B7.2), CD40 and the DC-specific activation marker CD83, as well as marked up-regulation of MHC class I and II molecules and the adhesion molecule CD54. Most of these changes occurred within 24 hr of treatment. Morphologically, CI-treated CML cells developed long dendritic projections similar to those seen in mature DCs. Functionally, CI-treated CML cells provided stimulation of allogeneic T lymphocytes 10- to 20-fold that of untreated CML cells or untreated monocytes. Fluorescent in situ hybridization of CI-activated CML cells confirmed their leukemic origin by displaying the typical bcr/abl fusion signal. No difference in bcr/abl translocation percentages between untreated and CI-treated CML nuclei was observed. These observations indicate that calcium mobilization may constitute a valuable approach for rapidly and reliably generating CML-derived DCs for immunotherapy of CML.
Recent clinical observations indicate that T lymphocytes can play an important therapeutic role in chronic myelogenous leukemia (CML) (1, 2). Donor lymphocyte infusions are effective in inducing complete cytogenetic remission in patients who have relapsed after allogeneic bone marrow transplantation, indicating that donor lymphocytes mediate a graft-vs.-leukemia response in these patients (3–5).
A successful MHC-restricted response against CML is believed to depend on sufficient activation of CD8+ and CD4+ T lymphocytes, which recognize their leukemic target cell through CML-associated tumor antigens in the context of either MHC class I or class II molecules (6–8). CML cells are known to express various tumor antigens, including the bcr/abl fusion protein, a neoantigen not shared by any normal cells (9–12). Other cancer-specific tumor proteins in CML may include mutant p53 and mutant N-ras (12). The efficiency of antigen presentation by the leukemia cell and its susceptibility to cytotoxic targeting by activated T lymphocytes is contingent on appropriate expression of accessory molecules by the tumor cell (8). Costimulatory signals delivered through molecules such as B7, which interact with CD28 on the T lymphocytes, are essential to enhance T cell cytotoxicity (13, 14). Dendritic cells (DCs) are known to be potent antigen-presenting cells (APCs), capable of inducing naïve T lymphocyte sensitization (15, 16). Human DCs can originate from myeloid-origin progenitor cells in the bone marrow and, in addition, can be derived from human peripheral blood monocytes (17–19). Treatment of purified CD14+ monocytes with the cytokines granulocyte/macrophage colony-stimulating factor and IL-4 for 7–10 days, as well as with agents that increase intracellular calcium concentration in monocytes for 24–48 hr, results in rapid acquisition of multiple features typical of activated DCs (18, 19).
In the current study we demonstrate that leukemic myeloid progenitor cells obtained from peripheral blood of CML patients in chronic phase, depleted of monocytes and lymphocytes, likewise can be efficiently activated into APCs, with characteristics of mature DCs, by treating these cells with calcium-mobilizing agents.
MATERIALS AND METHODS
Preparation of CD33+ CML Cells.
After obtaining informed consent, heparinized blood samples were collected from 15 patients with CML in chronic phase. Cytogenetic analysis of the bone marrow of these patients revealed 100% positivity for the Philadelphia chromosome. Cells were either collected at diagnosis or during treatment with hydroxyurea or IFN-α. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by using a discontinuous density gradient method (Organon Teknika–Cappel). Contaminating monocyte and lymphocyte fractions were separated from the CML cell pool by superparamagnetic microbeads preconjugated with mouse mAb to human CD14, CD3, CD19, and CD56. The wash and incubation buffer used was Ca2+/Mg2+-free Hanks’ balanced salt solution (BioWhittaker) with 0.5% human serum albumin (Sigma). Briefly, fresh PBMCs were incubated with anti-CD14-conjugated microbeads at a concentration of 20 μg/107 cells for 15 min at 4°C and then applied to VS+ columns positioned on Vario MACS magnets (Miltenyi Biotec, Auburn, CA). Nonlabeled CD14-negative cells were washed through the column, collected, and submitted to a second sorting process by incubating cells with CD3-, CD19-, and CD56-conjugated microbeads. Incubation was performed in the same manner and yielded a purified, nonlabeled CD33+/CD3−/CD14−/CD19−/CD56− fraction. CD14+ cells obtained in the first sort were set aside in some CML patients for allosensitization studies.
Activation of Purified CML Cells.
Purified CML progenitor cells were plated in 24-well plates at 1 × 106 cells/ml. Cultures were maintained in Iscove’s modified Dulbecco’s medium (BioWhittaker) with 5% AB serum (Sigma)/100 units/ml penicillin/100 μg/ml streptomycin/50 μg/ml gentamycin sulfate/0.5 μg/ml amphotericin B/1 mM sodium pyruvate/0.1 mM nonessential amino acids. CML cells were either incubated in medium alone or medium with calcium ionophore (CI) at a concentration of 375 ng/ml (A23187; Sigma), which, in initial dose titration studies, was found to be the optimal dose for these experiments.
Phenotypic Analysis of Cells by Flow Cytometry.
Cells were assessed for surface marker expression by fluorescent multicolor flow cytometry (FACScan; Becton Dickinson). Ca2+/Mg2+-free Hanks’ balanced salt solution with 1% FCS and 0.1% sodium azide was used as a diluent/wash FACS buffer. Quality control of purified CML preparations was monitored by flow cytometry, staining cells with fluorescein-conjugated antibodies to CD33 (PharMingen), CD3, CD14, CD20, and CD56 (Becton Dickinson). Immunophenotypic profile of cultured CML cells was evaluated at different time points (1–5 days) by double staining with the FITC-conjugated anti-CD33 as well as phycoerythrin (PE)-conjugated antibodies to HLA-DR, B7.1 (CD80), and CD54 (intercellular adhesion molecule-1), which were obtained from Becton Dickinson; HLA-ABC and B7.2 (CD86) from PharMingen; CD40 from Serotec; and CD83, which was obtained from Caltag (South San Francisco, CA). Antibodies were incubated for 30 min at 4°C. After a wash with cold FACS buffer, cells were resuspended, 1 μg of propidium iodide (PI) (Sigma) was added per tube to distinguish viable from nonviable cells, and then multicolor analysis was performed immediately on unfixed cells by using a 5-W argon laser emitting 200 mW of 488-nm light. Simultaneous measurements of FITC, PE, and PI emissions were performed by using 530, 575, and 650 filters with acquisition in logarithmic mode. Nonviable cells were delineated clearly in FL3 (650) vs. forward-scatter display and were excluded from final analysis. All viable cells were analyzed in these immunophenotypic studies.
Enrichment of B7.1-Positive CML Myeloid Progenitor Cells After Treatment with CI.
CI-treated purified CML cells negative for immunophenotypical expression of CD14 were selected for B7.1-expressing cells by using the FACStar Plus cell sorter (Becton Dickinson). Briefly, CML cells were harvested after 4 days in culture with CI stained with the phycoerythrin (PE)-conjugated anti-B7.1 and immediately positively selected by using a 5-W argon ion laser emitting 200 mW of 488-nm light. PE fluorescence was detected by using logarithmic amplification in the FL2 channel with a 575DF26 bandpass filter. Two thousand B7.1-positive cells/sec were collected and then submitted to fluorescence in situ hybridization (FISH) assays for detection of the bcr/abl fusion gene.
Photomicroscopy.
Untreated and CI-treated, purified CML cells were evaluated for the acquisition of morphological features of DCs by Nomarski differential interference contrast microscopy. At day 5 cultured cells were harvested and transferred onto Lab-Tek 8 glass chamber slides (Nunc) previously coated with 1% polylysine (Sigma) and incubated for 1 hr at 37°C in 5% CO2. Unfixed cells then were photographed with an Olympus IX70 microscope (Olympus, New Hyde Park, NY).
Allogeneic Lymphocyte Proliferation Assays. Mixed leukocyte response assays were performed by using untreated and CI-treated CML cells as stimulator cells and allogeneic T lymphocytes as responder cells. Human T lymphocytes were obtained from lymphocyte-rich elutriation fractions of healthy donors. The ability of CI-treated CML cells to stimulate allogeneic lymphocytes was compared with that of untreated or CI-treated CD14+ monocytes.
Stimulator cells subjected to either medium alone or CI (375 ng/ml) treatment were harvested after 4 days, washed twice, γ-irradiated with 10 Gy, and then cocultured in triplicate with T lymphocytes in 96-well, flat-bottomed plates (Costar). The T cell number was held constant at 1 × 105 cells per well while the irradiated CML cells and monocytes were added to each well in graded numbers ranging from 650 to 20,000 cells per well. Cocultures were incubated for 4 days, pulsed with 1 μCi/well [3H]thymidine, and, 18 hr later, harvested onto fiberglass filter mats and counted for T lymphocyte proliferation in a β plate reader (Wallac, Gaithersburg, MD).
Genetic Analysis of CML Cells by FISH.
CML cells subjected to either medium alone or CI (375 ng/ml) treatment were harvested after 5 days and fixed onto glass slides. Monocytes of healthy donors maintained similarly in culture served as a negative control. CI-treated, B7.1-positive CML cells were fixed onto glass slides in the same fashion immediately after the sorting process. Specimen slides then were denatured, and the dual-color bcr/abl translocation DNA probe (Vysis, Downers Grove, IL) was applied to examine interphase nuclei. The FISH procedures were performed according to the manufacturer’s recommendations and photographed with a fluorescence microscope using a tricolor filter. Normal cells were expected to display two green (bcr gene) signals and two orange (abl gene) signals. Leukemic nuclei were expected to display one green, one orange, and one yellow signal that resulted from the fusion of the bcr/abl gene. Five hundred nuclei were counted and the percentage of positive fusion signals (yellow signals) was calculated. Very close bcr and abl signals were not scored as fusion signals.
RESULTS
Patients.
All CML patients included in this study presented clinically with considerable expansion of WBC in their peripheral blood, typically cells of the granulocyte lineage. WBC counts ranged from 18 to 269 × 106/cm3. Ten patients had not received any treatment at blood draw, one patient was treated with IFN-α for 14 days, three patients were treated with hydroxyurea for less than 14 days, and one patient had received hydroxyurea for longer than 14 days.
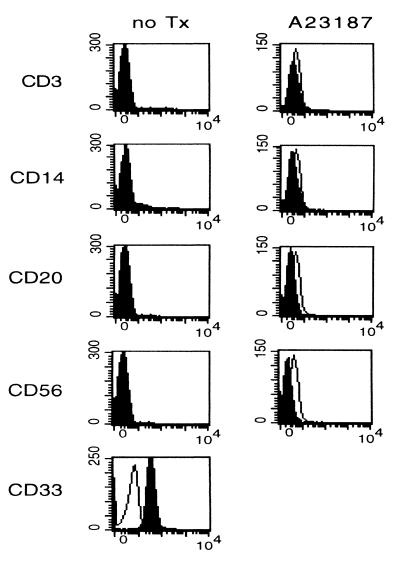
Immunophenotypical Analysis of Freshly Isolated CML Precursor Cells and Purified CML Cells Cultured in Standard Medium Alone. Immunocytochemical staining for CD33 of freshly isolated and untreated CML precursor cells from all patients demonstrated uniform expression, although this expression was dim compared with monocytes (Fig. 1). Furthermore, the purified cell population from 12 of 15 patients did not display any CD3, CD14, CD20, or CD56 lineage staining, confirming that these cells lacked lymphocyte or monocyte contamination and were of tumor origin (Fig. 1). Two patients expressed low levels of CD56 on their purified CML cells. A third patient showed a discrete monocytic phenotype. However, these CD14+ cells showed diminished staining for HLA-DR and negative staining for CD86, suggesting that these were leukemic cells of monocytic origin rather than contaminating monocytes, which are expected to stain positively for CD14+, HLA-DR, and CD86.
Figure 1.
Quality control of CML preparations after purification and after culture for 96 hr with CI. Progenitor cells were analyzed for their phenotype expression of CD3, CD14, CD20, CD56, and CD33 by flow cytometry. Open histograms represent the isotype-matched control; solid histograms represent staining for the specific antibodies.
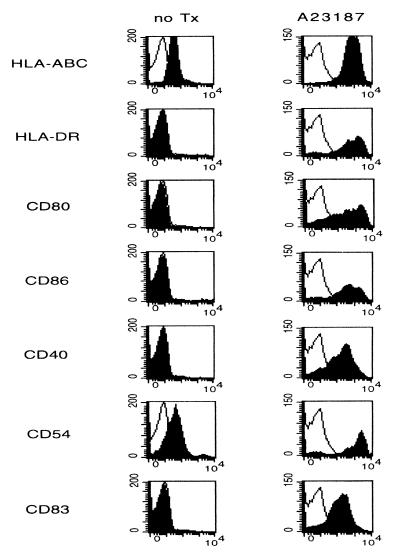
Fresh CML cells, as well as cells maintained in standard medium alone for up to 4 days, showed homogeneous surface expression of MHC class I molecules, whereas only minimal levels of HLR-DR were detectable. When incubated with a monomorphic mAb specific to HLA-DR, DP, and most DQ antigens, MHC class II expression increased, although it remained very dim (data not shown). Additionally, these cells stained for the adhesion molecule CD54 (intercellular adhesion molecule-1). The cells lacked expression of the costimulatory molecules B7.1 and B7.2 as well CD40 and the DC-associated activation marker CD83, again indicating that these cells did not display characteristics of normal monocytes or mature DCs (Fig. 2).
Figure 2.
Comparison of the immunophenotypes of purified CML cells cultured in standard medium alone and CML cells treated with CI (A23187). Cells were cultured for 4 days, harvested, and then analyzed for expression of DC surface markers HLA-A, B, and C, DR, CD80, CD86, CD40, CD54, and CD83. Open histograms represent the isotype-matched control, whereas solid histograms symbolize staining for the different antibodies. Untreated progenitor cells stained negatively for HLA-DR in this patient.
Immunophenotypical Analysis of CML Precursor Cells Treated with CI. Treatment with CI resulted in a homogeneous conversion of CML cells to a cellular phenotype closely resembling that of activated DCs, as evidenced by de novo expression of B7.1, B7.2, CD40, and CD83 as well as marked up-regulation of HLA-A, B, and C, HLA-DR, and CD54 by day 4 (Fig. 2). These effects were observed in CML progenitor cells of all 15 patients investigated regardless of their presenting WBC counts, prior treatment, or cell lineage characteristics. CI-treated cells retained their negativity for the B and T cell markers (Fig. 1). In some donors, CI induced dim staining of the monocytic marker CD14. The marked increase in HLA-DR expression occurred as early as 24 hr after treatment with CI and maximized 4 days thereafter. Notable de novo expression of the costimulatory molecules B7.1 and B7.2 was detectable 2–3 days into culture and peaked by day 4–5. Uniformly high CD40 and CD54 expression was maximal by day 4 and did not increase thereafter. De novo expression of the DC activation marker CD83 was highest 72 hr after the incubation with CI was initiated.
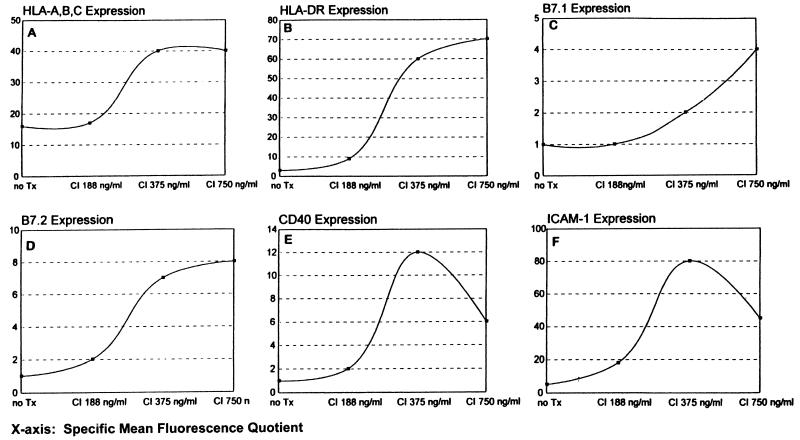
Dose-response studies indicated that maximal induction of activated DC characteristics in CML progenitor cells generally was achieved with a CI concentration of 375 ng/ml (Fig. 3); minimal toxicity was observed and harvest of cells after 72–96 hr typically yielded a 90% cell recovery, of which the cell viability ranged between 80% and 85% (data not shown).
Figure 3.
Dose-response analyses representative of CI’s effects on purified CML cells. Cell surface Ag expression data are plotted as specific mean fluorescence quotients, defined as the quotient between mean fluorescent intensity of cells stained with the specific antibody and mean fluorescent intensity of cells stained with the subclass isotype-matched control.
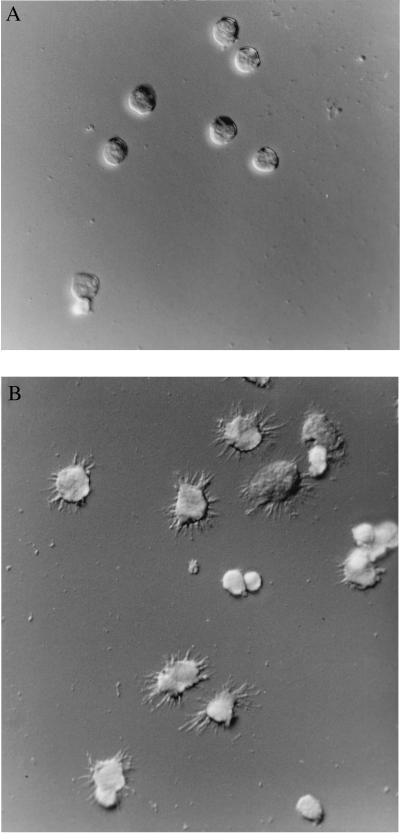
CI Induces Dendritiform Morphological Features in CML Progenitor Cells.
CI treatment causes CML progenitor cells to acquire typical morphological features of DCs, such as long dendritic projections and ruffled membranes, by day 5 (Fig. 4). Whereas untreated cells maintained their rounded, smooth surface morphology and arranged as dispersed, nonadherent cells in culture, CI-treated CML cells predominantly gathered in clusters as nonadherent cells with a larger cell surface and irregular shape.
Figure 4.
Photomicrographs representative of untreated and CI-treated CML cells. Cells were analyzed while viable under Nomarski optics at ×600. (A) CML cells cultured in standard medium alone. (B) CML cells cultured in medium supplemented with 375 ng/ml A23187.
Functional Properties of CI-Treated CML Cells Compared with Untreated and CI-Treated Monocytes.
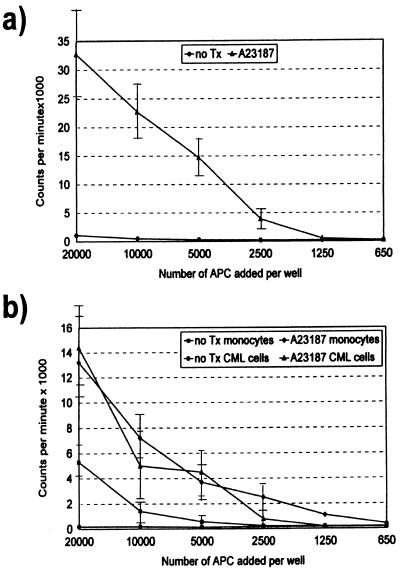
The mixed leukocyte response is an in vitro study used to assess naïve T lymphocyte proliferation (18). In previous studies, we have demonstrated that CD14+ peripheral blood monocytes can be induced to acquire multiple DC characteristics in response to CI treatment and that they are as efficient in sensitizing T lymphocytes to alloantigen as purified blood DC (19). We therefore were interested in determining the ability of CI-treated CML cells to stimulate allogeneic lymphocyte proliferation compared with untreated CML cells as well as untreated and CI-treated CD14+ monocytes. As shown in Fig. 5, CI-treated CML cells are potent stimulators of allogeneic T lymphocytes. Their allosensitizing capacity proved to be consistently superior to untreated CML cells and untreated monocytes. When compared with CI-treated normal monocytes, CI-treated CML cells generally were as effective in allosensitizing T cells. Untreated CML cells displayed no immunostimulatory activity toward MHC-mismatched peripheral blood T lymphocytes, consistent with the minimal expression of MHC and costimulatory molecules on these untreated leukemic cells (Fig. 5).
Figure 5.
Effects of CI treatment on T cell allosensitization potency of CD33+ CML cells and CD14+ monocytes. SEM of triplicate wells is displayed as error bars. (a) T cell allosensitization potency of CML cells treated with A23187 compared with untreated CML cells. (b) T cell allosensitization potency of both purified CML cells and monocytes treated with A23187 compared with untreated cells. CML cells and monocytes were purified from the same donor.
Detection of the t(9:22) on Interphase Nuclei by FISH.
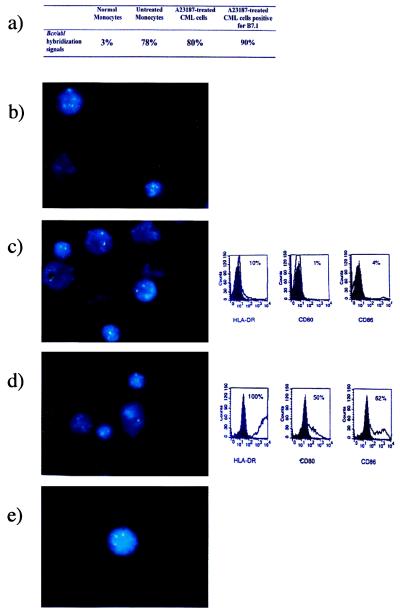
To ensure that converted CML cells were of leukemic origin, untreated and CI-treated CML precursor cells were analyzed for the presence of the bcr/abl gene by using FISH. Fig. 6b depicts nuclei of monocytes from normal donors, whereas Fig. 6 c and d shows nuclei of untreated and CI-treated CML cells, respectively. The untreated CML cells stained negatively for HLA-DR, CD80, and CD86 by FACS at the time the FISH studies were performed. Cells treated with CI demonstrated de novo expression of these DC markers, as can be seen in Fig. 6d. The yellow hybridization signal resulting from the fusion of the bcr and abl genes was observed in 75–95% of nuclei of the CML donors and did not differ between fresh, untreated, and CI-treated CML nuclei (Fig. 6a). Nuclei of monocytes in a healthy donor generally were 3% positive for the fusion gene (Fig. 6a). This number represents a false-positive value. Fig. 6e depicts a nucleus of a CML cell positively selected for B7.1. Overall, counts of nuclei expressing the fusion signal averaged 90%. That percentages of counted bcr/abl fusion gene signals in both untreated and CI-treated CML cells never reached 100% is attributable to the method of how cells were counted, where very close bcr/abl signals lacking the typical yellow signal were scored as negative.
Figure 6.
Representative photographs of nuclei analyzed for the bcr/abl gene rearrangement by FISH. (a) Percentages of bcr/abl hybridization signals in normal monocytes, untreated CML cells, and A23187-treated and B7.1-selected CML cells. (b) Nuclei of normal monocytes. (c) Nuclei of untreated CML cells. Phenotypic expression of untreated cells for DC surface markers HLA-DR, CD80, and CD86 at the same time point. Solid histograms represent the isotype control; open histograms symbolize the staining of the specific antibodies. (d) Nuclei of A23187-treated CML cells. Phenotypic expression of treated cells for HLA-DR, CD80, and CD86 is as described in c. (e) Nucleus of CI-treated and B7.1-enriched CML cell.
DISCUSSION
We recently have demonstrated that CI treatment of monocytes in healthy volunteers and cancer patients rapidly yields large numbers of DCs (19). Calcium signaling causes CD14+ monocytes within the myeloid origin mononuclear cell (MOMC) pool to display characteristics of DCs with down-regulation of CD14 expression, up-regulation of costimulatory molecule expression, and de novo expression of the DC activation marker CD83. CI-treated MOMCs consistently develop enhanced T cell sensitization efficiency both for primary and recall responses (19). Furthermore, CI also has been shown to cause acquisition of DC features in other myeloid derived cells, i.e., in the promyelocytic leukemia cell line HL-60 (20).
In this study, we have demonstrated that a purified CD33+ cell preparation from CML, depleted of other peripheral blood cell components and independent of prior chemotherapy, rapidly acquires immunophenotypical, functional, and morphological characteristics of DCs. The immunophenotypic changes induced by CI were consistent in every donor, with de novo expression of the costimulatory molecules CD80 and CD86, the DC activation markers CD83 and CD40, as well as rapid and significant up-regulation of HLA-ABC, HLA-DR, and CD54. These CI-treated CML cells were shown to be positive for the bcr/abl translocation by FISH with no difference in translocation expression between untreated and CI-treated CML nuclei.
Our findings are in agreement with studies of Choudhury et al. (21), which show that exposure of CML cells to a cytokine combination of granulocyte/macrophage colony-stimulating factor, IL-4, and tumor necrosis factor-α also led to the acquisition of a DC-like phenotype in these Ph+ cells. However, our study differs from that report, demonstrating that conversion with calcium-signaling agents can be achieved reliably in a CML progenitor cell population free of monocytes and B lymphocytes. Furthermore, conversion can be accomplished more rapidly and consistently as compared with treatment with the cytokines, and a procedure that requires selecting for DC-positive cells by CD1a sorting does not apply. In addition, the whole CML population is susceptible to calcium signaling independent of lineage or differentiation stage.
Allogeneic bone marrow transplantation in chronic phase presently is the only therapy successful in curing chronic myelogenous leukemia (22, 23). For a significant number of patients who are not eligible for bone marrow transplantation based on older age or lack of appropriately matched donors, treatment with chemotherapy or IFN-α remains their only treatment alternative to prolong life expectancy (24). Therefore, additional treatment options involving adoptive immunotherapy or vaccine therapy for this disease have become increasingly desirable. Vaccine therapy for treatment of CML can be approached either by pulsing CML-specific tumor peptides onto professional APCs or by modifying CML cells to render them more immunogenic for selective antileukemic T cell cytotoxicity. Recent in vitro studies suggest that tumor peptides derived from the chimeric bcr/abl protein pulsed onto professional DCs can sensitize CD4+ lymphocytes to recognize CML cell lysates and CML blast cells (8, 25). However, this approach to leukemia control may be suboptimal, because pulsing APCs with a single peptide may elicit a restricted antitumor T cell response and CML cells lacking this particular tumor antigen may escape immune surveillance. Therefore, the development of a vaccine based on conversion of CML progenitor cells into APCs may constitute a more effective approach to leukemia control.
In the present study, we have demonstrated that a single pharmacological agent interfering with intracellular Ca2+ homeostasis establishes an activation stimulus for myeloid-derived cells to abandon other developmental programs in favor of an enhanced evolution toward antigen presentation. Conversion is accomplished within a myeloid progenitor cell pool typically lacking other APCs such as monocytes or B lymphocytes. Furthermore, conversion can be achieved rapidly and homogeneously without the need for long incubation times and additional selection methods for DC-positive cells. These APCs may express both a wide variety of naturally derived, CML-relevant tumor peptides as well as distinctive, DC-associated accessory molecules important for successful antigen presentation and recognition, T cell activation, and leukemia-restricted cytotoxicity. Immunotherapy with DC-like CML cells generated after calcium signaling therefore may offer a viable alternative for patients who increasingly become resistant to conventional chemotherapy and who lack a suitable bone marrow donor.
Acknowledgments
We thank Lisa Moreau and Janet Finan from the Department of Pathology/Laboratory Medicine, Cytogenetics Laboratory, for their valuable assistance in the training to perform FISH analyses. We thank Dr. Jonni S. Moore and Charles H. Pletcher for their helpful advice regarding the flow cytometry studies, which were performed in the University of Pennsylvania Cancer Center Flow Cytometry and Cell Sorting Shared Resource. This work was supported in part by grants from the Harrington Foundation and the National Institutes of Health (CA-42232).
ABBREVIATIONS
- CML
chronic myelogenous leukemia
- DC
dendritic cell
- CI
calcium ionophore
- APC
antigen-presenting cell
- FISH
fluorescence in situ hybridization
References
- 1.Weiden P L, Flournoy N, Thomas E D, Prentice R, Fefer A, Bruckner C D, Storb R. N Engl J Med. 1997;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 2.Hale G, Waldmann H. Bone Marrow Transplant. 1994;13:597–611. [PubMed] [Google Scholar]
- 3.Porter D L, Roth M S, McGarigle C, Ferrara J L, Antin J H. N Engl J Med. 1994;330:100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 4.Drobyski W R, Keever C A, Roth M S, Koethe S, Hanson G, McFadden P, Gottschall J L, Ash R C, van Tuinen P, Horowitz M M, et al. Blood. 1993;82:2310–2318. [PubMed] [Google Scholar]
- 5.Kolb H J, Mitteruller J, Clemms C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 6.Nieda M, Nicol A, Kikuchi A, Kashiwase K, Taylor K, Suzuki K, Tadokoro K, Juji T. Blood. 1998;91:977–983. [PubMed] [Google Scholar]
- 7.Greco G, Fruci D, Accapezzato D, Barnaba V, Nisini R, Alimena G, Montefusco E, Vigneti E, Butler R, Tanigaki N, Tosi R. Leukemia. 1996;10:693–699. [PubMed] [Google Scholar]
- 8.Mannering S I, McKenzie J L, Fearnely D B, Hart D N J. Blood. 1997;90:290–297. [PubMed] [Google Scholar]
- 9.Honda H, Fujii T, Takatoku M, Mano H, Witte O N, Yazaki Y, Hirai H. Blood. 1995;85:2853–2861. [PubMed] [Google Scholar]
- 10.van Denderen J, ten Hacken P, Berendes P, van Ewijk W. Leuk Lymphoma. 1993;11:29–32. doi: 10.3109/10428199309047859. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Peace D J, Rovira D K, You S G, Cheever M A. Proc Natl Acad Sci USA. 1997;89:1468–1472. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim S H, Coleman S. Am J Hematol. 1997;54:61–67. doi: 10.1002/(sici)1096-8652(199701)54:1<61::aid-ajh9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Lanier L L, O’Fallon S, Somoza C, Phillips J H, Linsley P S, Okumura K, Ito D, Azuma M. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 14.Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier L L, Banchereau J. J Exp Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft M, Bradley L M, Swain S L. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 16.Crowley M, Inaba K, Steinman R M. J Exp Med. 1990;172:383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickl W F, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bellow-Fernandez C, Knapp W. J Immunol. 1996;157:3850–3859. [PubMed] [Google Scholar]
- 19.Czerniecki B J, Carter C, Rivoltini L, Koski G K, Kim H I, Weng D E, Roros J G, Hijazi Y M, Xu S, Rosenberg S A, et al. J Immunol. 1997;159:3823–3837. [PubMed] [Google Scholar]
- 20.Koski, G. K., Schwartz, G. N., Weng, D. E., Gress, R. E., Engels, F. H., Toskos, M., Czerniecki, B. J. & Cohen, P. A. (1999) Blood, in press. [PubMed]
- 21.Choudhury A, Gajewski J L, Liang J C, Popat U, Claxton D F, Kliche K-O, Andreeff M, Champlin R E. Blood. 1997;89:1133–1142. [PubMed] [Google Scholar]
- 22.Thomas E D, Clift R A. Blood. 1989;73:861–864. [PubMed] [Google Scholar]
- 23.Clift R A, Anasetti C. Baillieres Clin Haematol. 1997;10:319–336. doi: 10.1016/s0950-3536(97)80010-8. [DOI] [PubMed] [Google Scholar]
- 24.Hehlmann R, Heimpel H, Hasford J, Kolb H J, Pralle H, Hossfeld D K, Queisser W, Loffler H, Hochaus A, Heinze B, et al. Blood. 1994;84:4064–4077. [PubMed] [Google Scholar]
- 25.ten Bosch G J A, Joosten A M, Kessler J H, Melief C J M, Leeksma O C. Blood. 1996;88:3522–3527. [PubMed] [Google Scholar]