Abstract
The three most common methods of sterilization in use today are ethylene oxide exposure, γ-irradiation, and steam sterilization. Each of these methods has serious limitations for the sterilization of some materials used in medicine, especially thermally and hydrolytically sensitive polymers by themselves and in combination with proteins. In this work, we demonstrate a potential new method of sterilization by using supercritical fluid carbon dioxide. Using this method we achieve complete inactivation of a wide variety of bacterial organisms at moderate temperatures and in the absence of organic solvents or irradiation. Sterilization is a function of both the proximity to the fluid’s critical point and the chemical nature of the fluid itself. When biodegradable polymers poly(lactic-co-glycolic) acid and polylactic acid were included in the sterilization process, there was no effect on the inactivation efficiency, yet no physical or chemical damage to these thermally and hydrolytically labile materials was observed.
Three common methods of sterilization exist for medical materials: ethylene oxide exposure, γ-irradiation, and steam sterilization. With ethylene oxide, residual gas remains in the material that can cause hemolysis (1, 2) and other toxic and chemical reactions (3, 4) including molecular weight changes of biodegradable polymers (5). γ-Irradiation causes damage to polymers [for example, changes in shear and tensile strength, elastic modulus (4), and transparency (6)] and reduced activity in drugs, if they are incorporated for drug delivery, because of cross-linking or degradation. Steam sterilization is undesirable for biomaterials, particularly thermally and hydrolytically labile materials that cannot tolerate the temperatures required during autoclaving (7). Recently, supercritical fluid carbon dioxide has been used to inactivate viruses in bone allografts (8). A critical objective of any sterilization process would be the development of a process that could destroy high levels of bacteria. Here we examine the use of a supercritical fluid method for the inactivation of a variety of Gram-negative and Gram-positive bacteria in the presence and absence of model polymers poly(lactic-co-glycolic acid) (PLGA) and poly(lactic acid) (PLA).
We envision that supercritical fluid (SCF) sterilization might occur as a result of the fluid’s ability to penetrate cell walls [because of the unique mass transfer properties of SCFs (9)] and interact with intracellular components, rather than hydrostatic pressure of the fluid phase. Hydrostatic pressures between 1,200 and 8,000 bar have been used in an attempt to mechanically rupture yeast cells and spores (10–13); however, even when coupled with very high shearing forces and high temperatures, this method is inadequate for sterilizing heavily contaminated samples (5).
Processing in the near-critical region of carbon dioxide requires only moderate pressures (PC = 73.8 bar). Supercritical carbon dioxide has been used to inactivate yeast cells (14, 15) in food products; however, success has been limited for many other microorganisms (16–18). Researchers in the food industry have reported that sterilization of several bacterial microbes was achieved by using SCF carbon dioxide; however, total inactivation of microorganisms other than yeast cells, including Staphylococcus aureus, Clostridia sporogenes, Saccharomyces cerevisiae, Salmonella, Bacillus megaterium, and others (15–20) was incomplete. The “sterilization” resulted in only 2–3 log orders of reduction of the cells when starting with 108 colony-forming units (cfu)/ml. The standard test for sterilization requires that at least 106 cfu/ml of starting material be used and inactivated completely (5, 6).
MATERIALS AND METHODS
Bacteria Culturing.
The bacteria were cultivated in a medium containing 30 g/liter tryptone soya broth (code CM129) and incubated for 18–20 hr at 37°C. Bacillus cereus spores were suspended from colonies on selective agar. Legionella dunnifii was cultivated in a medium containing Legionella CYE Agar Base (code CM655) and Legionella BYCE Growth Supplement (code SR110) and incubated for 7 days at 37°C on a Petri dish. All cells were grown daily for experiments to ensure that cells were fresh and fully grown. Staphylococcus aureus, B. cereus, Listeria innocua, Salmonella salford, Proteus vulgaris, Legionella dunnifii, and Pseudomonas aeruginosa were obtained from the Australian Government Analytical Laboratory, and Escherichia coli was provided by the Biochemical Engineering Laboratory of University of New South Wales.
Preparation of 1-μm Microspheres.
The 1-μm microspheres were prepared by a supercritical fluid antisolvent technique (unpublished data). PLGA (50:50, RG504; Boehringer Ingelheim) and PLA (Sigma) were precipitated from an acetone solution at 5°C by using carbon dioxide as the antisolvent. The acetone/polymer solution was injected into the chamber, and carbon dioxide was flowed continuously. The precipitated samples were washed and dried by passing fresh CO2 at constant pressure through the apparatus for at least 30 min. After washing, the chamber was depressurized and sample was removed. The samples then were gold-coated, and the size and morphology of the precipitated polymer were analyzed with a scanning electron microscope (Hitachi 4500) and image analysis.
Preparation of Larger and Porous Microspheres.
The 7- and 20-μm solid microspheres and 10-μm porous microspheres of PLGA (50:50, RG504; Boehringer Ingelheim) were prepared by using a single emulsion solvent evaporation method (21, 33). Particle sizing was performed by using a Coulter Multisizer.
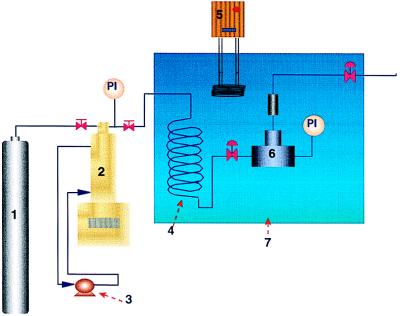
SCF-Sterilization Procedure. The sterilization apparatus used in these experiments is shown in Fig. 1. This sterilization unit was custom-made from stainless steel and was designed such that continuous pressurization/depressurization cycles could be carried out with no loss of media and no contamination of pressure lines via back diffusion.
Figure 1.
Schematic of SCF sterilization apparatus. (1) CO2 cylinder. (2) Syringe pump. (3) Pump header cooler and heater. (4) Preheating coil. (5) Heater. (6) Extraction vessel. (7) Water bath.
For each experiment, the unit was filled with 2–3 ml of freshly grown bacteria cells. In relevant experiments polymer microspheres were incubated with the bacteria broth overnight, before sterilization, to ensure a high degree of contamination. Polymer microspheres were added to the sterilization unit just before processing in experiments that were designed to determine the effect of sterilization conditions on the physical and chemical properties of the polymer. The reactor was sealed (Swagelok, Mortadale, Australia) and immersed in a constant temperature bath. Compressed carbon dioxide was passed through preheating coils to obtain the desired pressure and temperature. The sterilization unit was partially depressurized and repressurized (ΔP ≥100 bar, approximately 5 cycles/hr) during the experiment to provide a driving force for mass transport. The reactor was shaken mechanically throughout the experiment, and 2-mm glass beads (Selby-Anax, Nottinghill, Australia; range, 1.5–2.5 mm) were added for enhanced agitation. A 0.5-μm filter (stainless steel; Swagelok) was placed before the release valve to ensure that none of the sample was lost during the pressure cycles. After each sterilization experiment, the sample was removed directly from the bottom cylinder by loosening a high-pressure seal and pouring out the reactor contents.
The sterilization effect of the near-critical or supercritical CO2 was evaluated by determining the ratio of the number of living cells (N) after treatment to those initially added to the unit (No). The number of living cells was determined before and after the experiment by counting the cfus growing on the agar plates. Triplicate samples were averaged for each data point.
Degradation Analysis. The hydrolysis of PLGA was performed by the incubation of 10 vials, each containing 40 mg of PLGA in 40 ml of PBS (pH 7.4) in a shaking water bath at 37°C. Vials were removed at various time intervals, and particles were isolated, washed with distilled water three times, and dried in a vacuum. The reduction in polymer molecular weight was determined by using gas permeation chromatography (Shimadzu, Rydalmere, Australia). The instrument was calibrated by using narrow-molecular-weight-range (200–1.6 × 106) poly(methylmethacrylate) standards. All samples were dissolved in tetrahydrofuran and passed through a column in a single solvent batch to reduce experimental artifacts in molecular weight analysis.
Differential scanning calorimetry (TA Instruments 2010, Rydalmere, Australia) was used to estimate polymer phase transitions for SCF processed and unprocessed PLGA and PLA. Samples (4 mg) were heated (5°C/min) in aluminum pans from −20°C to 110°C and back down to −20°C to standardize their thermal history.
Fourier transform IR spectroscopy (HP 2000, Hewlett-Packard Australia, North Ryde, Australia) was used to collect spectra of PLGA and PLA samples before and after sterilization processing to determine whether the SCF method resulted in any chemical changes to the polymer. Samples were mixed with KBr and compressed into pellets for analysis. Spectra were recorded between 600 and 4,000 wave numbers.
RESULTS AND DISCUSSION
Here we show that SCF CO2, coupled with appropriate reactor design, provides an effective method of sterilization for thermally and hydrolytically labile biomedical materials by inactivation of a variety of bacterial microbes.
In our work, the following bacteria were subjected to the SCF sterilization procedure: S. aureus, B. cereus, L. innocua, S. salford, P. vulgaris, L. dunnifii, P. aeruginosa, and E. coli. The first three in the list are Gram-positive, and the last five are Gram-negative bacteria. These species were chosen based on their prevalence in medical contamination and/or their resistance to inactivation. In all experiments the reactors were loaded with at least 107–109 cfu/ml of the microorganism (with the exception of the Legionella strain, which was difficult to culture in concentrations higher than 104 cfu/ml) to stringently test the sterilization procedure. Complete sterilization of all microorganisms occurred in 0.6–4 hr at 205 bar and at temperatures generally between 25 and 40°C, with the exception of B. cereus, which required 60°C (see Table 1). Sterilization occurred in the absence of organic solvents, chemicals, or radiation.
Table 1.
The effect of CO2 at 205 bar on the inactivation of various microorganisms
| Microorganism | Temperature, °C | Time, hr | Cycle | Initial cfu/ml | Degree of inactivation, log |
|---|---|---|---|---|---|
| B. cereus | 34 | 0.6 | 3 | 5.1 × 107 | 2 |
| 34 | 2 | 6 | 5.7 × 107 | 1 | |
| 60 | 2 | 6 | 5.2 × 107 | 5 | |
| 60 | 4 | 6 | 1.8 × 108 | 8 | |
| L. innocua | 34 | 0.6 | 3 | 5.8 × 109 | 3 |
| 34 | 0.6 | 6 | 2.1 × 109 | 9 | |
| S. aureus | 34 | 0.6 | 3 | 2.5 × 109 | 3 |
| 34 | 0.6 | 6 | 1.2 × 109 | 7 | |
| 40 | 2 | 6 | 6.7 × 108 | 6 | |
| 40 | 4 | 6 | 1.9 × 109 | 9 | |
| S. salford | 34 | 0.6 | 3 | 1.5 × 109 | 3 |
| 34 | 0.6 | 6 | 1.0 × 109 | 3 | |
| 40 | 2 | 6 | 6.0 × 108 | 6 | |
| 40 | 4 | 6 | 2.2 × 109 | 9 | |
| P. aeruginosa | 34 | 0.6 | 3 | 7.4 × 108 | 6 |
| 40 | 1.5 | 6 | 2.9 × 108 | 6 | |
| 40 | 4 | 6 | 2.4 × 108 | 8 | |
| E. coli | 34 | 0.5 | 3 | 6.4 × 108 | 8 |
| P. vulgaris | 34 | 0.6 | 3 | 9.1 × 108 | 8 |
| L. dunnifii | 40 | 1.5 | 6 | 6.7 × 104 | 4 |
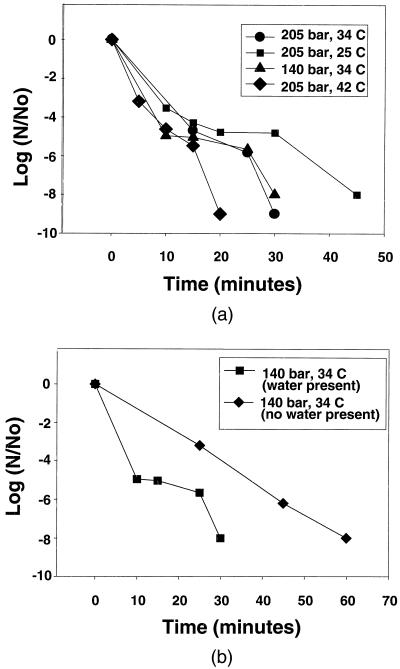
Although some of the organisms required up to 4 hr for complete inactivation, enhanced mass transfer conditions should be able to reduce this processing time significantly. To test this, optimal conditions for inactivation of one microorganism, E. coli, were determined. Experiments were optimized for the given reactor design by increasing the frequency of pressurization cycles and agitation (i.e., mass transfer) within the sterilization vessel until no discernible improvement in kinetics was observed. Inactivation kinetics may be optimized further with additional reactor designs that enhance contact between the materials and the fluid phase. The inactivation kinetics for E. coli are given in Fig. 2A. In all experiments, the initial loading of E. coli cells was between 108 and 109 cfu/ml, and the final data point of each experiment represents the sample that formed no cfus after 48 hr of incubation. Even at subcritical conditions (t = 25°C), the time required for complete inactivation of the E. coli was only 45 min, and at 42°C complete inactivation occurred in less than 20 min. The time required for complete SCF sterilization is significantly less than for ethylene oxide or irradiation methods (2) (several hours to overnight) and similar to steam autoclaving (at 120°C or higher) (5).
Figure 2.
(A) Sterilization kinetics of E. coli in SCF CO2 in the presence of water. (B) Comparison of sterilization kinetics of E. coli in the presence and absence of water at 34°C and 140 bar. (Solid lines indicate trends only.)
We carried out two experiments to test the importance of (i) proximity to the fluid’s critical point and (ii) the chemical/physical properties of CO2 on the effectiveness of sterilization. First, experiments were conducted under identical conditions by using nitrogen in place of CO2 to verify the sterilizing effect of the near-critical fluid properties on E. coli. Under these experimental conditions, nitrogen is well removed from its critical point and, consequently, does not exhibit the special gas-like mass transport properties and liquid-like densities that make near-critical fluids ideal for extraction (9). The critical properties of carbon dioxide are tc = 31.1°C and Pc = 73.8, whereas the critical properties of nitrogen are tc = −147°C and Pc = 33.9 bar. Therefore, although operating conditions are in the vicinity of the critical point for carbon dioxide, they are extremely well removed from the critical point of nitrogen. In all nitrogen experiments there was no sterilizing effect (i.e., >108 cfu/ml in samples before and after the sterilization treatment using nitrogen).
The importance of the chemical nature and/or size of carbon dioxide as the sterilizing fluid also was considered. The sterilization procedure was carried out with SCF tetrafluoroethane (TFE) (tc = 55°C, Pc = 40.6 bar), which has similar critical properties as CO2, but different chemical properties and size [chemical properties of CO2 and TFE, respectively: dipole moment, Dc = 0, DT = 2.1; solubility parameter, δc = 7.0, δT = 13.6 (22)]. When carried out at an equivalent reduced temperature and pressure (t = 38°C, P = 110 bar) as successful CO2 experiments, the TFE experiment resulted in no reduction in the number of viable cells after 45 min (approximately 4.3 × 108 cfu/ml before and after sterilization treatment). Total inactivation was achieved when using SCF CO2 under these conditions (no cfus present after 48 hr of incubation). Enhanced mass transfer properties characteristic of near-critical fluids, as well as the specific chemical and/or physical properties of CO2, are required for successful inactivation of microorganisms.
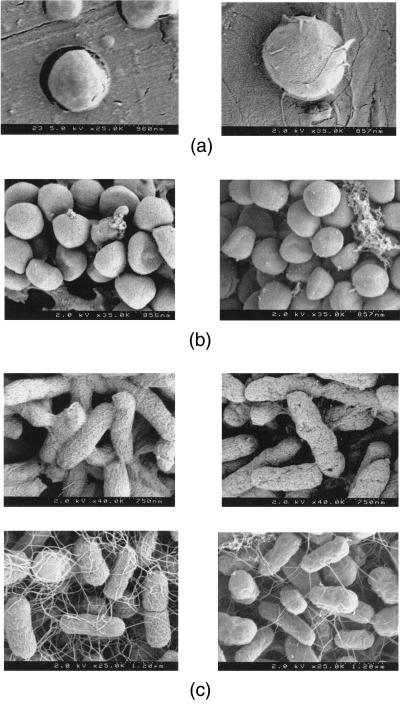
To test the results of our approach in the presence of biomaterials, samples of PLGA or PLA were added to the system. These are biodegradable polymers that have been used extensively in medicine, including carriers of pharmaceuticals (23). PLGA is the most widely used biodegradable polymer and is used for applications ranging from delivery of narcotic antagonists, local anesthetics, and steroid hormones (24) to surgical sutures (25) and in tissue-engineering applications as a biodegradable scaffolding (26, 27). The experiments were carried out with 1-, 7-, and 20-μm solid microspheres and 10-μm porous microspheres of PLGA or PLA and E. coli (109 cfu/ml) to validate the sterilization procedure with polymeric particles of varying size and morphology. The subsequent inactivation kinetics were unaffected by the presence of the polymer. Sterilization of the polymer was accomplished in 30 min at 205 bar and 34°C and 45 min at 205 bar and 25°C. In Fig. 3A, the 1-μm spheres of PLGA are shown before and after the sterilization process, illustrating that morphological changes in the materials were not observed from the supercritical fluid processing. Other researchers have determined recently that SCF CO2 cleaning of prostheses fabricated from polyester fabric (7 cm in length) resulted in minimal changes in the physical properties of the material (2% shrinkage in the longitudinal direction) (28).
Figure 3.
(A) PLGA microsphere before (Left) and after (Right) sterilization at 25°C and 205 bar for 1 hr. (B Upper) S. aureus before (Left) and after (Right) SCF-sterilization process. (Lower) P. aeruginosa before (Left) and after (Right) SCF process. (C) E. coli before (Left) and after (Right) SCF sterilization.
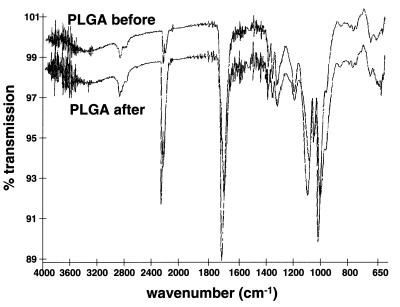
We also looked for chemical changes in PLGA and PLA resulting from SCF processing (t = 25–34°C, P = 205 bars). Fourier transform IR spectroscopy, gas permeation chromatography, and differential scanning calorimetry analysis confirmed that there were no detectable chemical changes in either the PLGA or PLA samples after the sterilization procedure compared with untreated samples. The unprocessed and SCF-processed (t = 24–40°C, P = 205 bar) PLGA and PLA samples revealed sample peak maxima occurring at equivalent elution volumes for both samples (gas permeation chromatography) and glass-transition temperatures of 50.94 and 50.39°C, respectively (differential scanning calorimetry). Characteristic IR triplet peaks for lactic acid, glycolic acid, and PLGA occur between 1,400 and 1,500 cm−1 (29). The ratio of these characteristic peaks does not change before and after SCF treatment of the PLGA (see Fig. 4). In addition, degradation analysis of PLGA was conducted to ensure that the SCF sterilization does not cause unusual degradation behavior of the processed polymers. The results (data not shown) indicate a steady decrease in the total mass and the molecular weight as the degradation proceeded and illustrates that the SCF-treated polymers degrade in an equivalent manner as the PLGA microspheres processed by non-SCF methods (30). Additionally, SCF treatment does not result in changes in initial mass or molecular weight of the polymers studied.
Figure 4.
Fourier transform IR spectroscopy analysis of unprocessed and SCF-sterilized PLGA (t = 34°C, P = 205 bar).
The experiments described illustrate that the SCF sterilization process is successful for a wide variety of microorganisms in the presence of biomaterials and that this process results in no physical or chemical damage to the materials. However, to optimize this procedure for eventual large-scale use, we must first understand the fundamental mechanisms of the SCF CO2 inactivation.
Fig. 3B shows scanning electron microscope photographs of S. aureus and P. aeruginosa cells (Gram-positive and Gram-negative, respectively) that illustrate that the cell walls remain largely unchanged before and after the sterilization process. These scanning electron microscope photographs are representative of all tested microorganisms. However, more defects of the cell wall are found in the Gram-negative cells after the sterilization process, indicating rupture of some cells; yet, evidence that some Gram-negative bacteria (E. coli) do not appear to suffer cell wall damage during SCF sterilization is shown in Fig. 3C. It is expected that the Gram-negative bacteria will rupture more easily than the Gram-positive bacteria because the cell wall is very thin compared with that of the Gram-positive bacteria (30). However, because most cells appear to have intact cell walls, we believe that the sterilization mechanism is not a rupture due to increased internal pressure of the cell or extraction of cell wall lipids.
We believe that the mechanism of sterilization involves the ability of SCF CO2 (or near-supercritical CO2) to diffuse readily into the cell and alter the pH within the cell. This mechanism has been suggested by others who have realized partial success in inactivating microorganisms with carbon dioxide (15). Carbon dioxide, in the presence of water, will react to produce carbonic acid that will result in a further reduction of pH within the cell. The proposed mechanism is consistent with experimental evidence that indicates minimal destruction of cell walls. The mechanism that we propose is consistent with experimental results that indicate that small amounts of water greatly enhance the sterilizing effect of SCF CO2 (Fig. 2B). Water also may increase the permeability of the cell wall so that the carbon dioxide may more readily diffuse through the lipoprotein barrier (thus effecting the kinetics). To test the importance of water, E. coli cultures were dried and submitted to the SCF CO2 sterilization process at 34°C and 140 bar for 2.5 hr. One milliliter of water was added to a second sample (prepared under identical conditions) to determine the number of viable cells in the dried samples (6.45 × 108 cfu/ml). One hundred percent inactivation was achieved for the dried samples that were submitted to the sterilization process; however, sterilization kinetics were strongly affected (see Fig. 2B).
From the experiments it is clear that the residence time and experimental conditions required for complete sterilization are sensitive to at least three factors: (i) the nature of the cell wall, both in terms of chemical nature and cell shape (e.g., the surface-to-volume ratio); (ii) efficiency of contact between the near- or supercritical CO2 phase and the solid “cell phase”; (iii) exposure to water. With further study, this technique may prove to be a useful method for sterilization of many types of materials and pharmaceutical formulations because of the mild (31, 32), nonreactive process conditions employed and the ability of SCF CO2 to inactivate a wide variety of microorganisms.
ABBREVIATIONS
- SCF
supercritical fluid
- cfu
colony-forming unit
- PLGA
poly(lactic-co-glycolic) acid
- PLA
polylactic acid
References
- 1.Clarke C P, Davidson W L, Johnson J B. J Surg. 1966;36:53–56. doi: 10.1111/j.1445-2197.1966.tb04398.x. [DOI] [PubMed] [Google Scholar]
- 2.Nair P D. J Biomat Appl. 1995;10:121–135. doi: 10.1177/088532829501000203. [DOI] [PubMed] [Google Scholar]
- 3.Guidoin R, Snyder R, King M, Martin L, Botzko K, Awad J, Marios M. Biomaterials. 1985;6:122–128. doi: 10.1016/0142-9612(85)90075-4. [DOI] [PubMed] [Google Scholar]
- 4.Clayton J L. Minimally Invasive Surg Nursing. 1996;10:13–20. [PubMed] [Google Scholar]
- 5.Konig C, Ruffieux K, Wintermantel E, Blasser J. J Biomed Mater Res Appl Biomat. 1997;38:115–119. doi: 10.1002/(sici)1097-4636(199722)38:2<115::aid-jbm5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Bjursten L, Freij-Larson C, Kober M, Wesslen B. Biomaterials. 1996;17:2265–2272. doi: 10.1016/0142-9612(96)00055-5. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey D J, Thirucote R R. J Biomat Appl. 1989;3:454–523. doi: 10.1177/088532828800300303. [DOI] [PubMed] [Google Scholar]
- 8.Fages J, Poirier B, Barbier Y, Frayssinet P, Joffret M L, Majewski W, Bonel G, Larzul D. ASAIO J. 1998;44:289–293. doi: 10.1097/00002480-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Eckert C A, Knutson B L, Debenedetti P G. Nature (London) 1996;383:313–318. [Google Scholar]
- 10.Lin H M, Chan E C, Chen C, Chen L F. Biotechnol Prog. 1991;7:201–204. [Google Scholar]
- 11.Hayakawa I, Kanno T, Tomita M, Fujio Y. J Food Sci. 1994a;59:159–163. [Google Scholar]
- 12.Hayakawa I, Kanno T, Yoshiyama K, Fujio Y. J Food Sci. 1994b;59:164–167. [Google Scholar]
- 13.Hashizume C, Kimura K, Hayashi R. Biosci Biotech Biochem. 1995;59:1455–1457. doi: 10.1271/bbb.59.1455. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Zhiying Y, Chen L. Biotech Prog. 1993;32:B29–B34. [Google Scholar]
- 15.Nakamura K, Enomoto A, Fukushima H, Nagai K, Hakoda M. Biosci Biotech Biochem. 1994;58:1297–1301. [Google Scholar]
- 16.Hass G J, Prescott H E, Dudley E, Dik R, Hintlian C, Keane L. J Food Safety. 1989;9:253–265. [Google Scholar]
- 17.Arreaola A G, Balaban M O, Wei C I, Peplow A, Marshall M. J Food Qual. 1991;14:275–284. [Google Scholar]
- 18.Ishikawa H, Shimoda M, Tamaya K, Yonekura A, Kawano T, Osajima Y. Biosci Biotech Biochem. 1997;61:1022–1023. doi: 10.1271/bbb.61.1022. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai H, Hata C, Nakamura K. Biosci Biotech Biochem. 1997;61:931–935. doi: 10.1271/bbb.61.1663. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto A, Nakamura K, Hakoda M, Amaya N. J Ferment Bioeng. 1997;83:305–307. [Google Scholar]
- 21.Jeffery H, Davis S S, O’Hagan D T. J Pharm Res. 1993;10:362–368. doi: 10.1023/a:1018980020506. [DOI] [PubMed] [Google Scholar]
- 22.Jackson K, Fulton J L. Langmuir. 1996;12:5289–5295. [Google Scholar]
- 23.Langer R. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 24.Rhine W D, Hsieh D S, Langer R. J Pharm Sci. 1980;69:265–270. doi: 10.1002/jps.2600690305. [DOI] [PubMed] [Google Scholar]
- 25.Cima L G, Ingber D E, Vacanti J P, Langer R. Biotech Bioeng. 1991;38:145–158. doi: 10.1002/bit.260380207. [DOI] [PubMed] [Google Scholar]
- 26.Langer R, Vacanti J P. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 27.Cima L G, Langer R. Chem Eng Prog. 1993;89:46–50. [Google Scholar]
- 28.Fages J, Poddevin N, King M W, Marois Y, Bronner J, Jakubiec B, Roy R, Mainard D, Laroche G, Delagoutte J P, et al. ASAIO J. 1998;44:278–288. doi: 10.1097/00002480-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Webber W L, Lago F, Thanos C, Mathiowitz E. J Biomed Mater Res. 1998;41:18–29. doi: 10.1002/(sici)1097-4636(199807)41:1<18::aid-jbm3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.McKane L, Kandel J. Applied Microbiology. New York: McGraw-Hill; 1996. [Google Scholar]
- 31.Winters M A, Knutson B L, Debenedetti P G, Sparks H G, Przybycien T M, Stevenson C L, Prestrelski S J. J Pharm Sci. 1996;85:586–594. doi: 10.1021/js950482q. [DOI] [PubMed] [Google Scholar]
- 32.Randolph T W, Randolph A D, Mebes M, Yeung S. Biotechnol Prog. 1993;9:429–435. doi: 10.1021/bp00022a010. [DOI] [PubMed] [Google Scholar]
- 33.Edwards D A, Hanes J, Caponetti G, Hrkach J, Ben-Jerbia A, Eskew M L, Mintzes J, Deaver D, Lotan N, Langer R. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]