Abstract
To determine whether the depletion of body fat caused by adenovirus-induced hyperleptinemia is mediated via the hypothalamus, we used as a “bioassay” for hypothalamic leptin activity the hypothalamic expression of a leptin-regulated peptide, cocaine- and amphetamine-regulated transcript (CART). The validation of this strategy was supported by the demonstration that CART mRNA was profoundly reduced in obese rats with impaired leptin action, whether because of ablation of the ventromedial hypothalamus (VMH) or a loss-of-function mutation in the leptin receptor, as in Zucker diabetic fatty rats. We compared leptin activity in normal rats made hyperleptinemic by adenovirus-leptin treatment (43 ± 9 ng/ml, cerebrospinal fluid leptin 100 pg/ml) with normal rats made hyperleptinemic by a 60% fat intake (19 ± 4 ng/ml, cerebrospinal fluid leptin 69 ± 22 pg/ml). CART was increased 5-fold in the former and 2-fold in the latter, yet in adenovirus-induced hyperleptinemia, body fat had disappeared, whereas in high-fat-fed rats, body fat was abundant. Treatment of the high-fat-fed rats with adenovirus-leptin further increased their hyperleptinemia to 56 ± 6 ng/ml without changing CART mRNA or food intake, indicating that leptin action on hypothalamus had not been increased. Nevertheless, their body fat declined 36%, suggesting that an extrahypothalamic mechanism was responsible. We conclude that in diet-induced obesity body-fat depletion by leptin requires supraphysiologic plasma concentrations that exceed the leptin-transport capacity across the blood–brain barrier.
Keywords: leptin, cerebrospinal fluid, obesity
Reduction in body fat induced by the intracerebroventricular administration of leptin (1–4) has led to the widespread assumption that the actions of leptin are regulated entirely by hypothalamic factors (5–9). However, there are now reasons to suspect that some effects of leptin, at least those that occur at high plasma concentrations, are mediated via extraneural actions. First, the plasma levels of radioiodinated leptin 20 min after intracerebroventricular injection equal or exceed those observed after its i.v. injection (10), evidence that intracerebroventricular administration of the peptide could have effects on peripheral tissues. Second, leptin receptors are widely expressed throughout the body (11–13). Third, direct in vitro action of leptin has been demonstrated in several tissues, including adipocytes (14–17). Finally, transport of leptin across the blood–brain barrier is saturable (18–21), suggesting that leptin effects that occur only at plasma concentrations above the saturation level represent direct actions on peripheral tissues.
A primary goal of this study was to determine whether the rapid disappearance of body fat (22) and adipocyte dedifferentiation caused by virus-induced hyperleptinemia (23) are mediated by actions of leptin on the hypothalamus or via direct peripheral actions. Three criteria for hypothalamic activity of plasma leptin were used: (i) evidence of transport across the blood–brain barrier reflected by an increase in cerebrospinal fluid (CSF) leptin proportional to its increase in plasma; (ii) an increase in a hypothalamic peptide, cocaine- and amphetamine-regulated transcript (CART), known to be up-regulated by leptin (24); and (iii) a decrease food intake. As a criterion for direct, nonhypothalamic action of leptin, we measured leptin mRNA in adipocytes, which normally is directly suppressed by recombinant leptin in vitro (25). We attribute leptin effects occurring in the absence of the first three criteria to a direct extrahypothalamic mechanism, whereas those occurring in the absence of suppression of leptin mRNA we assume are not the result of direct leptin action on adipocytes.
Using this approach, we find that increments of plasma leptin concentration within the physiologic range act on the hypothalamus without any evidence of direct action on adipocytes; on the other hand, increments of plasma leptin well above the physiologic range cause depletion of body fat through combined hypothalamic activity on food intake plus direct leptin on adipocytes.
METHODS
Animal Groups.
Ventromedial hypothalamus (VMH)-lesioned and sham-lesioned control rats. VMH-lesioning was performed at the Department of Neuroscience, University of Florida (Gainesville) as approved by the University of Florida Institutional Animal Care and Use Committee, which follows the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult Sprague–Dawley (Harlan–Sprague–Dawley) male rats weighing 250–300 g were housed in air-conditioned rooms (22–25°C) with lights on from 0500–1900 h. Food and water were available ad libitum. Rats were anesthetized with 50 mg/kg sodium pentobarbital (Abbott) administered i.p. The VMH was destroyed bilaterally by the electrolytic lesion procedure as described (26). Briefly, rats were placed in the stereotaxic instrument and positioned with the nose bar set 3.3 mm below the interaural line. The electrode, consisting of an insulated stainless steel insect pin with exposed tip, was positioned 2.6 mm behind the bregma and 0.6 mm lateral to the midline and was lowered to the base of the brain and then raised 0.5 mm. A direct anodal current of 2.5 mA for 15 sec was passed through the electrode aimed at the VMH with a rectal electrode serving as cathode. For sham controls, the same surgical procedures were performed except that no current was passed. Rats were allowed to recover for approximately 1 week. They then were flown to Dallas for further studies. Only animals that developed hyperphagia and rapid gain in body weight compared with sham-lesioned rats were used. Sham-lesioned animals were used to control for possible damage to nerve fibers by the procedure.
Rats with diet-induced obesity (DIO).
Sprague-Dawley rats purchased from Charles River Laboratories were housed in individual metabolic cages with constant temperature and 12 h of light and 12 h of darkness. Four-week-old rats were given either their usual diet of 50 g/d standard chow (Teklad FG Rodent diet, Harlan Teklad, Madison, WI), which contains 24.8% protein and 4% fat; (3.94 kcal/g; 1 kcal = 4.18 J) or a high-fat diet (Teklad FG Rodent diet, Harlan Teklad), which contains 60% fat, 7.5% carbohydrate and 24.5% protein (6.7 kcal/g).
Zucker diabetic fatty (ZDF) rats.
Obese ZDF/drt-fa/fa rats were bred in our laboratory from rats purchased from R. Peterson (University of Indiana, Indianapolis). Obesity is discernible at ≈4 weeks of age. The same diet used above was used except for a 6% content of fat.
Construction of Recombinant Adenovirus Containing the Leptin cDNA (AdCMV–leptin).
The recombinant adenovirus containing the rat leptin cDNA (AdCMV–leptin) was prepared as described (22). For control purposes, virus containing the bacterial β-galactosidase gene under control of the CMV promoter (AdCMV–β-gal) was prepared and used as described (27).
Collection of CSF Samples.
Animals were anesthetized with pentobarbital sodium, 5 mg/100 g of body weight, administered i.p. CSF was collected through a 28-gauge 1/2 needle inserted into the cisterna magna and then gently withdrawn. Usually, 50–200 μl of CSF were collected from each rat and assayed individually. All CSF samples were centrifuged to assess contamination with blood. Because plasma leptin levels are much higher than those of CSF, the presence of blood would result in spuriously high CSF readings. Those without gross pellet formation were stored at −20°C until assayed for leptin, whereas those contaminated with blood were discarded.
Collection of Hypothalami.
Immediately after sacrifice, the brain of each rat was exposed with its ventral side up, a 0.5 × 0.6 cm incision with a depth of 1–1.5 mm was made behind the optic chiasm, and a rectangular piece containing the hypothalamus was removed and immediately frozen in liquid nitrogen.
Leptin Measurements.
At the time of sacrifice, blood samples were collected from the inferior vena cava and placed in tubes coated with EDTA. Plasma was stored at −20°C. Plasma leptin was assayed by using the Linco leptin assay kit (Linco Research Immunoassay, St. Charles, MO). CSF leptin was assayed by using the Mediagnost kit (Túbingen, Germany).
Nuclear MRI.
The method of Stein et al. was used (28). Proton magnetic resonance spectroscopy and nuclear MRI data were obtained with 4.7-T 40-cm-bore system (Omega chemical shift imaging model, Bruker Instruments, Billerica, MA) by using a 6-inch (15.24 cm) diameter birdcage coil. Rats were placed supine within the coil and positioned in the center of the magnet. Proton spectra of the rat were resolved into water and fat resonances, the areas of which were quantified by using the nuclear magnetic resonance (nrm-1) software program (Tripos Associates, St. Louis), assuming equal line widths for both resonances. Proton images were obtained from the abdominal region of rat. Spin-echo transaxial images were acquired with the following parameters: two transients, recycle time = 500 msec, echo time = 16 msec, 2-mm slice thickness, 2-mm interslice gap, eight slices, a 140-mm field of view, and a 128 × 256 matrix. Images were analyzed by using NIH image software (National Institutes of Mental Health, Bethesda, MD).
Semiquantitation of CART mRNA and Leptin mRNA by Reverse Transcription–PCR.
CART and leptin mRNA expressions were analyzed by using reverse transcription–PCR semiquantitation in hypothalamus and in white adipose tissues. Briefly, total RNA was extracted by using TRIzol isolation kit (Life Technologies, Grand Island, NY) and treated with RNase-free DNase. First-strand cDNA was obtained by using the first-strand cDNA synthesis kit (CLONTECH). The following primers were used: CART, 5′-AGCGAGGAAGTCCAGCAC-3′ (sense, 1–18) and 5′-CCGAAGGAGGCTGTCACC-3′ (antisense, 413–430) (GenBank accession no. U10071) and β-actin, 5′-TTGTAACCAACTGGGACGATATGG-3′ (sense, 1552–1575) and 5′-GATCTTGATCTTCATGGTGCTAGG-3′ (antisense, 2967–2991) (GenBank accession no. J00691); leptin, sense primer 5′-GGAGGAATCCCTGCTCCAGC-3′ and antisense primer 5′-CTTCTCCTGAGGATACCTGG-3′. Linearity of the PCR was tested by amplification of 200 ng per reaction from 20–45 cycles. The linear range was found to be between 20 and 40 cycles. Two microliters of the first-strand cDNA was amplified for 35 cycles for CART and leptin and 25 cycles for β-actin by using the following parameters: 92°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min. The products were electrophoresed on a 1.2% agarose gel. After transferring to Hybond-N Nylon membrane (Amersham Pharmacia), DNA samples were hybridized with [32P]ATP-labeled specific probes (5′-ACTGCTCTCCAGCGTCACACTCACACAGCT-3′ for CART, 5′-GGTCAGGATCTTCATGAGGTAGTCTGTCAG-3′ for β-actin, and 5′-CGGATACCGACTGCGTGTGTGAAATGTCAT-3′ for leptin) and analyzed in the Molecular Imager (Bio-Rad Laboratories).
Statistical Analyses.
All values shown are expressed as mean ± SEM. Statistical analysis was performed by two-tailed unpaired with unequal variance Student’s t test.
RESULTS
Validation of CART mRNA Expression as an Index of Hypothalamic Leptin Activity. To determine whether measurement of hypothalamic CART mRNA does, in fact, provide a valid index of leptin action, we semiquantified the transcript in rats with absent and with enhanced leptin action. CART, one of several hypothalamic inhibitors of food intake (30), is widely expressed throughout the brain and is known to be regulated by leptin (24, 29). It is almost absent in the arcuate nucleus of animals with disrupted leptin signaling (29).
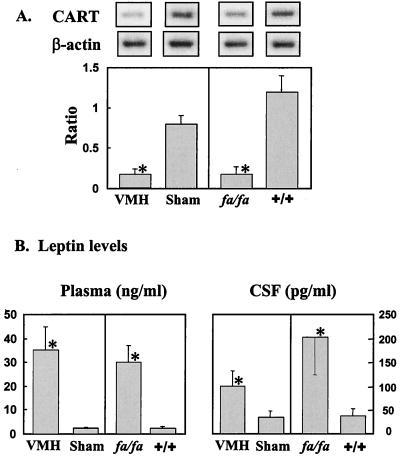
Two models with absent leptin action were studied: rats with obesity caused by electrolytic lesions of the ventromedial nucleus of the hypothalamus (VMH rats) (26) and rats with obesity caused by a loss-of-function mutation in the leptin receptor (ZDF rats) (31, 32). CART mRNA in hypothalamic tissue of both VMH-lesioned rats and ZDF rats was markedly reduced (P < 0.01; Fig. 1A) in the former, perhaps via destruction of CART neurons. In VMH-lesioned rats, CART mRNA levels were 20 ± 2% of sham-lesioned controls, despite plasma and CSF leptin levels of 35 ± 10 ng/ml and 100 ± 32 pg/ml, respectively (Fig. 1B).
Figure 1.
(A) Ratio of CART/β-actin mRNA in VMH-lesioned rats (n = 9) and sham-lesioned controls (n = 6) and in fa/fa (n = 12) and +/+ ZDF rats (n = 11). (B) Leptin levels in plasma and CSF of VMH-lesioned and sham-lesioned rats and in fa/fa and +/+ ZDF rats. ∗, P < 0.01.
In ZDF rats with elevated plasma and CSF leptin levels of 30 ± 7 ng/ml and 202 ± 79 pg/ml, respectively, CART mRNA was only 14 ± 2% of wild-type controls (P < 0.01; Fig. 1 A and B). Moreover, a further increase in the hyperleptinemia of the ZDF rats induced by infusing AdCMV–leptin did not raise the depressed CART mRNA, despite the increase of their plasma leptin levels to 50 ± 5.5 ng/ml, compared with 14 ± 1.0 ng/ml in ZDF rats infused with AdCMV–β-gal as a control. Food intake, another index of hypothalamic leptin activity, also was unaffected, as was body weight, even though CSF leptin levels of ZDF rats averaged 202 ± 79 pg/ml, the highest observed in any of our rat models (Fig. 1B).
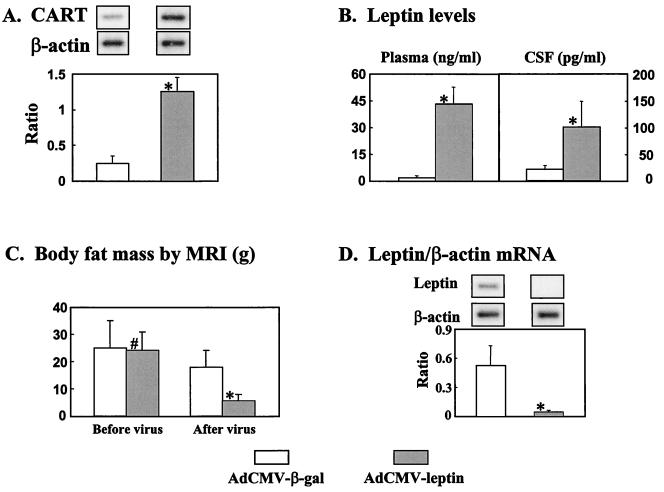
For further validation of the use of CART expression in the hypothalamus as an index of leptin action, we determined whether hyperleptinemia would augment hypothalamic CART expression in normal rats. We infused a group of four normal rats with either AdCMV–leptin or AdCMV–β-gal and measured plasma and CSF leptin levels and CART mRNA in the hypothalamus before the infusion and 6 days thereafter (Fig. 2). Plasma leptin rose to 43 ± 9 ng/ml on day 6, and CSF leptin averaged 100 ± 49 pg/ml (Fig. 2B). Food intake had decreased by 30–50%, and CART mRNA had increased 5-fold (Fig. 2A), evidence of leptin action on the hypothalamus. However, body fat declined by 22 ± 9 g (Fig. 2C), and leptin mRNA in white fat tissue was undetectable by day 6 (Fig. 2D), suggesting direct leptin action on adipocytes. These findings in normal animals are consistent with both hypothalamic and peripheral leptin actions.
Figure 2.
Effects in normal rats of adenovirus-induced hyperleptinemia (n = 6) or, as a control, adenovirus-induced β-gal (n = 5) overexpression on CART/β-actin mRNA ratio in hypothalamus (A), leptin levels in plasma and CSF (B); body fat mass determined by nuclear MRI (C); leptin/β-actin mRNA ratio in adipocytes, an index of autosuppression of leptin gene expression (D). ∗, P < 0.01; #, P > 0.05.
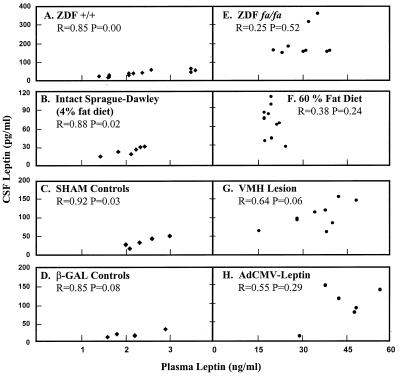
Correlations Between Plasma and CSF Leptin.
At plasma leptin levels below 4 ng/ml, there was a high degree of correlation between plasma and CSF leptin levels in all of the normal rats. This includes wild-type ZDF (+/+), intact or sham-lesioned Sprague–Dawley rats, and AdCMV–β-gal-infused controls on a normal diet (Figs. 3 A–C) (r = 0.85–0.92; P < 0.05). However, at plasma leptin levels in excess of 15 ng/ml, no correlation between plasma and CSF leptin was observed in any of the rat models studied (Figs. 3 E–H) (r = 0.25–0.64; P > 0.05). These hyperleptinemic models included the obese ZDF (fa/fa) rats, rats with VMH lesions, normal rats on a high fat diet, and normal rats made hyperleptinemic by AdCMV–leptin injection. This result suggests that leptin transport across the blood–brain barrier is saturated at plasma leptin levels >4 but <15 ng/ml.
Figure 3.
Relationship between leptin levels in plasma (x axis) and CSF (y axis) in normal wild-type ZDF (+/+) rats (A), intact Sprague–Dawley rats (B), sham-lesioned controls (C), and normal rats infused with adenovirus–β-gal (D) as a control for the adenovirus-leptin treated rats (H). fa/fa ZDF rats (E). Normal rats with obesity induced by a 60% fat diet (F). VMH-lesioned rats (G). Adenovirus-leptin-induced hyperleptinemia (H). There was high and significant correlation in rats with plasma leptin levels below 4 ng/ml. No correlation between plasma and CSF leptin levels was noted in hyperleptinemic rats with plasma values over 15 ng/ml.
Relationship Between Plasma and CSF Leptin and Its Hypothalamic and Nonhypothalamic Actions. The increases in CART expression and food intake seem to have reached an upper limit at plasma leptin levels <15 ng/ml, inasmuch as they were not affected by further increments in plasma leptin levels. Fat mass, on the other hand, was not affected by plasma leptin levels <15 ng/ml. Consequently, the profound changes in fat mass and in adipocyte leptin gene expression observed at plasma leptin levels >15 ng/ml may represent a direct peripheral action.
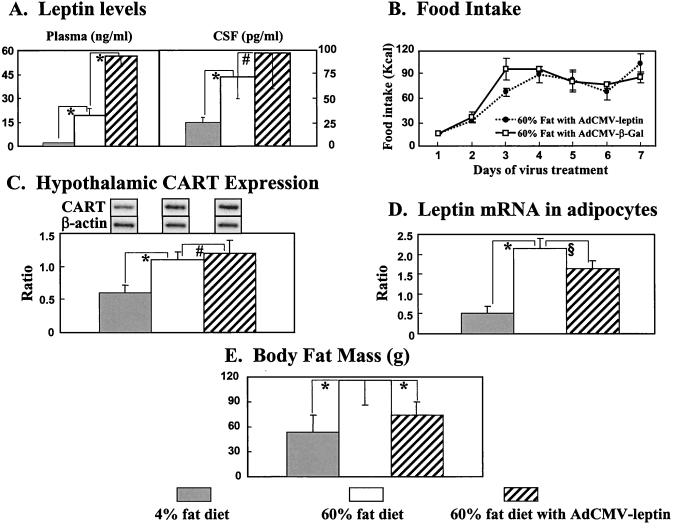
To separate more clearly the hypothalamic actions of leptin from its peripheral actions, it was necessary to select a hyperleptinemic rat model with normal leptin receptors. We chose the DIO rat. Sprague–Dawley rats were fed a 60% fat diet for 8 weeks. At the end of this period, they weighed 502 ± 11 g, compared with 376 ± 8 g in controls fed a 4% fat diet. Their plasma leptin levels averaged 19 ± 4 ng/ml, significantly above the 2 ± 0.2 ng/ml average of rats on the 4% fat diet (P < 0.01), and their CSF leptin levels averaged 69 ± 22 pg/ml, compared with 24 ± 6 pg/ml in controls on a 4% diet (Fig. 4A). DIO rats spontaneously altered their food intake so that their caloric intake was not significantly different from rats on a 4% fat diet (data not shown), suggesting that leptin-mediated hypothalamic control over food intake was intact. Also, CART mRNA was twice as high in the hypothalamus of the high-fat group as in rats fed the 4% fat diet (P < 0.01). The increase in CSF leptin was not significantly different from the increase observed in rats with virus-induced hyperleptinemia in which all fat disappeared (69 ± 22 pg/ml vs. 100 ± 49 pg/ml, P > 0.05). Despite comparable increases in CSF leptin, in DIO rats, the adipocytes were not depleted of fat (Fig. 4E), nor was the leptin mRNA of their adipocytes decreased (Fig. 4D). On the contrary, in DIO rats, the leptin mRNA/β-actin ratio averaged 2.2 ± 0.3 vs. 0.5 ± 0.2 in controls (P < 0.01), and body fat mass, measured by using nuclear MRI, was 116 ± 30, compared with 53 ± 21 in controls on a 4% fat diet (Fig. 4D).
Figure 4.
Effects of adenovirus-induced hyperleptinemia superimposed on preexisting hyperleptinemia caused by 60% fat feeding on plasma and CSF leptin levels (A), food intake (B), CART/β-actin mRNA ratio in hypothalamus (C), leptin/β-actin mRNA ratio in adipocytes (D) and body fat mass (E) measured by nuclear MRI (n = 4). ∗, P < 0.01; §, P < 0.05; #, P > 0.05.
These findings indicate, first, that there is substantial leptin transport across the blood–brain barrier in DIO rats, second, that leptin is acting, at least to some degree, on the hypothalamus to adjust caloric intake and to increase CART mRNA and, third, that there is no evidence of direct leptin action on adipocytes to deplete fat content and decrease fat mRNA.
To determine in DIO rats whether a further increase in the hyperleptinemia would raise their CSF leptin to still higher levels, reduce their food intake, and further increase hypothalamic CART mRNA, we infused them with AdCMV–leptin. Plasma leptin rose to 56 ± 6 ng/ml, compared with 19 ± 4 ng/ml in untreated DIO controls. CSF rose to 93 ± 36 pg/ml, which was not significantly different from the 69 pg/ml level of uninfused DIO rats (P = 0.19; Fig. 4A), suggesting that leptin transport capacity across the blood–brain barrier had been almost saturated by the relatively modest hyperleptinemia of DIO alone. Neither CART mRNA in the hypothalamus nor food intake were altered by the 36 ng/ml increase in plasma leptin induced by virus (Figs. 4 B and C), evidence that the hypothalamic action of leptin is not changed by the near tripling of plasma leptin levels.
Despite the lack of evidence for hypothalamic action, the body fat mass declined dramatically from 116 ± 30 g to 74 ± 16 g in 7 days, a surprising 6 g/day loss of body fat (Fig. 4E). This was twice the 3 g/day fat loss in normal lean rats made hyperleptinemic. Because the fat loss induced by the hyperleptinemia in the DIO rats was not associated with a significant increase in CSF leptin or in CART mRNA or with a decrease in food intake, it likely represents a direct effect of leptin on adipocytes. This possibility is supported by the small reduction (P < 0.05) in leptin mRNA in adipocytes (Fig. 4D).
The findings of this study are summarized in Table 1.
Table 1.
Comparison of effects of hyperleptinemia induced by high fat feeding, by adenovirus-mediated leptin gene transfer, and by combination of high-fat feeding and adenovirus-induced hyperleptinemia on CSF leptin levels, on hypothalamic parameters (food intake and hypothalamic CART mRNA), and on adipocytes (body-fat mass and leptin mRNA)
| Response | Dietinduced | Adenovirusinduced | Diet- plus adenovirus-induced |
|---|---|---|---|
| CSF leptin levels | ↑ | ↑ | ↔ |
| Hypothalamus | |||
| Food intake (g) | ↓ | ↓ | ↔ |
| CART mRNA | ↑ | ↑ | ↔ |
| Adipocytes | |||
| Body fat | ↑ | ↓ | ↓ |
| Leptin mRNA | ↑ | ↓ | ↓ |
DISCUSSION
We have used the hypothalamic level of CART expression as a bioassay for in vivo leptin activity on the hypothalamus of rats. Its use for this purpose was validated by demonstrating a relationship of CART mRNA to different levels of leptin activity. Marked hyperleptinemia induced by adenovirus-mediated overexpression of the leptin activity caused a 5-fold increase in CART expression, whereas absence of leptin activity as a result of ablation of the VMH or of a loss-of-function mutation of the leptin receptor (26, 31, 32) profoundly reduced CART expression. As a bioassay for direct in vivo leptin activity in adipocytes, we semiquantified leptin mRNA of fat tissue, which had been shown previously to be autosuppressed in vitro by recombinant leptin (25).
Using this strategy to assay hypothalamic and peripheral actions of leptin, we studied the hyperleptinemic DIO model of obesity. DIO caused by the feeding of a 60% fat diet was associated with hyperleptinemia of 19 ± 4 ng/ml and a mean CSF leptin of 69 ± 22 pg/ml; CART mRNA in the hypothalamus was twice normal in DIO, evidence that the hypothalamus does respond, at least partially, to the hyperleptinemia of DIO. But a further increase in the hyperleptinemia of DIO rats to a level of 56 ± 6 ng/ml, induced by adenovirus-mediated overexpression of the leptin gene, did not elicit a further increase in either CSF leptin levels or CART mRNA, and it did not reduce food intake. Taken together, these results suggest that in DIO, the capacity for leptin transport across the blood–brain barrier and consequent leptin activity on the hypothalamus is saturated by the preexisting hyperleptinemia and cannot be additionally increased by a further rise in plasma leptin. Nonetheless, the nearly 3-fold increase in plasma leptin induced by adenovirus-leptin treatment reduced the nuclear MRI-determined fat mass by 36%. This reduction of 42 g of body fat in 7 d may represent a direct peripheral action of leptin on adipocytes. The slight but significant reduction in adipocyte leptin mRNA is taken as presumptive evidence of direct leptin action on adipocytes. The possibility that resistance to leptin action at the central and/or peripheral levels exits in DIO was not excluded in this study.
These results permit differentiation of hypothalamic leptin actions from extrahypothalamic effects believed to be direct. Physiologic levels of leptin acting on hypothalamic centers influence appetite through various neurotransmitters, as extensively reviewed (8, 30). Physiologic concentrations of leptin may also regulate homeostasis of fatty acids and triacylglycerol in nonadipocytes (33) but do not deplete body fat or autosuppress leptin expression. The fat-depleting effect of leptin appears to be direct and occurs in completely denervated white fat tissue (34). It is observed only at leptin concentrations well above the physiologic range and, although irrelevant to leptin physiology, may be important in the pharmacologic approach to obesity.
Acknowledgments
We thank Dr. Joel Elmquist, D.V.M., Ph.D., of Harvard University, for critical review of this manuscript, Tess Perico, who provided excellent secretarial assistance, and Kay McCorkle, for excellent technical support. This work was supported by Veterans Affairs Institutional Research Support Grant SMI 821–109, National Institutes of Health (NIH) Grant DK-02700-37, the NIH/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research program and Novo Nordisk A/S, Dagsvaerd, Denmark, and NIH Grants DK37273 and NS32727. Animal care was in accordance with institutional guidelines.
ABBREVIATIONS
- CART
cocaine- and amphetamine-regulated transcript
- VMH
ventromedial hypothalamus
- ZDF
Zucker diabetic fatty
- CSF
cerebrospinal fluid
- DIO
diet-induced obesity
References
- 1.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 2.Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. Diabetes. 1996;45:1446–1450. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- 3.Shi Z Q, Nelson A, Whitcomb L, Wang J, Cohen A M. Metabolism. 1998;47:1274–1280. doi: 10.1016/s0026-0495(98)90336-5. [DOI] [PubMed] [Google Scholar]
- 4.Seeley R J, van Dijk G, Campfield L A, Smith F J, Burn P, Nelligan J A, Bell S M, Baskin D G, Woods S C, Schwartz M W. Horm Metab Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- 5.Stephens T W, Basinski M, Bristow P K, Bue-Valleskey J M, Burgett S G, Craft L, Hale J, Hoffmann J, Hsiung H M, Kriauciunas A, et al. Nature (London) 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W H, Kimura M, Walczewska A, Karanth S, McCann S M. Proc Natl Acad Sci USA. 1997;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung C C, Clifton D K, Steiner R A. Endocrinology. 1997;138:4489–5592. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 9.Friedman J M, Halaas J L. Nature (London) 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 10.Maness L M, Kastin A J, Farrell C L, Banks W A. Endocrinology. 1998;139:4556–4562. doi: 10.1210/endo.139.11.6319. [DOI] [PubMed] [Google Scholar]
- 11.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 12.Hoggard N, Mercer J G, Rayner D V, Moar K, Trayhurn P, Williams L M. Biochem Biophys Res Commun. 1997;232:383–387. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 13.Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M. Biochem Biophys Res Commun. 1997;238:648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 14.Siegrist-Kaiser C A, Pauli V, Juge-Aubry C E, Boss O, Pernin A, Chin W W, Rohner-Jeanrenaud F, Burger A G, Zapf J, Meier C A. J Clin Invest. 1997;100:2858–2864. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimabukuro M, Koyama K, Chen G, Wang M Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tena-Sempere M, Pinilla L, Gonzalez L C, Dieguez C, Casanueva F F, Aguilar E. J Endocrinol. 1999;161:211–218. doi: 10.1677/joe.0.1610211. [DOI] [PubMed] [Google Scholar]
- 17.Nemecz M, Preininger K, Englisch R, Furnsinn C, Schneider B, Waldhausl W, Roden M. Hepatology. 1999;29:166–172. doi: 10.1002/hep.510290110. [DOI] [PubMed] [Google Scholar]
- 18.Caro J F, Kolaczynski J W, Nyce M R, Ohannesian J P, Opentanova I, Goldman W H, Lynn R B, Zhang P L, Sinha M K, Considine R V. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 19.Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz M W, Peskind E, Raskind M, Boyko E J, Porte D., Jr Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 21.Koistinen H A, Karonen S L, Iivanainen M, Koivisto V A. Eur J Clin Invest. 1998;28:894–897. doi: 10.1046/j.1365-2362.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Koyama K, Yuan X, Lee Y, Zhou Y T, O’Doherty R, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y T, Wang Z W, Higa M, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1999;96:2391–2395. doi: 10.1073/pnas.96.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen P, Judge M E, Thim L, Ribel U, Christjansen K N, Wulff B S, Clausen J T, Jensen P B, Madsen O D, Vrang N, et al. Nature (London) 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 25.Wang M Y, Lee Y, Unger R H. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 26.Dube M G, Kalra P S, Crowley W R, Kalra S P. Brain Res. 1995;690:275–278. doi: 10.1016/0006-8993(95)00644-6. [DOI] [PubMed] [Google Scholar]
- 27.Herz J, Gerard R D. Proc Natl Acad Sci USA. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein D T, Babcock E E, Malloy C R, McGarry J D. Int J Obes Metab Disord. 1995;19:804–810. [PubMed] [Google Scholar]
- 29.Elias C F, Lee C, Kelly J, Aschkenasi C, Ahima R S, Couceyro P R, Kuhar M J, Saper C B, Elmquist J K. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 30.Kalra S P, Dube M G, Pu S, Xu B, Horvath T L, Kalra P S. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 31.Phillips M S, Liu Q, Hammond H A, Dugan V, Hey P J, Caskey C J, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 32.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Biochem Biophys Res Commun. 1996;222:19–26. doi: 10.1006/bbrc.1996.0691. [DOI] [PubMed] [Google Scholar]
- 33.Unger R H, Zhou Y T, Orci L. Proc Natl Acad Sci USA. 1999;96:2327–2332. doi: 10.1073/pnas.96.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z-W, Zhou Y-T, Lee Y, Higa M, Kalra S P, Unger R H. Biochem Biophys Res Commun. 1999;260:653–657. doi: 10.1006/bbrc.1999.0918. [DOI] [PubMed] [Google Scholar]