Abstract
The proteasome regulates cellular processes as diverse as cell cycle progression and NF-κB activation. In this study, we show that the potent antitumor natural product epoxomicin specifically targets the proteasome. Utilizing biotinylated-epoxomicin as a molecular probe, we demonstrate that epoxomicin covalently binds to the LMP7, X, MECL1, and Z catalytic subunits of the proteasome. Enzymatic analyses with purified bovine erythrocyte proteasome reveal that epoxomicin potently inhibits primarily the chymotrypsin-like activity. The trypsin-like and peptidyl-glutamyl peptide hydrolyzing catalytic activities also are inhibited at 100- and 1,000-fold slower rates, respectively. In contrast to peptide aldehyde proteasome inhibitors, epoxomicin does not inhibit nonproteasomal proteases such trypsin, chymotrypsin, papain, calpain, and cathepsin B at concentrations of up to 50 μM. In addition, epoxomicin is a more potent inhibitor of the chymotrypsin-like activity than lactacystin and the peptide vinyl sulfone NLVS. Epoxomicin also effectively inhibits NF-κB activation in vitro and potently blocks in vivo inflammation in the murine ear edema assay. These results thus define epoxomicin as a novel proteasome inhibitor that likely will prove useful in exploring the role of the proteasome in various in vivo and in vitro systems.
In eukaryotes, protein degradation is mediated predominantly through the ubiquitin pathway in which proteins targeted for destruction are ligated to the 76-aa polypeptide ubiquitin (1). Once targeted, ubiquitinated proteins then serve as substrates for the 26S proteasome, a multicatalytic protease that cleaves proteins into short peptides through the action of its three major proteolytic activities (2). Although it has a general function in intracellular protein turnover, proteasome-mediated degradation also plays a key role in many processes such as MHC class I antigen presentation, apoptosis, cell division, and NF-κB activation (for review, see ref. 3).
The proteasome is a 700-kDa, cylindrical-shaped multicatalytic protease complex composed of 28 subunits organized into four rings. In yeast and other eukaryotes, seven different α subunits form the outer rings, and seven different β subunits comprise the inner rings (4). In addition to the ubiquitously expressed β subunits, higher vertebrates also possess three IFN-γ-inducible β subunits (LMP7, LMP2, and MECL1), which replace their normal counterparts, X, Y, and Z, respectively, thus altering the catalytic activities of the proteasome (5).
Through the use of different peptide substrates, three major proteolytic activities have been defined for the eukaryote 20S proteasome: chymotrypsin-like activity, which cleaves after large hydrophobic residues; trypsin-like activity, which cleaves after basic residues; and peptidyl-glutamyl peptide hydrolyzing activity (PGPH), which cleaves after acidic residues. Two additional, less-characterized activities also have been ascribed to the proteasome: BrAAP activity, which cleaves after branched-chain amino acids, and SNAAP activity, which cleaves after small neutral amino acids (6). The proteasome proteolytic activities appear to be contributed by different catalytic sites, because inhibitors, point mutations in β subunits, and the exchange of IFN-γ-inducing β subunits alter these activities to various degrees (7–9).
Several classes of proteasome inhibitors have been identified and currently are employed to study the physiological roles of the proteasome (10). Specific peptide aldehydes of substrate analogs have been found to form reversible covalent adducts with the proteasome and inhibit certain proteolytic activities (11). Peptide aldehydes also have been used to study IκB-α processing (12), antigen presentation (13), and induction of stress response (14). Although they have been used widely in recent years to study proteasome function, peptide aldehydes also inhibit lysosomal and Ca+2-activated proteases (15), thus complicating a precise dissection of their effects on cells. Other proteasome inhibitors include peptides possessing a carboxyl vinyl sulfone moiety, such as Z-Leu-Leu-Leu-vinyl sulfone, which acts as a “suicide substrate” (16). However, these vinyl sulfone-based inhibitors have similar limitations as the peptide aldehydes insofar as they have been reported to bind and inhibit intracellular cysteine proteases (i.e., cathepsin S) (16, 17) in addition to their action against the proteasome.
The structurally distinct proteasome inhibitor lactacystin initially was isolated from an Actinomycetes strain based on its ability to promote neurite outgrowth (18). Schreiber and coworkers (19) demonstrated that lactacystin and its related clasto-β-lactone covalently bind to the N-terminal threonine of the 20S proteasome subunit X. Subsequent studies demonstrated that lactacystin irreversibly modifies all catalytic β subunits (20). Like the peptide aldehydes and vinyl sulfones, lactacystin also inhibits proteases other than the proteasome, namely, cathepsin A (21) and tripeptidyl peptidase II (22).
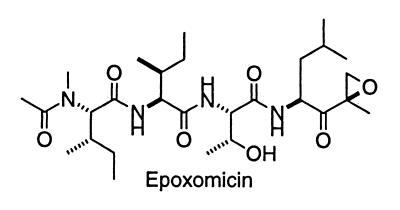
The α′,β′-epoxyketone containing natural product epoxomicin (Fig. 1) was isolated from an Actinomycetes strain based on its in vivo antitumor activity against murine B16 melanoma tumors (23). Despite this potent activity, the mechanism of epoxomicin’s biological action has remained unknown. We have identified the proteasome as the intracellular protein target of this potent antitumor agent. Using a synthetic biotinylated affinity derivative, we show here that epoxomicin covalently binds the LMP7, X, Z, and MECL1 catalytic β subunits of the proteasome and selectively inhibits the three major proteasome proteolytic activities at different rates. Moreover, we present in vitro and in vivo evidence that epoxomicin effectively inhibits NF-κB-mediated proinflammatory signaling. Given its unique specificity and potency, this antitumor, antiinflammatory natural product represents a class of cell-permeable, irreversible inhibitors distinct from those currently in use and, thus, might prove useful in in vivo and in vitro analyses of proteasome function.
Figure 1.
Structure of epoxomicin.
MATERIALS AND METHODS
Materials.
Streptavidin-horseradish peroxidase, calpain, cathepsin B, and streptavidin agarose were purchased from Sigma. Suc-LLVY-AMC and Boc-LRR-AMC were purchased from Bachem and Peptides International, respectively. Z-LLE-AMC, clasto-lactacystin β-lactone, NLVS, cathepsin B substrate III (Z-RR-AMC), and anti-p53 antisera were obtained from Calbiochem. NeutrAvidin beads were purchased from Pierce. Anti-IκB-α and antiubiquitin antisera were obtained from Santa Cruz Biotechnology and Zymed, respectively. Epoxomicin and epoxomicin-biotin were synthesized as described (24). The human B cell lymphoma cell lines LCL 721.45 and LCL 721.174 were kindly provided by P. Cresswell (Yale University).
Cell Culture and Treatments. Human umbilical vein endothelial cells (HUVECs) (provided by J. Pober, Yale University), were cultured in DMEM (GIBCO/BRL) containing 10% FBS and 50 μg/ml endothelial cell growth factor (Sigma). The transformed human kidney epithelial cell line (HEK-293) and HeLa cell line were grown in DMEM containing 10% FBS. The mouse thymoma cell line EL4 was cultured in RPMI 1640 medium (GIBCO/BRL) plus 10% FBS. All cell culture media were supplemented with 50 μg/liter penicillin and 50 μg/liter streptomycin.
For IκB and ubiquitin Western blot analysis, 60% confluent HeLa monolayers were treated with 10 μM epoxomicin or Z-LLL-H for 2 hr. Tumor necrosis factor α (TNF-α) (10 ng/ml) was added to one set of the drug-treated plates and alone to a separate dish of cells. Cells were harvested after 15 min. p53 stabilization was analyzed by immunoprecipitating with mouse anti-p53 antisera from HUVECs treated with DMSO, 100 nM epoxomicin, or 5 μM Z-LLL-H for 6 hr followed by immunoblot analysis with rabbit anti-p53 antisera. For electrophoretic mobility-shift assays (EMSAs), epoxomicin was added to HeLa cells in duplicate and TNF-α (10 ng/ml) was added to one set of the drug-treated plates and also alone to a separate dish of cells. Cells were harvested after 1 hr, and nuclear lysates were prepared as described (25).
Purification of Epoxomicin-Binding Proteins.
Ten liters of EL4 cells (106cells/ml) was harvested and resuspended in 50 ml of RPMI 1640 medium containing 10% FBS. Epoxomicin-biotin was added to a final concentration of 8 μM, and cells were incubated at 37°C for 4 hr. Cells were harvested and homogenized by using a Powergen homogenizer in lysis buffer (25 mM Hepes, pH 7.4/5 mM EGTA/50 mM NaF) plus protease inhibitors (10 μg/ml leupeptin, pepstatin, and soybean trypsin inhibitors and 1 mM PMSF). The high-speed (100,000 × g) supernatant was loaded onto a 1-ml streptavidin-agarose column to remove endogenous biotinylated proteins. The flow-through fraction then was incubated for 10 min with 50 ml of DE52 beads preequilibrated with lysis buffer, washed twice with 50 ml of lysis buffer containing 0.1 M NaCl, and eluted with 50 ml of lysis buffer containing 0.3 M NaCl. SDS was added to the eluant at a final concentration of 0.5%, boiled for 10 min, and diluted 2.5-fold by using lysis buffer. The diluted solution was loaded onto a 0.4-ml NeutrAvidin agarose column. The flow-through fraction was collected and reloaded onto the same column three times. After extensive washes, epoxomicin-biotin-binding proteins were eluted by boiling the NeutrAvidin agarose in 0.4 ml of 1× SDS sample buffer. The purified protein complexes were separated by SDS/PAGE, excised, and identified by the W. M. Keck Foundation Biotechnology Resource Laboratory (Yale University) by using LCQ (MS/MS) and automated Edman degradation of internal tryptic peptides.
Enzyme Kinetic Assays.
For proteasome inhibition assays, peptide-AMC substrates (5 μM Suc-LLVY-AMC, 5 μM Z-LLE-AMC, and 5 μM Boc-LRR-AMC) and inhibitors in DMSO were added to assay solutions at a final DMSO concentration of 1%. The following assay buffer was used: 20 mM Tris⋅HCl, pH 8.0/0.5 mM EDTA (plus 0.035% SDS for Suc-LLVY-AMC and Z-LLE-AMC assays). Bovine red blood cell proteasome was added to the assay buffer containing substrates and inhibitors at a final volume of 100 μl at room temperature (23°) in a Dynex (Chantilly, CA) Microfluor II 96-well plate and the fluorescence emission immediately was measured at 460 nm (λex, 360 nM) by using a Cytofluor (Perspective Biosystems, Framingham, MA) fluorescence plate reader for 50 min. kobs/[I] values were obtained using kaleidagraph by nonlinear least-squares fit of the data to the following equation: fluorescence = vst + [(vo − vs)/kobs][1 − exp(−kobst)], where vo and vs are the initial and final velocities, respectively, and kobs is the reaction rate constant. Dilutions of bovine erythrocyte 20S proteasome (2.5 mg/ml) were as follows: 1:1,200 final dilution for Suc-LLVY-AMC activity, 1:3,000 for Z-LLE-AMC, and 1:800 for Boc-LRR-AMC. Inhibition reactions were performed as described previously (26). For calpain inhibition assays, the enzyme was used at 1 unit/ml, and Suc-LLVY-AMC was used at a final concentration of 10 μM in assay buffer containing 20 mM Tris, pH 8.0/1 mM CaCl2/2 mM DTT. Cathepsin B was used at a concentration of 0.005 unit/ml in 100 mM sodium acetate/5 mM EDTA, pH 5.5, and cathepsin substrate III was used as substrate at 40 μM. Kinetic assays were performed as described for the proteasome.
EMSAs.
EMSAs were performed as described (25). In brief, consensus DNA-binding oligonucleotide sequences for transcription factor NF-κB from Santa Cruz Biotechnology were labeled with [γ-32P]ATP and incubated with equal amounts of nuclear lysates. Protein–DNA complexes were separated on 4% polyacrylamide gels under nondenaturing and nonreducing conditions. The gels were dried and exposed to a PhosphorImaging screen (Molecular Dynamics) for quantitation of radioactivity in retarded bands. Results are representative of experiments performed at least twice.
Assay for Contact Sensitivity (CS).
CS and irritant-response assays to picrylchloride (a generous gift of P. Asekanse, Yale University) challenge were performed essentially as described (27), with slight modifications. In brief, for the CS assay, mice were injected i.p. daily for 6 days with vehicle or epoxomicin (0.58 mg/kg body weight) solubilized in 10% DMSO/PBS. Six days after abdomen immunization with picrylchloride, ear thickness measurements (0 hr) of both ears were made in triplicate with an engineer’s micrometer (Peacock dial thickness gauge; Ozaki Manufacturing, Tokyo). Mice subsequently were challenged on both ear lobes by application of 15 μl of a 0.8% solution of picrylchloride (solubilized in high-grade extra virgin olive oil). Ear swelling measurements again were made 24 hr post-ear challenge. In a second assay, elicitation of inflammatory response to the nonspecific vascular activation and permeability effects of picrylchloride (irritant response) were determined by using two groups of four nonimmunized mice. The 0-hr ear thickness measurements were made, a single high-dose injection of epoxomicin (2.9 mg/kg) was delivered i.p. to one group, and the control group was treated with vehicle. Ear thickness was measured 24 hr post-ear challenge.
RESULTS
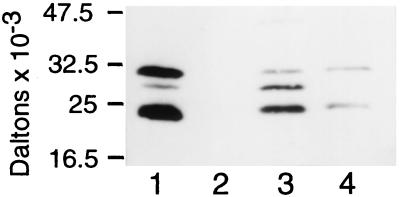
Identification of Epoxomicin-Binding Proteins as β Subunits of the Proteasome. Given epoxomicin’s potent cytotoxic activity and the biological importance of the epoxide moiety in α′,β′-epoxyketone-containing natural products (28), we hypothesized that epoxomicin mediates its biological activity through the covalent interaction with an intracellular protein(s). To identify this epoxomicin receptor(s), we synthesized a biotinylated epoxomicin analog to serve as an affinity chromatography reagent (24). Epoxomicin-biotin was incubated with the murine lymphoma EL4 cell line for 4 hr, cell lysates were analyzed by SDS/PAGE, and subsequently, the membrane-immobilized cellular lysates were probed with streptavidin-HRP. Two major (23- and 30-kDa) and one minor (28-kDa) newly biotinylated bands were detected (Fig. 2). These proteins were shown to interact specifically with epoxomicin-biotin because pretreatment of cells with a 5-fold molar excess of epoxomicin prevents biotin binding to these proteins upon subsequent challenge with epoxomicin-biotin (Fig. 2, lane 2).
Figure 2.
Epoxomicin biotin binds four proteasome catalytic subunits. EL4 murine thymoma cells (3 × 105) were incubated with 5 μM epoxomicin-biotin without (lane 1) and with (lane 2) 50 μM epoxomicin pretreatment. Human B cell lymphoma cells LCL 721.45 (lane 3) and LCL 721.174 (lane 4) were labeled with 5 μM epoxomicin-biotin. Biotinylated proteins were visualized by using avidin-horseradish peroxidase and chemiluminescence.
After large-scale purification using avidin affinity chromatography, the three purified epoxomicin-binding proteins were digested with trypsin and the resulting tryptic peptides were subjected to matrix-assisted laser desorption ionization–MS (MALDI-MS) analysis. Comparison of these results with peptide masses generated from theoretical tryptic digests of putative ORFs in GenBank revealed significant matches between two of the three epoxomicin-binding proteins (23 and 28 kDa) and two murine proteosomal subunits. The 23-kDa band was identified as LMP7, a IFN-γ-inducible catalytic β subunit of the 20S proteasome. The percentage of the LMP7 protein sequence covered was 31.9% for the tryptic peptides from the 23-kDa band. MALDI-MS analysis of tryptic peptides from the 28-kDa band identified this epoxomicin-binding protein as the IFN-γ-inducible proteasome catalytic subunit, MECL1, with 29% coverage between the tryptic peptides and the MECL1 protein sequence. Although the tryptic peptide masses of the 30-kDa band matched 19.9% of the predicted protein sequence of the Z catalytic subunit of the proteasome, this was less than the 20% match criteria used to confirm protein identity. Therefore, automated Edman degradation was performed on an internal tryptic peptide from this 30-kDa protein. The resulting 11-aa sequence was identical to residues 185–195 of the murine Z β catalytic proteasome subunit (data not shown). Thus, epoxomicin was shown to bind covalently the LMP7, MECL1, and Z proteasome catalytic subunits. Given that epoxomicin binds both Z and its IFN-γ-inducible counterpart MECL1, we tested whether epoxomicin also binds in an analogous manner to the subunit X, which is the housekeeping counterpart of LMP7. Epoxomicin-biotin binding to the human B cell line LCL 721.45 gave a similar pattern as observed with the murine lymphoma line EL4 (Fig. 2, lane 3). However, epoxomicin-biotin binding to a LCL 721.45 derivative line (5), which does not express LMP7 or LMP2, resulted in two epoxomicin-biotin-binding proteins of 23 and 30 kDa (Fig. 2, lane 4). Immunoblot analysis confirmed that these bands are the X and Z proteasome subunits, respectively (not shown).
Proteasome/Protease Inhibition Studies. The identification of the major epoxomicin-binding proteins as proteasome β subunits suggested that epoxomicin mediates its cell biological effects via inhibition of proteasome catalytic function. To test this hypothesis, purified bovine erythrocyte proteasome was assayed enzymatically by using a variety of different substrates and inhibitors. To evaluate the rates of proteolytic inactivation by inhibitors, kassociation (kobs/[I]) values were determined by using fluorogenic peptide substrates over a range of inhibitor concentrations. Epoxomicin most potently inhibits the chymotrypsin-like activity of the 20S proteasome with a kassociation value of 35,400 M−1⋅s−1 (Table 1). This rate of inactivation is greater than 4- and 5-fold faster than clasto-lactacystin β-lactone and the potent vinyl sulfone NLVS, respectively. Interestingly, epoxomicin and clasto-lactacystin β-lactone displayed near-identical inhibitory activities against the trypsin-like and the PGPH activities. The greater inhibition of chymotrypsin-like activity relative to the trypsin-like activity is consistent with the more rapid covalent modification of the LMP7/X subunits than the MECL1/Z subunits by epoxomicin-biotin (data not shown).
Table 1.
Inhibition of proteasome catalytic activities
| Compound |
kassociation = kobs/[I] (M−1s−1)
|
||
|---|---|---|---|
| Chymotrypsin-like activity | Trypsin-like activity | PGPH activity | |
| Epoxomicin | 35,400 ± 1400 | 287 ± 71 | 34 ± 4.8 |
| (40–80 nM) | (6–10 μM) | (25–75 μM) | |
| clasto-lactacystin | 8,530 ± 280 | 253 ± 41 | 37 ± 4.7 |
| β-Lactone | (200–400 nM) | (6–10 μM) | (25–75 μM) |
| NLVS | 6,790 ± 919 | 5.3 ± 2.8 | 6.4 ± 2.3 |
| (200–400 nM) | (50–100 μM) | (50–100 μM) | |
The rate of covalent inhibition (kassociation) of the three major proteasome catalytic activities were determined for epoxomicin, clasto-lactacystin β-lactone, and peptide vinyl sulfone, NLVS. A range of concentrations was used to determine the kassociation for inhibition of individual enzymatic activities.
We also investigated whether epoxomicin shared protease inhibitory specificities with two other classes of peptide-based proteasome inhibitors (i.e., peptide aldehydes and peptide vinyl sulfones). Although several peptide aldehydes potently inhibit the proteasome, they also inhibit other intracellular, nonproteasomal proteolytic activities such as calpain and lysosomal proteases (cathepsins) (13). Epoxomicin did not display any inhibitory activity against the calcium-dependent protease calpain, papain, chymotrypsin, trypsin, and cathepsin B at concentrations of up to 50 μM, whereas the vinyl sulfone NLVS inhibited cathepsin B enzymatic activity at 10 μM with a kassociation value of 191 M−1⋅s−1.
Epoxomicin Induces the Accumulation of p53 and Ubiquitinated Proteins.
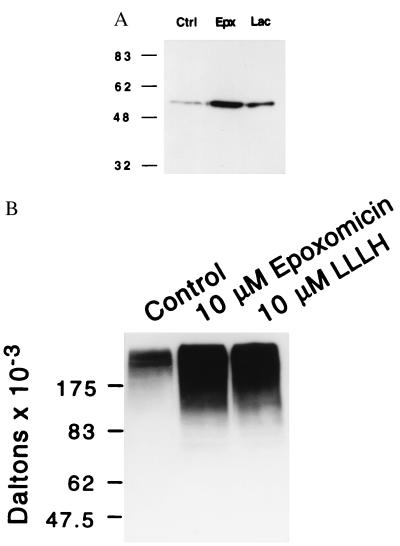
We next investigated the ability of epoxomicin to inhibit in vivo proteasome function in cultured cells. Incubation of HUVECs with epoxomicin for 6 hr resulted in a 30-fold increase in the levels of p53 protein, a known target of the proteasome (Fig. 3A). In contrast, incubation with a 50-fold-higher concentration of lactacystin resulted in only a 10-fold increase of p53 levels over that of untreated cells. Incubation of cells with epoxomicin for longer than 48 hr results in greater than 95% cellular apoptosis (not shown). Because inhibition of proteasome function should result in increased levels of ubiquitinated proteins, we also analyzed the effect of epoxomicin on total ubiquitinated protein accumulation. Incubation of HeLa cells with 10 μM epoxomicin for 2 hr resulted in the accumulation of multiple, higher-molecular-mass bands recognized by antiubiquitin antisera. These upper-molecular-mass bands also were observed in cellular lysates of cells treated with the peptide aldehyde inhibitor, Z-LLL-H (Fig. 3B, lane 3).
Figure 3.
Accumulation of p53 (A) and ubiquitinated proteins (B) in epoxomicin-treated cells. (A) α-p53 immunoblot analyses of HUVECs treated with 100 nM epoxomicin (Epx), 5 μM lactacystin (Lac), or vehicle (ctrl) for 6 hr. (B) α-Ubiquitin immunoblot analyses of HeLa cells treated with 10 μM epoxomicin or peptide inhibitor Z-LLL-H for 2 hr.
Epoxomicin Prevents IκB Degradation and Activation of NF-κB DNA-Binding Activity.
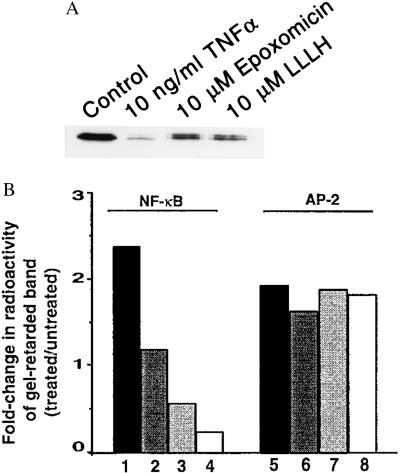
The targeting of IκBα, the cytoplasmic inhibitory subunit complexed to NF-κB, for degradation by the proteasome is known to occur in response to a variety of stress activators (29). In this study, we investigated whether epoxomicin blocks TNF-α-induced activation of NF-κB by stabilizing IκBα. Western blot analysis revealed that 10 ng/ml TNF-α potently induced the degradation of IκBα in HeLa cells within 15 min of treatment. However, pretreatment with 10 μM epoxomicin for 2 hr inhibited IκBα degradation by 10-fold (Fig. 4A), to a level similar to that produced by treating TNF-α-stimulated cells with 10 μM of the peptide aldehyde inhibitor Z-LLL-H. Next, using EMSAs, we tested whether epoxomicin inhibits NF-κB DNA-binding activity. EMSAs of nuclear lysates derived from HeLa cell cultures treated with TNF-α alone showed greater than 2-fold induction of NF-κB DNA-binding activity. Epoxomicin pretreatment for 1 hr produced a significant dose-dependent reduction in TNF-α-stimulated NF-κB DNA-binding activity (Fig. 4B). These effects were selective because we determined that the DNA-binding activity of activator protein 2 (AP-2) was unaltered. Additionally, incubation of HEK 293 cells with 100 nM epoxomicin for up to 5 hr did not inhibit the PMA-stimulated DNA binding of transcription factor AP-2 (data not shown). These results suggest that epoxomicin targets NF-κB activity via inhibition of proteasome-mediated degradation of IκBα.
Figure 4.
Epoxomicin inhibits activation of NF-κB. (A) IκBα degradation induced by TNF-α is prevented by epoxomicin. HeLa cells were treated with epoxomicin (10 μM) or Z-LLL-H (10 μM) for 2 hr and subsequently treated with TNF-α (10 ng/ml) for 15 min. Western blot analysis of cell lysates was performed to measure IκBα levels as in Fig. 3. (B) EMSA analysis of NF-κB DNA-binding activity. HeLa cells were treated with increasing concentrations of epoxomicin for 2 hr, and, subsequently, 10 ng/ml TNF-α was added to drug-treated cells or to untreated cultures and incubated for 1 hr. Equal amounts of protein from nuclear extracts prepared from untreated and treated cultures were incubated with a radiolabeled NF-κB oligonucleotide or a control AP-2 oligonucleotide and fractionated on 4% polyacrylamide gels. Dried gels were exposed to a PhosphorImaging screen. The amount of radioactivity in the transcription factor-retarded bands was quantitated and represented as fold-change of treated over that of untreated samples. Shown are TNF-α alone (lanes 1 and 5) and TNF-α plus epoxomicin (100 nM, lanes 2 and 6; 1 μM, lanes 3 and 7; and 10 μM, lanes 4 and 8).
Epoxomicin Potently Reduces CS in Vivo.
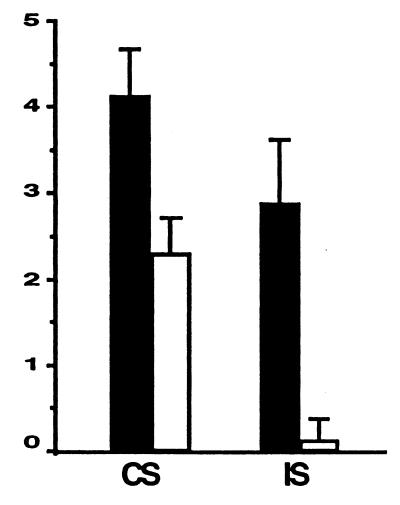
CS is an inflammatory response to certain classes of chemical compounds and haptens (30). Based on inhibition of NF-κB activation in cell culture, we hypothesized that epoxomicin would have antiinflammatory activities in vivo. To test this hypothesis, epoxomicin was evaluated in the picrylchloride mouse model of CS. Mice immunized with picrylchloride were challenged after 6 days by application of picrylchloride on their ears. Ear thickness measurements were made at 0 and 24 hr after picrylchloride ear challenge. As shown in Fig. 5, daily treatment with epoxomicin at a nontoxic dose of 0.58 mg/kg per day reduced the CS response by 44% relative to the control group of mice treated with vehicle alone. Because the hapten can elicit a nonspecific, irritation-related inflammatory response (31), we explored the effects of epoxomicin on skin irritation-mediated inflammation by using nonimmunized mice. In a second experiment, mice were pretreated with epoxomicin at a dose five times higher than that used previously to test the idea that a single injection of the drug could reduce inflammation in response to picrylchloride ear challenge. Epoxomicin administered at 2.9 mg/kg potently inhibited the irritant-associated inflammatory response by 95% when ear edema measurements were made 24 hr postchallenge (Fig. 5).
Figure 5.
Epoxomicin inhibits inflammation in vivo. (A) CS to picrylchloride. BALB/c mice were immunized with picrylchloride by topical application to shaved chest and abdomen. One group of immunized mice (n = 4) was treated with epoxomicin daily from the time of immunization until the time of ear challenge, whereas the control immunized group (n = 4) was treated with vehicle. On day 6 postimmunization, all mice were skin-challenged by painting both ears with 0.8% picrylchloride. Ear swelling responses were measured before (0 hr) and 24 hr after ear challenge. (B) Irritant sensitivity (IS) response to picrylchloride. Nonimmunized BALB/c mice were injected with epoxomicin (n = 4) or vehicle (n = 4) before ear challenge with 0.8% picrylchloride, and ear swelling measurements were made 0 and 24 hr post-ear challenge. Results in CS and IS assays are expressed as 24-hr measurement minus 0-hr measurement.
DISCUSSION
Once thought merely to dispose of denatured and misfolded proteins, the proteasome is now recognized as a master proteolytic machinery that regulates the levels of diverse intracellular proteins through their degradation in a signal-dependent manner. Hence, there is great interest in identifying cell-permeable reagents that can specifically perturb the activities of the proteasome and thereby be used as probes to study the role of the proteasome in biological processes. Toward these goals, we have employed a chemical–genetic approach to identify natural products that target the proteasome. We report here the identification of a cell-permeable, natural product, epoxomicin, that potently, selectively, and irreversibly inhibits proteasome activity. Epoxomicin modifies four catalytic subunits of the 20S proteasome, resulting in inhibition primarily of the chymotrypsin-like activity; the trypsin-like and PGPH activities also were inhibited at approximately 100- and 1,000-fold-slower rates, respectively. Furthermore, in comparison with other potent, irreversible proteasome inhibitors, epoxomicin inhibits the chymotrypsin-like activity 80-fold faster than lactacystin (data not shown) and 4-fold faster than clasto-lactacystin β-lactone. However, unlike several other peptide-based inhibitors, epoxomicin does not inhibit nonproteasomal proteases such as trypsin, chymotrypsin, cathepsin B, papain, and calpain at concentrations of up to 50 μM. Epoxomicin also is shown to inhibit NF-κB activation and to stabilize p53 levels in cell culture. Moreover, we have demonstrated the potent antiinflammatory activity of epoxomicin in a mouse model of cutaneous inflammation. Thus, this cell-permeable natural product lends itself as a unique molecular probe, which has the versatility to explore proteasome function in normal biological and pathological processes.
A number of active site-directed, carboxyl-terminally modified peptides have been shown to inhibit the 20S proteasome. These include peptide aldehydes (13, 32), peptide vinyl sulfones (16), peptide boronic acids (33), glyoxals (34), and α′,β′-epoxyketone-containing peptides (35). Given that peptide aldehydes, vinyl sulfones, and boronic acid proteasome inhibitors share with epoxomicin many structural similarities, it was somewhat unexpected to find that epoxomicin lacks inhibitory activity against papain, chymotrypsin, cathepsin B, or calpain, a characteristic that compromises the utility of several proteasome inhibitors in cellular studies of proteasome function (17, 36). The specificity of epoxomicin for the proteasome may be attributed to the selective activation of the epoxyketone pharmacophore within the active sites of the proteasome, relative to the active sites of other proteases. We speculate that activation of the epoxide occurs in situ via hydrogen bonding between oxygen of the epoxide of this covalent-binding suicide substrate and an appropriately configured, proton-donating amino acid side-chain moiety around the active site. This mechanism is consistent with our findings that the aldehyde derivative of epoxomicin, which also inhibits the proteasome, harbors cross-reactivity toward other cellular proteases (unpublished results). The crystallization of the proteasome complexed with epoxomicin undoubtedly will shed light on this conundrum.
Because NF-κB is a key regulator of inflammation, it is an attractive target for antiinflammatory therapeutic intervention. Although several peptide aldehyde proteosomal inhibitors have been shown to target NF-κB activation (37), their potential cross-reactivity with other cellular proteases limits their use in studying the biological processes of proteasome function and also raises concerns for their use in studying pathological disease processes. In comparison with these other proteasome inhibitors, epoxomicin is unique, demonstrating potent and specific inhibition of the proteasome and its functions. We showed that epoxomicin targets NF-κB-mediated signaling by demonstrating that the natural product stabilized levels of IkBα, resulting in inhibition of the DNA-binding activity of NF-κB in the nucleus. This transcriptional inhibition is specific because we demonstrated that DNA-binding activity of AP-2, which also was stimulated by TNF-α and PMA, was not affected by epoxomicin up to 10 μM. In addition, we also observed a similar specificity in other cell types; treatment of HEK 293 cells with epoxomicin results in inhibition of PMA-induced DNA-binding activities of NF-κB and AP-1, but not that of AP-2 (data not shown). In this respect, both Jun and Fos, components of the AP-1 complex, are also known to be degraded by the proteasome (38).
To address the role of the proteasome in pathological inflammatory processes, we explored epoxomicin’s potential as an in vivo antiinflammatory agent. CS is a cutaneous immune inflammatory response that is mediated by CD4+ T cells in the classical delayed-type hypersensitivity (DTH) reaction (39). The DTH response in mice immunized with such haptens as picrylchloride (2,4,6-trinitro chlorobenzene) or 2,4,6-trinitrobenzene sulfone has been well characterized (40), being mediated by the proinflammatory cytokines IFN-γ and TNF-α. The DTH response, which is initiated by antigen-specific and nonspecific factors, leads to local increase in vascular permeability, in part, by serotonin release that enables circulating CD4+ effector T cells to migrate into local sites of antigen challenge. Using the picrylchloride model of CS, we demonstrated that epoxomicin administration at nontoxic doses (as judged by absence of weight loss) was found to reduce CS significantly. Because picrylchloride also exerts a degree of nonspecific irritation and nonspecific inflammation, we tested the efficacy of epoxomicin in the skin irritation assay by using nonimmunized mice. A single bolus of epoxomicin abrogated 95% of the inflammatory response. Presumably, approximately 25% of ear swelling in the picrylchloride-preimmunized BALB/c mice is derived from priming mechanisms involving immune function (compare ear-swelling values of vehicle-treated animals in CS and irritant assays).
The biological consequences of proteasome inhibition are numerous. At the cellular level, the accumulation of polyubiquitinated proteins, cell morphological changes, and apoptosis has been reported upon treatment of cells with various proteasome inhibitors. In our analyses, we have observed that p53 levels are stabilized more than 30-fold by epoxomicin treatment. In addition, our results showing an accumulation of polyubiquitinated proteins in epoxomicin-treated cells provide evidence that the proteasome is the target of epoxomicin. Proteasome inhibition also has been suggested as a possible antitumor therapeutic strategy (26, 41, 42). That epoxomicin initially was identified in a screen for antitumor compounds (23) validates the proteasome as an antitumor chemotherapeutic target. Moreover, in parallel with this study, we have found that another antitumor natural product, eponemycin, targets the proteasome, although less potently (26).
In conclusion, we have demonstrated that the cell-permeable natural product epoxomicin specifically targets the 20S proteasome, thereby potently and irreversibly inhibiting its proteolytic activities. Moreover, unlike other peptide-based proteasome inhibitors currently in use, epoxomicin inhibits the proteasome selectively relative to other intracellular proteases. In addition, we show that epoxomicin demonstrates antiinflammatory activity in vivo. While this manuscript was in preparation, the dipeptide boronic acid proteasome inhibitor, PS-341, was shown to inhibit NF-κB activation in vitro and attenuate inflammation in the rat model of Streptococcal cell wall-induced polyarthritis (43). Together with our findings, these results suggest that proteasome inhibition may prove a viable strategy for antiinflammation therapy.
Acknowledgments
We thank George DeMartino for the generous gift of bovine 20S proteasome and Gabriel Fenteany for helpful comments on the manuscript. This work was supported by the National Institutes of Health (CA74967). C.M.C. is a Burroughs Wellcome Fund New Investigator. M.E. was supported by a postdoctoral fellowship from the Swedish Natural Science Research Council, 1996–1998.
ABBREVIATIONS
- TNF
tumor necrosis factor
- EMSA
electrophoretic mobility-shift assay
- CS
contact sensitivity
- AP-1 and -2
activator protein 1 and 2
- HUVEC
human umbilical vein endothelial cell
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Varshavsky A. Trends Biochem Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 2.Hilt W, Wolf D H. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 3.Ciechanover A. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik H D, Huber R. Nature (London) 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll J, Brown M G, Finley D, Monaco J J. Nature (London) 1993;365:262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- 6.Orlowski M, Cardozo C, Michaud C. Biochemistry. 1993;32:1563–1572. doi: 10.1021/bi00057a022. [DOI] [PubMed] [Google Scholar]
- 7.Bogyo M, Shin S, McMaster J S, Ploegh H L. Chem Biol. 1998;5:307–320. doi: 10.1016/s1074-5521(98)90169-7. [DOI] [PubMed] [Google Scholar]
- 8.Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski U H, Kloetzel P M. J Exp Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinemeyer W, Gruhler A, Mohrle V, Mahe Y, Wolf D H. J Biol Chem. 1993;268:5115–5120. [PubMed] [Google Scholar]
- 10.Lee D H, Goldberg A L. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 11.Read M A, Neish A S, Luscinskas F W, Palombella V J, Maniatis T, Collins T. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 12.Palombella V J, Rando O J, Goldberg A L, Maniatis T. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 13.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 14.Coux O, Tanaka K, Goldberg A L. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 15.Mellgren R L. J Biol Chem. 1997;272:29899–29903. doi: 10.1074/jbc.272.47.29899. [DOI] [PubMed] [Google Scholar]
- 16.Bogyo M, McMaster J S, Gaczynska M, Tortorella D, Goldberg A L, Ploegh H. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bromme D, Klaus J L, Okamoto K, Rasnick D, Palmer J T. Biochem J. 1996;315:85–89. doi: 10.1042/bj3150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omura S, Takeshima H. Tanpakushitsu Kakusan Koso. 1996;41:327–336. [PubMed] [Google Scholar]
- 19.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 20.Craiu A, Gaczynska M, Akopian T, Gramm C F, Fenteany G, Goldberg A L, Rock K L. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowska H, Wojcik C, Omura S, Worowski K. Biochem Biophys Res Commun. 1997;234:729–732. doi: 10.1006/bbrc.1997.6434. [DOI] [PubMed] [Google Scholar]
- 22.Geier E, Pfeifer G, Wilm M, Lucchiari-Hartz M, Baumeister W, Eichmann K, Niedermann G. Science. 1999;283:978–981. doi: 10.1126/science.283.5404.978. [DOI] [PubMed] [Google Scholar]
- 23.Hanada M, Sugawara K, Kaneta K, Toda S, Nishiyama Y, Tomita K, Yamamoto H, Konishi M, Oki T. J Antibiotics. 1992;45:1746–1752. doi: 10.7164/antibiotics.45.1746. [DOI] [PubMed] [Google Scholar]
- 24.Sin N, Kim K, Elofsson M, Meng L, Auth H, Crews C M. Bioorg Med Chem Lett. 1999;9:2283–2288. doi: 10.1016/s0960-894x(99)00376-5. [DOI] [PubMed] [Google Scholar]
- 25.Mohan R, Rinehart W B, Bargagna-Mohan P, Fini M E. J Biol Chem. 1998;273:25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- 26.Meng L, Kwok B H B, Sin N, Crews C M. Cancer Res. 1999;59:2798–2801. [PubMed] [Google Scholar]
- 27.Tsuji R F, Kikuchi M, Askenase P W. J Immunol. 1996;156:4444–4450. [PubMed] [Google Scholar]
- 28.Sugawara K, Hatori M, Nishiyama Y, Tomita K, Kamei H, Konishi M, Oki T. J Antibiot. 1990;43:8–18. doi: 10.7164/antibiotics.43.8. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, May M J, Kopp E B. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 30.Weston W L. Ann Allergy. 1976;37:346–352. [PubMed] [Google Scholar]
- 31.Grabbe S, Steinert M, Mahnke K, Schwartz A, Luger T A, Schwarz T. J Clin Invest. 1996;98:1158–1164. doi: 10.1172/JCI118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinitsky A, Michaud C, Powers J C, Orlowski M. Biochemistry. 1992;31:9421–9428. doi: 10.1021/bi00154a014. [DOI] [PubMed] [Google Scholar]
- 33.McCormack T, Baumeister W, Grenier L, Moomaw C, Plamondon L, Pramanik B, Slaughter C, Soucy F, Stein R, Zuhl F, Dick L. J Biol Chem. 1997;272:26103–26109. doi: 10.1074/jbc.272.42.26103. [DOI] [PubMed] [Google Scholar]
- 34.Lynas J F, Harriott P, Healy A, McKervey M A, Walker B. Bioorg Med Chem Lett. 1998;8:373–378. doi: 10.1016/s0960-894x(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 35.Spaltenstein A, Leban J J, Huang J J, Reinhardt K R, Viveros O H, Sigafoos J, Crouch R. Tetrahedron Lett. 1996;37:1343–1346. [Google Scholar]
- 36.Coutts S J, Kelly T A, Snow R J, Kennedy C A, Barton R W, Adams J, Krolikowski D A, Freeman D M, Campbell S J, Ksiazek J F, Bachovchin W W. J Med Chem. 1996;39:2087–2094. doi: 10.1021/jm950732f. [DOI] [PubMed] [Google Scholar]
- 37.Traenckner E B, Wilk S, Baeuerle P A. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treier M, Staszewski L M, Bohmann D. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 39.Van Loveren H, Askenase P W. J Immunol. 1984;133:2397–2401. [PubMed] [Google Scholar]
- 40.Piguet P F, Grau G E, Hauser C, Vassalli P. J Exp Med. 1991;173:673–679. doi: 10.1084/jem.173.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolfe M, Chiu M I, Pagano M. J Mol Med. 1997;75:5–17. doi: 10.1007/s001090050081. [DOI] [PubMed] [Google Scholar]
- 42.Spataro V, Norbury C, Harris A L. Br J Cancer. 1998;77:448–455. doi: 10.1038/bjc.1998.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palombella V J, Conner E M, Fuseler J W, Destree A, Davis J M, Laroux F S, Wolf R E, Huang J, Brand S, Elliott P J, et al. Proc Natl Acad Sci USA. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]