Abstract
Elaborate courtship displays are relatively common features of the masculine reproductive behavior in birds. However, little is known about their neural and hormonal control. One bird that performs such a display is the golden-collared manakin (Manacus vitellinus) of Panamanian forests. Adult males, but not females, perform a physically intense display requiring substantial neuromuscular control of the wings and legs. We tested the hypothesis that steroid sensitivity is a property of neurons in the manakin spinal cord. Males and females were captured from active courtship leks, treated with drugs to block steroidogenesis, injected with 3H-labeled testosterone, and the spinal cords were removed and processed for autoradiography. Sex steroid-accumulating cells were widely distributed in the spinal cords in each of six males and in one of five females. Cells, including presumptive motoneurons, reached their highest density in the ventral horns of the cervical and lumbosacral enlargements, regions associated with motor control of the wings and legs. These results suggest that neurons in the adult manakin spinal cord can express sex-steroid receptors, but do so less in females than in males. This evidence for androgen sensitivity and sexual dimorphism in the adult avian spinal cord suggests that sex steroids may control diverse behaviors in male birds in part by acting directly on the spinal neural circuits.
To reproduce, males perform behaviors to attract and stimulate females, defend territories and mates, copulate, and care for young. Sex steroids can control the development and expression of many of these behaviors by direct actions on the central nervous system. Many studies focus on the actions of sex steroids on the hypothalamus, where there exists a relatively conserved population of neurons expressing androgen receptors (AR) and estrogen receptors (ER) within circuits controlling masculine copulatory behaviors (1–3). Steroids can also act directly on motoneurons (4, 5). For example, AR can be expressed in mammalian motoneurons of the lumbar spinal cord that innervate muscles controlling the penis (6–9). By a combination of actions of estrogens and androgens on the brain and on the spinal cord, male mammals are stimulated to copulate and are functionally able to do so.
Steroids also influence neurons controlling other reproductive behaviors (4, 10), such as the widely studied neural circuitry controlling song located in the telencephalon of the oscine passerine songbirds (11, 12). Given that song is a significant acoustic signal coordinating reproduction in these birds, it is not surprising that the song-control circuitry is influenced by sex steroids via the expression of AR and ER. But song is just one of a suite of avian reproductive behaviors. Male birds can exhibit an impressive repertoire of visual and acoustic reproductive displays (13). In some species, these visual displays can be dramatic, including acrobatic movements that are enhanced by conspicuous physical ornaments. Despite the performance of these behaviors by birds across many taxa, little is known about their hormonal and neural control. Given that some of these displays (i) involve coordinate usage of several neuromuscular systems controlling posture and movements of the wings, legs, and tail; (ii) are often performed by males and not females; and (iii) are used in reproductive contexts, we would predict that in displaying birds, the spinal motoneurons controlling behaviorally relevant muscles would be sensitive to steroid hormones and would be anatomically and/or physiologically sexually dimorphic.
To test these hypotheses, we have performed tritiated testosterone (3H-T) autoradiography on the spinal cords of adult male and female golden-collared manakins (Manacus vitellinus), a common bird species of central Panamanian forests. Manakins are a family of suboscine, passerine birds that are common in forests of the New World tropics. Males of several manakin species, including the golden-collared manakins, perform elaborate courtship displays involving short flights with midair acrobatics and intense jumping and dancing movements. In addition, the wings of some species (including golden-collared manakins) possess sexually dimorphic feather structures (14) that assist in producing loud snapping sounds by the rapid flipping of their wings (13, 14). We report that male golden-collared manakins show widespread accumulation of 3H-T or its metabolites in the spinal cord, including in many large motoneurons, and this pattern of sex-steroid accumulation is different in females. Androgen accumulation in spinal motoneurons in adult birds suggests that steroid sensitivity may be present in those neural pathways of the spinal cord that generate a range of avian courtship behaviors.
METHODS
Golden-collared manakins (six males; five females) were captured in mist nets from active courtship leks located in central Panama in June, 1995 and September, 1996. (All protocols for animal use have been approved by the Chancellor’s Animal Research Committee and were collected under permit from Instituto Nacionale de Recursos Naturales Renovables, government of Panama.) To reduce endogenous androgen production, birds were injected immediately with an inhibitor of one of two steroidogenic enzymes, either trilostane (an inhibitor of 3-β-hydroxysteroid dehydrogenase/isomerase, Sterling-Winthrop Research Insitute, 2 males and 1 female) or ketoconazole (an inhibitor of 17-α-hydroxylase/C17–20 lyase, Janssen; 4 males and 4 females). Studies on the effectiveness of these two inhibitors in birds are published elsewhere (15, 16). After 24 hr (trilostane) or 8–12 hr (ketoconazole), the birds were injected with 60–80 μCi (1 Ci = 37 GBq) of 3H-T (specific activity 102.5 Ci/mmol; New England Nuclear) and sacrificed 90 min later by decapitation. The gonads were visually inspected and sizes noted. The vertebral columns were immediately dissected free of surrounding tissues and fixed briefly (≈1 min) in formalin (3.7% formaldehyde in 0.9% PBS) to assist with removal of the spinal cord (which remained unfixed). The spinal cords were then cut lengthwise, into halves or thirds, flash-frozen with crushed dry ice onto cork with Tissue-Tek (Sakura Finetek, Torrance, CA), and stored in dry ice at −80°C. The cords were transported in dry ice to the United States and prepared for autoradiography.
Cords were sectioned longitudinally in the dark on a cryostat (at −17°C) and thaw-mounted onto microscope slides. For autoradiographic analysis, two consecutive sections were cut at 6 μm and thaw-mounted onto separate slides previously dipped in Kodak NTB-2 photographic emulsion (series 1 and 2, respectively). The third consecutive section was sectioned at 20 μm and thaw-mounted on slides (Fisher Superfrost Plus). These slides were later stained with thionin and used to assist with preparation of a spinal cord map. The fourth consecutive section (cut at 6 μm) was usually discarded because of poor quality; this cycle was repeated through the entire cord. Although only one series was analyzed from a given bird, there was usually 38 μm of tissue between each section in a given series. Slides from series 1 and 2 were sealed in light-tight, desiccated containers and stored at 4°C. After 3, 6, 9, or 12 months, autoradiographic slides were immersed consecutively in Kodak D-19 developer, distilled H2O, and Kodak fixer, thionin stained, dehydrated in a graded series of alcohols, and coverslipped for later analysis.
For each bird, the slide series demonstrating optimal cellular-to-background silver grain density was chosen for analysis. Cells that appeared to have high levels of accumulation with a distinct nucleus were analyzed over both the nucleus and soma (which included the nucleus). Cells that appeared to have high levels of accumulation but lacked an obvious nucleus were analyzed for grain accumulation over the whole soma. These were measured by capturing the cellular image (at ×1,000) on a Macintosh computer by using a Zeiss Axioskop microscope linked to a Sony charge-coupled device video camera. By using National Institutes of Health image Version 1.61, the circumference of the nucleus, soma, or selected region of neuropil was traced, and silver grain size was defined. We computed both the area and the number of silver grains contained within the tracing. Silver-grain density was determined by dividing the area measurement into the number of silver grains counted. A similar measurement was then made over the remaining neuropil adjacent to the cell in the captured microscope image (background). The average background area measured was six times larger than the average motoneuron somal area. We then calculated the ratio of silver-grain density over the soma or nucleus to background density. Data were recorded for all cells in which this cellular or nuclear-grain density was ≥3 times the background; accumulation was considered significant when cellular or nuclear-grain densities were ≥5 times background (17, 18), and these cells are hereafter referred to as sex steroid-accumulating cells.
A map of the position of all sex steroid-accumulating cells in the spinal cord was created for each individual bird; afterward, a single composite map was created summarizing the general distribution of all birds. Cellular position in the rostrocaudal plane was established by observing landmarks such as visible dorsal-root ganglion, the beginning of the cervical enlargement that starts at C10 in the pigeon (19), and the lumbosacral enlargement that starts at L1 in the pigeon (20). Cells in the dorsoventral axis were located by estimating the proximity of the cell to Lamina IX (20), a morphologically prominent motoneuronal cell column throughout the ventral horn.
RESULTS
Sex steroid-accumulating cells were found in the manakin spinal cord with substantially more cells in males than in females. Accumulation of sex steroid was found in cells of the spinal cords of all six males (on average, 209 cells over the entire cord). By contrast, only one female approached this number (with 101 cells found). Only four, two, two, and zero accumulating cells per spinal cord were found in the remaining four females.
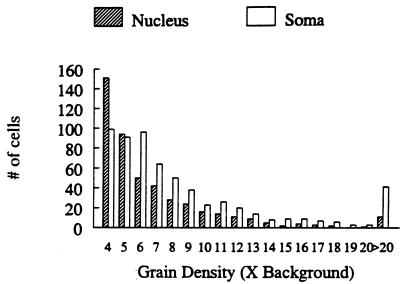
Cells with a visible nucleus comprised 59.5% of the total number of sex steroid-accumulating cells (Fig. 1). Of the nucleated cells, 95% had a greater density of silver grains over the nucleus alone as compared with the whole soma (which includes the nucleus). Fig. 2 illustrates the total number of nucleated and nonnucleated cells with nuclear or somal accumulation (respectively) greater than 3× background. Many cells in males were present with accumulation between 3 and 5× background (64.0 on average) but were not accepted as representing significant accumulation based on our criteria. In the female in which we found a large number of cells meeting the 5× criteria, 39 additional cells met the 3× criteria. If these cells do indeed express AR or ER, but at low levels, then our results may underestimate the total number of sex steroid-accumulating cells in the manakin spinal cord (21). No additional cells that met the 3× criteria were found in the remaining females.
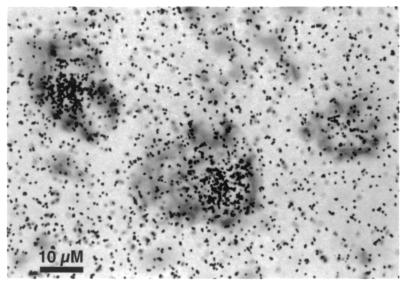
Figure 1.
Representative sex steroid-accumulating, thionin-stained neurons from the ventral cervical enlargement of an adult male golden-collared manakin.
Figure 2.
Motoneuronal silver grain density (cellular silver grain number as a multiple of background silver grain number) over nuclei (nucleated cells) and somas (nonnucleated cells) after injection of 3H-labeled testosterone into adult male and female golden-collared manakins.
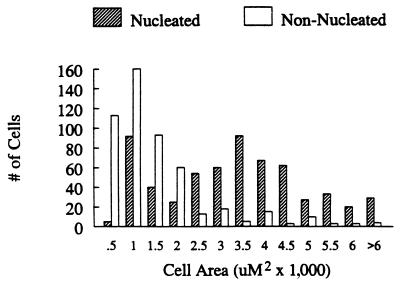
Sex steroid-accumulating cells with an obvious nucleus fell into two general size categories, small and large cells with mean areas of ≈1,000 μm2 and 4,000 μm2, respectively (Fig. 3). Most large cells were located ventrally, whereas small cells were distributed widely, especially in middle and dorsal levels of the cord.
Figure 3.
Areas of nucleated (hatched bars) and nonnucleated (open bars) sex steroid-accumulating cells in the spinal cords of adult male and female golden-collared manakins.
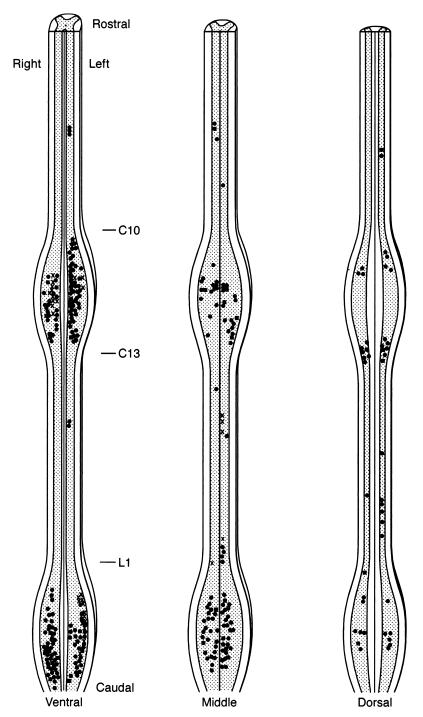
Even though sex steroid-accumulating cells were found in all six males, only four males provided histology that was qualitatively sufficient to allow for precise localization and mapping. Sex steroid-accumulating cells of the remaining two males were present with their size and position consistent with the large motoneurons of the ventral cervical and lumbosacral enlargements. For purposes of description, the spinal cord was subdivided rostrocaudally into the high cervical, cervical enlargement, midthoracic, and lumbosacral enlargement regions and dorsoventrally into the ventral, middle, and dorsal regions (Fig. 4). Cells were included on the map without distinguishing between those with and without nuclei. The relative abundance and distribution of sex steroid-accumulating cells in the spinal regions of the four males and five females is summarized in Table 1.
Figure 4.
Representative map showing the spatial distribution of sex steroid-accumulating cells in the golden-collard manakin spinal cord. Each symbol represents three cells, with isolated cells omitted; black dots represent cells found in males; X represents cells found in females (see text for details). The cord is illustrated at three levels; all cells found within a given level are illustrated: Ventral, the entire cord ventral to the bifurcation of the ventral horns, including most of lamina IX and much of lamina VIII of Leonard and Cohen (1975); dorsal, the entire cord dorsal to the beginning of the bifurcation of the dorsal horns out to their tips, including all of lamina I–IV of Leonard and Cohen (1975) and part of lamina V; middle, the remaining cord between the ventral and dorsal levels. Table 1 illustrates individual differences in the pattern of accumulation.
Table 1.
Relative abundance of sex steroid-accumulating cells in the manakin spinal cord
| Spinal cord region | Bird no. | Ventral
|
Middle
|
Dorsal
|
|||
|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | ||
| High cervical | 1 | + | − | + | − | + | − |
| 2 | + | − | + | − | + | − | |
| 3 | + | − | − | − | − | − | |
| 4 | − | − | + | + | − | − | |
| 5 | − | − | − | ||||
| Cervical enlargement | 1 | + | ++ | ++ | − | − | − |
| 2 | +++++ | − | ++ | − | +++ | − | |
| 3 | + | − | ++ | − | − | − | |
| 4 | − | − | +++ | − | − | − | |
| 5 | − | − | − | ||||
| Mid thoracic | 1 | − | − | − | + | − | − |
| 2 | − | − | − | − | − | − | |
| 3 | + | − | + | − | ++ | − | |
| 4 | − | − | + | − | − | − | |
| 5 | − | − | − | ||||
| Lumbasacral enlargement | 1 | − | ++ | − | − | − | − |
| 2 | − | − | − | + | − | − | |
| 3 | +++++ | − | ++ | − | − | + | |
| 4 | + | − | +++ | − | ++ | − | |
| 5 | − | − | − | ||||
M, male; F, female; −, no cells; +, 1–5 cells; ++, 16–50 cells; +++, 51–100 cells; ++++, 101–200 cells; +++++, 201–224 cells.
Within the high cervical cord (rostral to the cervical enlargement), accumulating cells were found in all four males and in none of the females. Here, the number of cells were roughly evenly distributed dorsoventrally, with the middle third of the high cervical region having the most cells relative to the ventral and dorsal regions. Localization was mostly confined to an area midway between the hindbrain and the cervical enlargement (see Fig. 4), approximately C5.
In the cervical enlargement (C10–C13), sex steroid-accumulating cells were abundant in the ventral third for three of the four males and for one female. Relative to the ventral third, there were comparatively fewer cells in the middle third, and accumulation was present in all four males and none of the females. In the ventral and middle third of the cervical enlargement, cells were found along the entire rostrocaudal length. In the dorsal third, we found one male with two distinct clusters of cells, one each in the rostral and caudal portions of the cervical enlargements. We found no sex steroid-accumulating cells in this region of any other bird.
A small number of sex steroid-accumulating cells were present in the midthoracic region, approximately T3, midway between the cervical and lumbosacral enlargements. Only one male showed a few accumulating cells in the ventral third of the cord. Small cell clusters were found in two males and two females in the middle third of the cord, and a group of cells from one male was found in the dorsal third of the cord.
Sex steroid-accumulating cells were widespread in the lumbosacral enlargement (L1–L6) throughout its full rostrocaudal extent. A majority of these cells were found in the ventral third of the enlargement and were found in one male and in one female. Numerous labeled cells were also found in medial portions of the middle third of the lumbosacral enlargement, especially in two males. One of these males also had a significant number of cells in the dorsal third of the enlargement. We were unable to reliably distinguish the glycogen body on our slides.
DISCUSSION
These results suggest that some neurons in the manakin spinal cord express AR as described in other vertebrates (6, 9, 22). It is also possible that 3H-T was aromatized into [3H]estradiol and that ER are expressed in the manakin spinal cord, as they are in rats (23). The presence of widespread sex-steroid accumulation in cells in the spinal cords of males compared with females suggests that there is a sex difference in the magnitude of AR and/or ER expression or in the number of AR- and/or ER-expressing cells. It is also possible that sex steroid availability in the manakin spinal cord may differ between males and females because of sex differences in steroid metabolism (24, 25). Presumably, sex steroids act on spinal neural circuits in manakins to control expression of male-specific behaviors.
Many sex steroid-accumulating cells in the manakin spinal cord are located in lamina IX (20) and are of a large size, consistent with that of motoneurons (Fig. 2). Similar AR-expressing motoneurons have been found in rat (23, 26) and in Xenopus (27), leading us to suspect that these ventral motoneurons in manakins are also androgen-, and not estrogen-, sensitive. Some smaller sex steroid-accumulating cells found in dorsal regions of the manakin spinal cord may be sensory neurons binding estrogen as reported in rats (23) and ring doves (28). Subpopulations of AR-expressing motoneurons are functionally associated with androgen-dependent muscles and together control androgen-dependent penile reflexes in rodents (6, 25, 9) and amplexus in Xenopus (22, 27). Therefore, we assume that at least some of these sex steroid-accumulating motoneurons in manakins are also part of androgen-dependent neuromuscular systems. Because in manakins the majority of sex steroid-accumulating cells are found in the cervical and lumbosacral enlargements, and motoneurons in these enlargements largely control muscles of the upper and lower extremities (19, 29), these cells may be involved in multiple behavioral functions, perhaps innervating muscles controlling the elaborate dancing and wing-snapping of these birds.
The presence of sex differences in sex-steroid accumulation in the manakin spinal cord is consistent with the idea that sex steroids act directly on spinal neural circuits to control the expression of these courtship behaviors. Males, but not females, court actively, presumably because testosterone circulates at higher levels in males than in females. We suspect that androgens, or their metabolites, act centrally to increase the motivation to court and peripherally to increase the neuromuscular capacity to perform the displays. Androgens also act on neurons centrally and peripherally in frogs and rats to activate masculine reproductive behaviors (5, 30, 31). In the spinal cords of these species, sex differences in AR expression in part underlie the observed sex differences in behavior (5).
The basis of the sex differences in sex-steroid binding in the manakin spinal cord is unknown. The differences could arise from sex differences in the numbers of some AR-expressing cells, as observed in the rat (6, 21), in which fewer AR-expressing motoneurons exist in the female lumbar spinal cord because females lack two of the target muscles (5). It is unlikely that the female manakin possesses fewer motoneurons if they are innervating essential muscles of the wings and legs. It is more likely that higher levels of circulating testosterone in male manakins transiently up-regulate spinal AR to a greater degree than in females, as is presumed to occur in Xenopus (27). Although we cannot exclude the possibility that some of the differences we observe reflect permanent sex differences in sex-steroid binding or in other cellular attributes, we assume that neuromuscular control of the wings and legs is temporally adapted for greater use by sex steroids when males are actively courting.
We found considerable variability in the numbers of sex steroid-accumulating cells across males and the extent to which androgens were bound by cells (Table 1). Cells were found most consistently in the cervical enlargement, but even here one male showed none. This variability in spinal cord steroid sensitivity may have been produced by differences in circulating testosterone if males were in different reproductive condition. Some birds were caught in September, a time when males are beginning to cease courtship activity (14). Our data indicate that the male with the smallest testes had the lowest number of sex steroid-accumulating cells, with greater numbers of accumulating cells found in males with somewhat larger testes. Because androgens can directly regulate AR in the spinal cord (9), males with low plasma testosterone levels before capture could have displayed less sex-steroid accumulation. No differences in ovary size were observed that account for differences observed across females. Variability might also have been produced artificially if trilostane or ketoconazole reduced endogenous androgen synthesis differently across birds, creating disproportionate competition for androgen-binding sites with endogenous nonradioactive testosterone. We cannot exclude the possibility that different effects of these drugs on testicular or ovarian steroidogenesis might also have contributed to observed differences in spinal cord sex-steroid accumulation.
The identification of steroid-sensitive neural circuits throughout the spinal cord of the golden-collared manakin suggests that sex steroids may have a broader role in modulating avian neuromuscular systems than previously thought. Although birds have been widely studied with respect to steroid actions on the brain, no avian neuromuscular system has been fully exploited to evaluate steroid control of motoneurons and sexually dimorphic muscles that they might innervate. As in other species, sex steroids may regulate the interrelationship of motoneurons and their targets, possibly stimulating plasticity in both (32–34). Insofar as physical displays and mechanical sounds are characteristic parts of the behavioral repertoire of a vast number of bird species, the results presented here support the view that sex steroids act on the spinal cord to activate these behaviors. Further studies defining the neuromuscular control of male courtship are necessary to establish the role of hormones in regulating these behaviors.
Acknowledgments
We thank Dr. Fritz Hertel for help with capturing birds, Margaret Kowalczyk for assistance with figures, and Drs. Art Arnold, Reggie Edgerton, and Colin Saldanha for comments on this manuscript; we also thank Sterling-Winthrop, Inc. for the gift of trilostane and Janssen Pharmaceutica for the gift of ketoconazole. This work was performed in association with the Smithsonian Tropical Research Institute in Panama, and we thank the scientific and support staff for their assistance. We also thank Instituto Nacionale de Recursos Naturales Renovables and the government of Panama for permitting us to perform this research. This work was supported by grants from the UCLA Academic Senate.
ABBREVIATIONS
- AR
androgen receptor
- ER
estrogen receptor
- 3H-T
[3H]testosterone
References
- 1.Yahr P. In: Neurobiological Effects of Sex Steroid Hormones. Miceyvch P E, Hammer R P Jr, editors. Vol. 1. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 40–56. [Google Scholar]
- 2.Simerly R B. In: Neurobiological Effects of Sex Steroid Hormones. Miceyvch P E, Hammer R P Jr, editors. Vol. 1. Cambridge, U.K.: Cambridge Univ. Press; 1995. pp. 85–116. [Google Scholar]
- 3.Balthazart J, Tlemcani O, Ball G F. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- 4.Kelley D B. J Neurobiol. 1986;17:231–248. doi: 10.1002/neu.480170307. [DOI] [PubMed] [Google Scholar]
- 5.Breedlove S M. J Neurosci. 1992;12:4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breedlove S M, Arnold A P. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 7.Forger N G, Breedlove S M. Proc Natl Acad Sci USA. 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurz E M, Sengelaub D R, Arnold A P. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto A, Arai Y, Prins G S. J Neuroendocrinol. 1996;8:553–559. doi: 10.1046/j.1365-2826.1996.04899.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnold A P, Nottebohm F, Pfaff D W. J Comp Neurol. 1976;165:487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- 11.Brenowitz E A. J Neurobiol. 1997;33:517–531. doi: 10.1002/(sici)1097-4695(19971105)33:5<517::aid-neu3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Schlinger B A. J Neurobiol. 1997;33:619–631. [PubMed] [Google Scholar]
- 13.Johnsgard P A. Arena Birds: Sexual Selection and Behavior. Washington, DC: Smithsonian Institute Press; 1994. [Google Scholar]
- 14.Chapman F M. Bull Am Mus Nat Hist. 1935;68:472–521. [Google Scholar]
- 15.Cam V, Schlinger B A. Horm Behav. 1998;33:31–39. doi: 10.1006/hbeh.1998.1434. [DOI] [PubMed] [Google Scholar]
- 16.Schlinger B A, Lane N I, Grisham W, Thompson L. Gen Comp Endocrinol. 1999;113:46–58. doi: 10.1006/gcen.1998.7179. [DOI] [PubMed] [Google Scholar]
- 17.Kelley D B. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold A P. J Histochem Cytochem. 1981;29:207–211. doi: 10.1177/29.1A_SUPPL.7288157. [DOI] [PubMed] [Google Scholar]
- 19.Sokoloff A, Deacon T, Goslow G E., Jr Anat Rec. 1989;225:35–40. doi: 10.1002/ar.1092250106. [DOI] [PubMed] [Google Scholar]
- 20.Leonard R B, Cohen D H. J Comp Neurol. 1975;163:159–180. doi: 10.1002/cne.901630203. [DOI] [PubMed] [Google Scholar]
- 21.Breedlove S M, Arnold A P. J Comp Neurol. 1983;215:211–216. doi: 10.1002/cne.902150208. [DOI] [PubMed] [Google Scholar]
- 22.Erulkar S D, Kelley D B, Jurman M E, Zemlan F P, Schneider G T, Krieger N R. Proc Natl Acad Sci USA. 1981;78:5876–5880. doi: 10.1073/pnas.78.9.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumpf W E, Sar M. J Steroid Biochem. 1979;11:801–807. doi: 10.1016/0022-4731(79)90015-3. [DOI] [PubMed] [Google Scholar]
- 24.Jurman M E, Erulkar S D, Krieger N R. J Neurochem. 1982;38:657–661. doi: 10.1111/j.1471-4159.1982.tb08681.x. [DOI] [PubMed] [Google Scholar]
- 25.Breedlove S M. Prog Brain Res. 1984;61:147–170. doi: 10.1016/S0079-6123(08)64433-7. [DOI] [PubMed] [Google Scholar]
- 26.Sar M, Stumpf W E. Science. 1977;197:77–79. doi: 10.1126/science.867053. [DOI] [PubMed] [Google Scholar]
- 27.Perez J, Cohen M A, Kelley D B. J Neurobiol. 1996;30:556–568. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Vargas M C, Stumpf W E, Sar M. J Comp Neurol. 1976;167:83–103. doi: 10.1002/cne.901670106. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser L. J Physiol (Paris) 1977;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley D B, Pfaff D W. Horm Behav. 1976;7:159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- 31.Kelley D B. Annu Rev Neurosci. 1988;11:225–251. doi: 10.1146/annurev.ne.11.030188.001301. [DOI] [PubMed] [Google Scholar]
- 32.Regnier M, Herrera A A. J Neurobiol. 1993;24:1215–1228. doi: 10.1002/neu.480240908. [DOI] [PubMed] [Google Scholar]
- 33.Rand M N, Breedlove S M. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto A. Brain Res Bull. 1997;44:539–547. doi: 10.1016/s0361-9230(97)00240-2. [DOI] [PubMed] [Google Scholar]