Abstract
Objective:
To evaluate the effectiveness of refractive correction alone for the treatment of untreated anisometropic amblyopia in children 3 to <7 years old.
Design:
Prospective, multicenter, noncomparative intervention.
Participants:
84 children 3 to <7 years old with untreated anisometropic amblyopia ranging from 20/40 to 20/250.
Methods:
Optimal refractive correction was provided and visual acuity was measured with the new spectacle correction at baseline, and at 5-week intervals until visual acuity stabilized or amblyopia resolved.
Main Outcome Measures:
Maximum improvement in best-corrected visual acuity in the amblyopic eye and proportion of children whose amblyopia resolved (interocular difference of 1 line or less) with refractive correction alone.
Results:
Amblyopia improved with optical correction by 2 or more lines in 77% of the patients and resolved in 27%. Improvement took up to 30 weeks for stabilization criteria to be met. After stabilization, additional improvement occurred with spectacles alone in 21 of 34 patients followed in a control group of a subsequent randomized trial, with amblyopia resolving in 6. Treatment outcome was not related to age, but was related to better baseline visual acuity and lesser amounts of anisometropia.
Conclusion:
Refractive correction alone improves visual acuity in many cases and results in resolution of amblyopia in at least one third of 3 to <7-year-old children with untreated anisometropic amblyopia. While most cases of resolution occur with moderate (20/40 to 20/100) amblyopia, the average 3-line improvement in visual acuity resulting from treatment with spectacles may lessen the burden of subsequent amblyopia therapy for those with denser levels of amblyopia.
Precis
Refractive correction alone improves visual acuity in many cases and results in resolution of amblyopia in at least one third of children 3 to <7 years old with untreated anisometropic amblyopia.
Introduction
Amblyopia is a frequent cause of monocular vision loss in children. A difference in refractive error between the two eyes (anisometropia) is a common cause of amblyopia, being present as the only identifiable amblyogenic factor in 37% of cases and present concomitantly with strabismus in an additional 24% of clinical populations.1
Although there have been reports that refractive correction alone results in improved vision in anisometropic amblyopia,2-7 it is generally held that the majority of cases will need additional treatment because refractive correction alone will not be sufficient to completely treat the amblyopia. Thus, patching or pharmacological treatment is often prescribed simultaneously or soon after the refractive correction is provided.
We conducted a prospective study of the treatment of untreated anisometropic amblyopia in children 3 to <7 years old with spectacle correction alone. The objectives were to determine (1) the incidence of resolution of amblyopia, (2) the time course of visual acuity improvement, and (3) factors associated with resolution of amblyopia with spectacles alone.
Methods
This study, supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, was conducted by the Pediatric Eye Disease Investigator Group (PEDIG) at 34 clinical sites. The protocol and HIPAA-compliant informed consent forms were approved by the respective institutional review boards. The parent or guardian of each study patient gave written informed consent. Study oversight was provided by an independent data and safety monitoring committee. The major aspects of the protocol are summarized herein. The complete protocol is available at http://public.pedig.jaeb.org.
This study was the first phase (spectacle phase) of a two-phase study. In the second phase, patients were randomly assigned to two hours of patching plus near activities with continued spectacle wear or to a control group with continued spectacle wear only.
Screening Visit and Prescription of Spectacles
We enrolled patients age 3 to <7 years with a history of untreated anisometropic amblyopia characterized by an interocular acuity difference of 3 or more logMAR (logarithm of the minimum angle of resolution) lines, anisometropia of ≥0.50 diopter (D) of spherical equivalent and/or ≥1.50 D difference between the eyes in astigmatism, no prior spectacle wear or other treatment for amblyopia, no ocular cause for reduced acuity, and no myopia more than a spherical equivalent of -6.00 D in the amblyopic eye. For this study of anisometropic amblyopia, patients were excluded if they had any measurable heterotropia in primary gaze at distance or near fixation in their prescribed spectacles or a documented history of strabismus. Testing for eccentric fixation to detect a microtropia with identity was not required.8
Spectacles were prescribed based on a cycloplegic refraction using cyclopentolate 1%. Anisometropia, astigmatism, and myopia were fully corrected. Hyperopia gt;3.00 D spherical equivalent was either fully corrected or symmetrically under-corrected by no more than +1.50 D in both eyes. Hyperopia ≤3.00 D spherical equivalent was corrected at investigator discretion.
Baseline Examination
The prescribed spectacles were not worn prior to the day of the baseline examination, which occurred within 30 days of the screening visit. After the spectacles had been worn for 10-30 minutes, corrected visual acuity was measured in each eye by a study-certified vision tester using the ATS single-surround HOTV letter protocol9 presented on the Electronic Visual Acuity Tester.10 This test provides visual acuity scores in one logMAR line increments. To be included in the study, it was necessary for the spectacle-corrected amblyopic eye visual acuity to be between 20/40 and 20/400 inclusive, the sound eye acuity to be 20/40 or better, and the interocular acuity difference to be 3 or more logMAR lines. If the baseline visual acuity was worse than the screening visual acuity in a patient with hyperopic refractive error, acuity testing was repeated with a -1.00 D lens to determine if an inability to relax accommodation sufficiently to accept the prescribed hyperopic spectacles was responsible for the worsening of vision. If testing with the -1.00D lens improved acuity, a symmetrically reduced hyperopic correction was prescribed and the patient did not wear any spectacles until 10-30 minutes prior to the rescheduled baseline examination visit.
Follow-up Visits
Protocol-specified follow-up visits were conducted every 5 (±1) weeks as long as the amblyopic eye acuity (1) had improved at least one line (0.1 logMAR) from the prior visit and (2) was still at least one line worse than the sound eye acuity. At every visit, visual acuity was measured in each eye and if amblyopic eye visual acuity had not improved at least one line from the prior 5-week visit, the amblyopic eye was retested. Follow up was complete when neither measurement of the amblyopic eye acuity showed improvement of at least one line from the prior visit. Amblyopia was considered “resolved” in patients achieving an interocular acuity difference of 1 line or less; these patients continued using their spectacle correction, did not have other treatment prescribed, and had a final follow up visit 4-6 months later.
At the time of stabilization, patients who had an interocular difference of 2 or more lines entered the randomized trial phase described in a companion report. Data from the patients randomized to the control group are included in this report to assess the proportion of patients who showed additional visual acuity improvement with continued spectacle wear after having not shown improvement over the prior 5-week period. Complete results of the randomized trial are reported separately.11
Statistical Methods
The sample size was not predetermined for this first phase of the study. Patients were enrolled until the recruitment goal of the randomized clinical trial (second phase) was reached. Four patients eligible at the baseline visit but with incomplete follow up were excluded from analyses.
For each patient, the maximum acuity improvement was computed and the visit at which this occurred was identified. For purposes of analysis, resolution of amblyopia was defined as improvement of the amblyopic eye acuity to be no more than one line worse than the sound eye. The proportion of patients whose amblyopia resolved was computed and the 95% confidence interval calculated. The associations of age, baseline amblyopic eye visual acuity, degree of anisometropia, and type of anisometropia (sphere only, cylinder only, both) with the maximum improvement and resolution of amblyopia were assessed using analysis of covariance and logistic regression, respectively.
All reported P values are two-tailed. Analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC).
In addition to patients with previously untreated anisometroic amblyopia, the spectacle phase preceding the randomized trial included patients with previously treated amblyopia that required a change in spectacles as well as patients with previously untreated amblyopia due to strabismus with or without anisometropia. Only patients with previously untreated anisometropic amblyopia are included in this report.
Results
Between February 2004 and December 2004, 84 patients with untreated anisometropic amblyopia ranging from 20/40 to 20/250 were enrolled into this study at 34 sites. The number of patients enrolled per site ranged from 1 to 9 (median = 2). The mean age of the patients at study entry was 5.2 ±0.9 years; 46% were female and 75% were white. The mean visual acuity measurement in the amblyopic eye at study entry was 0.60 logMAR (approximately 20/80) and in the sound eye was 0.04 logMAR (approximately 20/20-2). Table 1 provides the baseline characteristics of the cohort.
Table 1.
Baseline Demographic and Clinical Characteristics (N=84)
| n (%) | |
|---|---|
| Gender: Female | 39 (46) |
| Race / Ethnicity | |
| White | 63 (75) |
| African-American | 7 (8) |
| Hispanic or Latino | 7 (8) |
| Asian | 3 (4) |
| American Indian/Alaskan Native | 1 (1) |
| More than one race | 3 (4) |
| Age | |
| 3 to <4 years | 7 (8) |
| 4 to <5 years | 25 (30) |
| 5 to <6 years | 35 (42) |
| 6 to <7 years | 17 (20) |
| Mean (SD*) years | 5.2 (0.9) |
| Best-Corrected Distance Visual Acuity in Amblyopic Eye | |
| 20/250 | 1 (1) |
| 20/200 | 2 (2) |
| 20/160 | 5 (6) |
| 20/125 | 10 (12) |
| 20/100 | 13 (15) |
| 20/80 | 16 (19) |
| 20/63 | 19 (23) |
| 20/50 | 9 (11) |
| 20/40 | 9 (11) |
| Mean (SD) logMAR†, Snellen equivalent | 0.60 (0.19), 20/80 |
| Best-Corrected Distance Visual Acuity in Sound Eye | |
| 20/32 | 14 (17) |
| 20/25 | 14 (17) |
| 20/20 | 47 (56) |
| 20/16 | 9 (11) |
| Mean (SD) logMAR, Snellen equivalent | 0.04 (0.09), 20/20-2 |
| Interocular Acuity Difference Mean (SD) lines | 5.6 (2.1) |
| Refractive Error in Amblyopic Eye | |
| -4.00D to <0 | 4 (5) |
| 0 to <+1.00D‡ | 1 (1) |
| +1.00 to <+2.00D | 4 (5) |
| +2.00 to <+3.00D | 3 (4) |
| +3.00 to <+4.00D | 9 (11) |
| ≥+4.00D | 63 (75) |
| Mean (SD) spherical equivalent | 4.51 (2.07) |
| Refractive Error in Sound Eye | |
| -0.50D to <0 | 3 (4) |
| 0 to <+1.00D | 18 (21) |
| +1.00 to <+2.00D | 40 (48) |
| +2.00 to <+3.00D | 13 (15) |
| 3+.00 to <+4.00D | 6 (7) |
| ≥+4.00D | 4 (5) |
| Mean (SD) spherical equivalent | 1.57 (1.17) |
| Anisometropia definition met | |
| Cylinder only (≥1.50D difference) | 3 (4) |
| Spherical equivalent only (≥0.50D difference) | 67 (80) |
| Spherical equivalent and cylinder | 14 (17) |
| Anisometropia (calculated difference in spherical equivalent) at enrollment§ | |
| 0.50 to <1.00D | 7 (9) |
| 1.00 to <2.00D | 11 (14) |
| 2.00 to <3.00D | 13 (16) |
| 3.00 to <4.00D | 24 (30) |
| ≥4.00D | 26 (32) |
| Mean (SD) spherical equivalent | 3.21 (1.46) |
SD = Standard Deviation
logMAR = logarithm of the minimum angle of resolution
D=diopte r
3 patients meeting cylinder criteria only are excluded
Visual Acuity Improvement
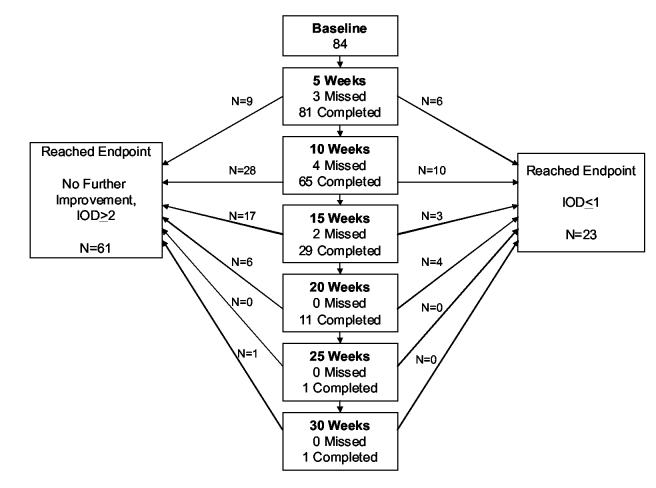
Figure 1A shows the disposition of patients during follow up. At the initial 5-week visit, visual acuity improvement from the spectacle-corrected baseline acuity averaged 1.8 ± 1.3 lines, with 59% of the patients having improvement of 2 or more lines of acuity and 7% meeting the definition for resolution (amblyopic eye acuity no more than one line worse than sound eye) (Table 2).
Figure 1A.
Study flow chart. A: Flow chart of spectacle phase (N=84) showing visit schedule, visit completion, and time points for when study endpoints were reached.
Table 2.
Improvement at 5 Weeks in Amblyopic Eye Visual Acuity Overall and by Baseline Amblyopic Eye Visual Acuity
| Baseline Amblyopic Eye Visual Acuity |
|||
|---|---|---|---|
| Overall N=81* |
20/40 to 20/100 N=63 |
20/125 to 20/250 N=18 |
|
| n(%) | n(%) | n(%) | |
| Improvement in Amblyopic Eye Acuity from Baseline to 5 Weeks | |||
| <=0 lines (no improvement or worsened) | 9 (11) | 6 (10) | 3 (17) |
| 1 line | 24 (30) | 21 (33) | 3 (17) |
| 2 lines | 31 (38) | 24 (38) | 7 (39) |
| ≥3 lines | 17 (21) | 12 (19) | 5 (28) |
| Mean (SD†) lines | 1.8 (1.3) | 1.8 (1.3) | 1.8 (1.4) |
| Change in Interocular Acuity Difference‡ from Baseline to 5 Weeks | |||
| -5 lines | 2 (2) | 2 (3) | 0 |
| -4 lines | 3 (4) | 2 (3) | 1 (6) |
| -3 lines | 8 (10) | 5 (8) | 3 (17) |
| -2 lines | 21 (26) | 16 (25) | 5 (28) |
| -1 line | 28 (35) | 23 (37) | 5 (28) |
| 0 lines | 12 (15) | 11 (17) | 1 (6) |
| >=1 line | 7 (9) | 4 (6) | 3 (17) |
| Mean (SD) lines change | -1.3 (1.4) | -1.3 (1.3) | -1.4 (1.5) |
| Resolution at 5 Weeks (interocular acuity difference‡ 1 line or less) | 6 (7) | 6 (10) | 0 |
3 patients who missed the 5-week visit are not included
SD= Standard Deviation
Interocular acuity difference at 5 weeks evaluated using better sound eye acuity at baseline or 5-week visit
Visual acuity improvement continued beyond the initial 5 weeks of spectacle wear for 39 (48%) of the 81 patients completing the initial 5-week visit, with the longest duration of improvement being 30 weeks in one patient (Table 3). The change from the spectacle-corrected baseline acuity to the best acuity achieved at any visit averaged 2.9 ± 1.8 lines, with 65 (77%) of the patients having improvement of 2 or more lines of acuity and 23 (27%, 95% confidence interval 18% to 38%) having resolution of their amblyopia (Table 3). Among the 23 patients meeting the resolution criteria, the amblyopic eye was one line worse than the sound eye in 14 (61%) and the same or better than the sound eye in 9 (39%).
Table 3.
Best-Measured Visual Acuity in Amblyopic Eye Overall and by Baseline Amblyopic Eye Visual Acuity
| Baseline Amblyopic Eye Visual Acuity |
|||
|---|---|---|---|
| Overall N=84 |
20/40 to 20/100 N=66 |
20/125 to 20/250 N=18 |
|
| n(%) | n(%) | n(%) | |
| Improvement from Baseline to Best-Measured Acuity | |||
| 0 lines (no improvement or worsened) | 9 (11) | 6 (9) | 3 (17) |
| 1 line | 10 (12) | 9 (14) | 1 (6) |
| 2 lines | 15 (18) | 11 (17) | 4 (22) |
| ≥3 lines | 50 (60) | 40 (61) | 10 (56) |
| Mean (SD*) lines | 2.9 (1.8) | 2.9 (1.7) | 2.8 (2.0) |
| Change in Interocular Acuity Difference† from Baseline to Visit of Best-Measured Acuity | |||
| >=-5 lines | 9 (11) | 7 (11) | 2 (11) |
| -4 lines | 12 (14) | 9 (14) | 3 (17) |
| -3 lines | 20 (24) | 15 (23) | 5 (28) |
| -2 lines | 15 (18) | 13 (20) | 2 (11) |
| -1 line | 15 (18) | 12 (18) | 3 (17) |
| 0 lines | 13 (15) | 10 (15) | 3 (17) |
| Mean (SD) lines change | -2.4 (1.7) | -2.4 (1.7) | -2.6 (1.9) |
| Interocular Acuity Difference† at Visit of Best- Measured Acuity | |||
| <=0 lines (amblyopic eye same or better than sound eye) | 9 (11) | 9 (14) | 0 |
| 1 line | 14 (17) | 13 (20) | 1 (6) |
| 2 lines | 13 (15) | 13 (20) | 0 |
| ≥ 3 lines | 48 (57) | 31 (47) | 17 (94) |
| Mean (SD) lines | 3.2 (2.4) | 2.4 (1.8) | 5.9 (2.3) |
| Visit of Best-Measured Acuity Baseline | 9 (11) | 6 (9) | 3 (17) |
| 5wks (3-7) | 33 (39) | 27 (41) | 6 (33) |
| 10wks (8-12) | 28 (33) | 23 (35) | 5 (28) |
| 15wks (13-17) | 6 (7) | 4 (6) | 2 (11) |
| 20wks (18-22) | 6 (7) | 4 (6) | 2 (11) |
| >=25wks | 2 (2) | 2 (3) | 0 |
| Resolution at Visit of Best-Measured Acuity (interocular acuity difference† 1 line or less) | 23 (27) | 22 (33) | 1 (6) |
SD= Standard Deviation
Interocular acuity difference evaluated using better sound eye acuity at baseline or visit of best-measured amblyopic eye acuity
This table does not include data on the additional long-term follow-up visit (4-6 months) for patients whose amblyopia resolved.
Resolution of amblyopia was related to better baseline amblyopic eye acuity (P=0.02), occurring in 16 (43%) of the 37 patients with spectacle-corrected baseline acuity of 20/40 to 20/63, in 6 (21%) of the 29 patients with baseline acuity of 20/80 to 20/100, in 1 (10%) of the 10 patients with baseline acuity of 20/125, and in none of the 8 patients with baseline acuity of 20/160 to 20/250. Resolution was inversely related to the magnitude of anisometropia even after adjusting for baseline acuity (P=0.03), but was not related to age (P=0.40) or type of anisometropia (sphere only, cylinder only, both) (P=0.66) (Table 4).
Table 4.
Best-Measured Acuity in Amblyopic Eye According to Baseline Factors (N=84)
| N | Mean (SD*) Lines Improved from Baseline to Visit of Best- Measured Acuity | P Value† | Resolution at Visit of Best- Measured Acuity (IOD‡ within 1 line) n(%) | P Value† | |
|---|---|---|---|---|---|
| Age (years) | 0.88 | 0.40 | |||
| 3-<4 | 7 | 3.3 (1.8) | 1 (14) | ||
| 4-<5 | 25 | 2.5 (1.5) | 7 (28) | ||
| 5-<6 | 35 | 3.0 (2.1) | 12 (34) | ||
| 6-<7 | 17 | 2.9 (1.6) | 3 (18) | ||
| Degree of Anisometropia§ | 0.07 | 0.03 | |||
| 0.50 to <1.00D ∥ | 7 | 3.1 (1.2) | 4 (57) | ||
| 1.00 to <2.00D | 11 | 2.8 (1.5) | 5 (45) | ||
| 2.00 to <3.00D | 13 | 3.5 (1.9) | 5 (38) | ||
| 3.00 to <4.00D | 24 | 2.6 (2.0) | 5 (21) | ||
| >=4.00D | 26 | 2.8 (1.9) | 2 (8) | ||
| Baseline Amblyopic Eye Acuity | 0.21 | 0.02 | |||
| 20/40 | 9 | 1.3 (1.1) | 2 (22) | ||
| 20/50 | 9 | 2.9 (1.1) | 6 (67) | ||
| 20/63 | 19 | 3.0 (1.6) | 8 (42) | ||
| 20/80 | 16 | 2.8 (1.6) | 2 (13) | ||
| 20/100 | 13 | 3.8 (2.2) | 4 (31) | ||
| 20/125 | 10 | 3.4 (2.2) | 1 (10) | ||
| 20/160-20/250 | 8 | 2.1 (1.7) | 0 | ||
| Anisometropia Definition Met | 0.54 | 0.66 | |||
| Spherical equivalent only (≥0.50D difference) | 67 | 3.0 (1.9) | 17 (25) | ||
| Spherical equivalent and cylinder | 14 | 2.4 (1.3) | 4 (29) | ||
| Cylinder only (≥1.50D difference) | 3 | 2.3 (1.2) | 2 (67) |
SD = Standard Deviation
P value for improvement to best-measured acuity outcome from analysis of covariance adjusted for baseline acuity, P value for resolution outcome from logistic regression analysis adjusted for baseline acuity
Interocular acuity difference evaluated using better sound eye at baseline or visit of bestmeasured amblyopic eye acuity
3 patients meeting only cylinder criteria are not included
D = diopter
This table does not include data on the additional long-term follow-up visit (4-6 months) for patients whose amblyopia resolved.
Continuation of Follow Up in the Randomized Trial Phase of the Study
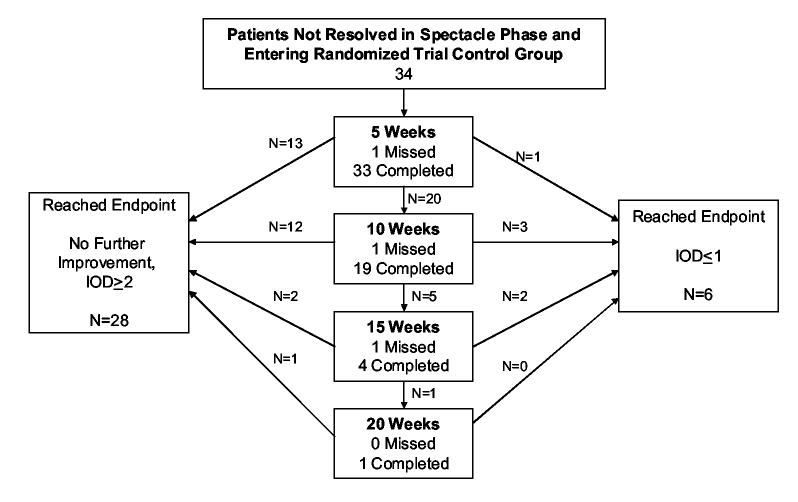
Patients with stabilized amblyopic eye acuity and an interocular acuity difference of 2 or more lines at the last visit entered a randomized trial comparing spectacle wear and patching versus spectacle wear alone (control group). In 21 (62%) of 34 patients assigned to the control group, acuity improved 1 or more lines after another 5 weeks of spectacle wear and in 8 (24%), acuity improved further at the second 5-week visit (10 weeks from randomization). In one patient, acuity improved even further at the third 5-week visit (15 weeks from randomization).
The mean additional improvement in amblyopic eye acuity in the 34 control group (spectacle-only) patients during the randomized trial phase was 1.2 ± 1.1 lines. Resolution of the amblyopia occurred in 6 (18%) of the 34 patients during this phase (Figure 1B).
Long-term Follow Up for Resolved Cases in the Spectacle Phase of the Study
Fourteen of the 23 patients whose amblyopia resolved in the spectacle phase of the study were reexamined after a median of 19 weeks (range 10 to 25 weeks). Amblyopic eye acuity at this visit was unchanged in 8 patients, better in 2, and one line worse in 4.
Discussion
In this prospective observational study of 84 untreated anisometropic amblyopic children 3 to <7 years old, we found that refractive correction with spectacles alone improved amblyopic eye visual acuity an average of 2.9 lines. Visual acuity improved from baseline by 2 or more lines in 77% of the patients and by 3 or more lines in 60%. Resolution of amblyopia occurred in 27% of the cohort during the study. Additionally, amblyopia resolved in 6 of 34 (18%) patients with residual amblyopia who continued to be treated with spectacles only in the control group of the succeeding randomized trial. The observed improvement (2.9 lines on average) substantially exceeded any expected learning effect or age effect.9, 10, 12 These results demonstrate that refractive correction alone is a powerful amblyopia treatment modality for young children with anisometropic amblyopia, and that in some cases of moderate amblyopia, refractive correction may be the only treatment needed for a successful outcome.
Visual acuity improvement with refractive correction alone in children with anisometropic amblyopia has been observed in both retrospective and pilot studies.2-5, 7 However, only recently has the magnitude and time course of this phenomenon been evaluated prospectively.6 Stewart and colleagues6 found a mean improvement of nearly 3 lines in 18 patients with anisometropic amblyopia treated with spectacles. They have termed this effect “refractive adaptation,” but we prefer to refer to it as optical treatment of amblyopia.
The time course in our study for improvement to best amblyopic eye visual acuity was variable. The majority (83%) of patients stopped improving before 15 weeks, but one patient improved for 30 weeks. In the study of Stewart et al.,6 improvement occurred for an average of 15.6 weeks in their subgroup of patients with anisometropic amblyopia.
The finding of visual acuity improvement over many weeks for children with anisometropic amblyopia treated with refractive correction alone can guide (1) clinicians on the expected duration of treatment when using spectacles alone as the treatment and (2) investigators who want to control for the treatment effect of refractive correction when evaluating the effectiveness of other amblyopia treatments. In earlier randomized trials of patching and pharmacological treatment conducted by PEDIG, optimum spectacle correction was required for a minimum of 4 weeks prior to enrollment.13-16 The results of the present study suggest that in these previous trials, some of the visual acuity improvement was due to optical treatment of amblyopia with spectacles. Although this would have the effect of reducing the statistical power to detect a treatment group difference, these trials were highly powered and this effect of spectacles therefore should not alter the primary conclusions of those studies.
Patients who stopped improving based on serial visual acuity measurements were enrolled in the control group of the randomized trial (complete results reported in a companion paper).11 We were surprised that a substantial proportion of these patients (62%) demonstrated further improvement (1 line or greater) of amblyopic eye visual acuity after another 5 weeks of spectacle wear, and some continued to show further improvement 10 and 15 weeks from randomization. Therefore, the criterion that we used in this study to define visual acuity stabilization (i.e., less than 1 line improvement from the prior 5-week visit on at least two tests of acuity) was not sufficient.
There are several possible explanations for the continued improvement after apparent stability. These include the possibility of test-retest variability including poor test performance on that particular day and the inability to detect acuity improvements of less than one logMAR line with the ATS visual acuity protocol.9 It is also possible that visual acuity improvement may temporarily plateau for a time period and then subsequently improve again. Based on these considerations, one may want to wait for two or perhaps even three 5-week follow-up cycles with no further improvement before assuming that all acuity improvement has been obtained from the wearing of the spectacle lenses. Alternatively, the clinician might choose to lengthen the time interval between visits to 10 or 12 weeks.
We evaluated the influence of age, degree of anisometropia, and baseline amblyopic eye visual acuity on the response to treatment. The beneficial effect of spectacle correction was consistent throughout the 3 to <7 years age range. We are not aware of other studies that have evaluated the association of age at presentation with treatment outcome for patients with anisometropic amblyopia who were treated with spectacles alone. Regarding other patient characteristics, better baseline acuity in the amblyopic eye was related to an increased chance for resolution of amblyopia. Severe amblyopia was unlikely to resolve with spectacle correction alone. Higher rates of resolution were associated with lesser amounts of anisometropia. Again, we are not aware of other studies that have evaluated the relationship of baseline visual acuity or magnitude of anisometropia with treatment outcome for children with anisometropic amblyopia treated with spectacle correction alone.
In translating our results into clinical practice, the findings must be viewed in the context of the clinical profile of the cohort enrolled into the study: children 3 to <7 years old who had untreated anisometropic amblyopia with visual acuity that ranged from 20/40 to 20/250. Only 4 of the patients had myopic anisometropia of any degree and patients with high unilateral myopia were excluded; therefore, no conclusions can be drawn about myopic children. Although all of our patients were classified as having anisometropic amblyopia with no accompanying strabismus, it is possible that some of our patients may have had an undetected microtropia.8, 17
Based on this study of untreated 3 to <7 year old children with anisometropic amblyopia, one can expect most patients treated with refractive correction alone to have two lines or more of improvement in visual acuity, while at least one third will actually experience resolution of amblyopia if treated long enough with spectacles. Although anisometropic amblyopic children with severe amblyopia and /or higher degrees of anisometropia are much less likely to resolve with optical correction alone, many such patients could begin their prescribed occlusion or pharmacological regimen with better vision if first treated with spectacles alone. This, in turn, might decrease the burden of treatment on the child and parents and enhance compliance with patching treatment, because denser levels of amblyopia have been reported to be associated with poorer treatment compliance.18-20
Figure 1B.
Study flow chart. B: Flow chart of children continuing in randomized trial control group (N=34). IOD - Interocular acuity difference evaluated using better sound eye at baseline or visit.
Acknowledgements
Writing Committee: Lead authors: Susan A. Cotter, O.D.; Allison R. Edwards, M.S.; David K. Wallace, M.D.; Roy W. Beck, M.D., Ph.D; Additional writing committee members (alphabetical): Robert W. Arnold, M.D.; William F. Astle, M.D.; Carmen N. Barnhardt, O.D.; Eileen E. Birch, Ph.D.; Sean P. Donahue, M.D., Ph.D.; Donald F. Everett, M.A.; Joost Felius, Ph.D.; Jonathan M. Holmes, B.M., B.Ch.; Raymond T. Kraker, M.S.P.H; B. Michele Melia, Sc.M.; Michael X. Repka, M.D.; Nicholas A. Sala, D.O.; David I. Silbert, M.D.; Katherine K. Weise, O.D., M.B.A.
The Pediatric Eye Disease Investigator Group
Clinical Sites that Participated in this Protocol
Sites are listed in order by number of patients enrolled into the study. The number of patients enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, and (V) for Visual Acuity Tester.
Erie, PA - Pediatric Ophthalmology of Erie
Nicholas A. Sala (I); Rhonda M. Hodde (C); V. Lori Zeto (C); Cindy E. Tanner (V)
Anchorage, AK - Ophthalmic Associates
Robert W. Arnold (I); Mary Diane Armitage (C); Nancy H. Brusseau (V); Maru V. Gindling (V); Karen M. Lowe (V)
Lancaster, PA - Family Eye Group
David I. Silbert (I); Don D. Blackburn (I); Eric L. Singman (I); Noelle S. Matta (C); Cristina M. Corradino (V); Troy J. Hosey (V); Diane M. Jostes (V); Alyson B. Keene (V); Michelle M. Lindsey (V); Tonji L. Nelson (V); Sylvia R. Wright (V)
Fullerton, CA - Southern California College of Optometry
Susan A. Cotter (I); Carmen N. Barnhardt (I); Raymond H. Chu (I); Monique M. Nguyen (I); Susan M. Shin (I); Erin Song (I); Tracy Leonhardt (C); Rebecca Bridgeford (C)
Birmingham, AL - University of Alabama at Birmingham School of Optometry
Robert P. Rutstein (I); Marcela Frazier (I); Kristine T. Hopkins (I); Wendy L. Marsh-Tootle (I); Katherine K. Weise (I); Cathy H. Baldwin (C)
Dallas, TX - Pediatric Ophthalmology, P.A.
David R. Stager, Sr. (I); Joost Felius (C); June M. Gartlir (V); Lyn F. Parker (V)
Boise, ID - Katherine Ann Lee, M.D., PA.
Katherine A. Lee (I); Bonita R. Schweinler (C); Larry W. Plum (V)
Calgary - Alberta Children's Hospital
William F. Astle (I); Maria del Pilar Echeverri (I); Anna L. Ells (I); Vivian E. Hill (I); Heather J. Peddie (C); April D. Ingram (C); Trena L. Beer (V); Charlene Dawn Gillis (V); Cheryl R. Hayduk (V); Catriona I. Kerr (V); Heather M. Vibert (V)
Saint Paul, MN - Associated Eye Care
Susan Schloff (I); Ann Marie Hickson (I); Valori E. Olson (C);
West Des Moines, IA Wolfe Eye Clinic
Donny W. Suh (I); Kim S. Walters (C); Jan M. Jansen (C); Shannon L. Craig (V); Rhonda J. Swisher (V); Laura J. Watkin (V)
Wilmette, IL - Pediatric Eye Associates
Deborah R. Fishman (I); Lisa C. Verderber (I); JoAnn Spieker (C)
Chapel Hill, NC - UNC Dept. of Ophthalmology
David K. Wallace (I); John David Wright, Jr. (I); Beth A. Parente (C); Melissa W. Compton (V); Madonna R. Petty (V); Marguerite I. Sullivan (V)
Baltimore, MD - Wilmer Institute
Michael X. Repka (I); Alex X. Christoff (C); Carole R. Goodman (C); Xiaonong Liu (C); Noelle S. Matta (V)
Milwaukee, WI - Medical College of Wisconsin
Jane D. Kivlin (I); Mark S. Ruttum (I); Veronica R. Picard (C); Margaret L. Willett (V)
Wichita, KS - Grene Vision Group
David A. Johnson (I); Ruth D. Dannar (C); Amy Wheeler (V)
Miami, FL - Bascom Palmer Eye Institute
Susanna M. Tamkins (I); Eva M. Olivares (C); Bruce D. Bailey (V); Mirna Garcia (V); Sarah E. Hill (V); Ana C. Rosa (V);
Beachwood, OH - The Cleveland Clinic Foundation, Ophthalmology at Beachwood
Diane L. Tucker (I); Laurie A. Slaby (C)
Canton, OH - Eye Centers of Ohio
Elbert H. Magoon (I); Paula A. Kannam (C); Kathy Ann Earl (C); Lynn A. McAtee (C); Margie Andrews (V); Caroline M. Hoge (V); Debby Ann Null (V); Denise Richards (V); Judy A. Swartz (V)
Columbus, OH - Pediatric Ophthalmology Associates, Inc.
Richard W. Hertle (I); Don L. Bremer (I); Cybil M. Bean (I); Mary Lou McGregor (I); Gary L. Rogers (I); Rae R. Fellows (C); Rebecca A. Murray (V); Teresa M. Rinehart (V); Angela M. Serna (V); Laura Jean Shenberger (V)
Madison, WI - University of Wisconsin, Dept. of Ophthalmology & Visual Sciences
Yasmin S. Bradfield (I); Thomas D. France (I); Erika D. Soderling (C); Alyson J. Pohlman (C); Kristin A. Anderson (V); Gail V. Morton (V); Jacque W. Shimko (V)
Minneapolis, MN - University of Minnesota
C. Gail Summers (I); Erick D. Bothun (I); Stephen P. Christiansen (I); Ann M. Holleschau (C); Sally M. Cook (C); Sara J. Downes (V); Kathy M. Hogue (V); Kim S. Merrill (V)
St. Louis, MO - Cardinal Glennon Children's Hospital
Oscar A. Cruz (I); Bradley V. Davitt (I); Emily A. Miyazaki (C)
Waterbury, CT - Eye Care Group, PC
Andrew J. Levada (I); Tabitha L. Matchett (C); Cheryl Schleif (C); Lisa A. Marcil (V); Nicole G. Rannazzisi (V); Shelley K. Weiss (V)
Dallas, TX - Pediatric Ophthalmology, P.A.
Priscilla M. Berry (I); Joost Felius (C); June M. Gartlir (V); Lyn F. Parker (V)
Grand Rapids, MI - Pediatric Ophthalmology, P.C.
Patrick J. Droste (I); Robert J. Peters (I); Jan Hilbrands (C); Sandra K. Rogers (V); Maggie A. Santure (V)
Rockville, MD
Stephen R. Glaser (I); Jenifer A. Aventuro Luck (I); Paige E. Glaser (C); Jill R. Emmons (V); Andrea M. Matazinski (C)
Baltimore, MD - Greater Baltimore Medical Center
Mary Louise Z. Collins (I); Maureen A. Flanagan (C); Cheryl L. McCarus (C); Jaime N. Brown (V); Pamela A. Huston (V); Dorotea R. Maranto (V); Noelle S. Matta (V)
Bloomington, IN - Indiana School Of Optometry
Don W. Lyon (I); Kathryn Gray (V); Danielle F. Warren (V)
Fall River, MA - Center for Eye Health Truesdale Clinic
John P. Donahue (I); Mary E. Silvia (C); Christine J. Bazinet (V); Marisa F. Sousa (V)
Indianapolis, IN - Indiana University Medical Center
Daniel E. Neely (I); Gavin J. Roberts (I); Michele E. Whitaker (C); Donna J. Bates (V); Jay G. Galli (V); Elizabeth A. Hynes (C); Lisa K. Keenan (V)
Milford, CT - Eye Physicians & Surgeons, PC
Darron A. Bacal (I); Marla Doheny (C); Donna Martin (C)
Nashville, TN - Vanderbilt Eye Center
Sean P. Donahue (I); Lori Ann F. Kehler (I); David G. Morrison (I); Sandy A. Owings (C); Ronald J. Biernacki (V); Neva J. Fukoda (V)
Philadelphia, PA - Children's Hospital of Philadelphia
Brian J. Forbes (I); Monte D. Mills (I); Graham E. Quinn (I); Alexandra Huebner (C); Melissa L. Ehnbom (V); Michelle C. Maniscalco (V); Malinda A. News (V); David R. Phillips (V); Sonia Zhu (V)
Portland, OR - Casey Eye Institute
David T. Wheeler (I); Ann U. Stout (I); Kimberley A. Beaudet (C); Paula K. Rauch (C); Annika S. Joshi (V)
Providence, RI - Rhode Island Eye Institute
John P. Donahue (I); Cristy J. Garcia (V); Nicole L. Waterman (V)
Sacramento, CA - The Permanente Medical Group
James B. Ruben (I); Dipti Desai (C); Sue Ann Parrish (C)
Willoughby, OH - Ophthalmology Consultants, Inc.
Bernard D. Perla (I); Christine A. Hochrein (C); Pam R. Meyer (V); Danielle M. Zahler (V)
Albuquerque, NM - Goldblum Family Eye Care Center, P.C.
Todd A. Goldblum (I); Rachel Baca (C); Angela Alfaro (V); Antoinette Ramirez (V)
Atlanta, GA - The Emory Eye Center
Scott R. Lambert (I); Amy K. Hutchinson (I); Rachel A. Reeves (C); Kathy M. Brown (V)
Boston, MA - New England College of Optometry
Erik M. Weissberg (I); Nicole M. Quinn (I);
Memphis, TN - Southern College of Optometry
Erin R. Nosel (I); Kristin K. Anderson (I); Lindsay C. Moran (C); Christopher W. Lievens (V)
New York, NY - State University of New York, College of Optometry
Robert H. Duckman (I); Norman B. Medow (P); Marilyn Vricella (V)
Philadelphia, PA - Pennsylvania College of Optometry
Mitchell M. Scheiman (I); Karen E. Pollack (C); Brandy J. Scombordi (C)
Salt Lake City, UT - University of Utah/Moran Eye Center
David C. Dries (I); Scott A. Larson (I)
Brooklyn, NY - SUNY Downstate Medical Center
Janine N. Smith (I); Inara A. Kabba (V)
Columbus, OH - The Ohio State University
Marjean T. Kulp (I); Andrew J. Toole (V)
Dallas, TX - UT Southwestern Medical Center
David R. Weakley, Jr. (I); Clare L. Dias (C); Eileen E. Birch (V); Christina S. Cheng (V); Joost Felius (V)
Houston, TX - Texas Children's Hospital
Evelyn A. Paysse (I); David K. Coats (I); Jane Covington Edmond (I); Kimberly G. Yen (I); Michele L. Fulton (C); Maria S. Castanes (C); La-Shunna D. Jamerson (V); Alma D. Sanchez (V)
Iowa City, IA - University of Iowa Hospitals and Clinics
Ronald V. Keech (I); Richard J. Olson (I); Wanda I. Ottar Pfeifer (C); Pamela J. Kutschke (V)
San Diego, CA - Abraham Ratner Children's Eye Center, UCSD
Shira L. Robbins (I); David B. Granet (I); Erika F. Castro (C)
Marlton, NJ
Michael F. Gallaway (I); Alan Bacho (V); Debbie L. Killion (V)
New York, NY - New York Eye and Ear Infirmary
Lisabeth S. Hall (I); Katy W. Tai (C); Larisa Heiser (V); Susanne Schudel (V); Sara Shippman (V)
Oklahoma City, OK - Dean A. McGee Eye Institute, University of Oklahoma
Lucas Trigler (I); Dana M. Jones (I); R. Michael Siatkowski (I); Jesse S. Hart (C); Lisa M Ogilbee (C); Connie J. Dwiggins (V)
Rochester, MN - Mayo Clinic
Jonathan M. Holmes (I); Brian G. Mohney (I); Melissa L. Rice (I); Rebecca A. Nielsen (C); Julie A. Holmquist (V); Pamela Kirsh (V); Rose M. Kroening (V); David A. Leske (V); Marna L. Levisen (V); Deborah K. Miller (V); Debbie M. Priebe (V); Julie A. Spitzer (V)
Wilmington, DE - Delaware Vision Academy, L.L.C.
Don D. Blackburn (I); Noelle S. Matta (C); Patti E. MacDonald (V)
PEDIG Coordinating Center - Tampa, FL: Roy W. Beck, Gladys N. Bernett, Nicole M.
Boyle, Christina M. Cagnina-Morales, Esmeralda L. Cardosa, Danielle L. Chandler, Laura E. Clark, Sharon R. Constantine, Quayleen Donahue, Mitchell Dupre, Allison R. Edwards, Kyle L. Baccus Horsley, Heidi A. Gillespie, Raymond T. Kraker, Shelly T. Mares, Amanda R. McCarthy, B. Michele Melia, Pamela S. Moke, Jim L. Pyner, Heidi J. Strayer
National Eye Institute - Bethesda, MD: Donald F. Everett
PEDIG Executive Committee: Michael X. Repka (chair), Jonathan M. Holmes (vice-chair), Roy W. Beck, Eileen E. Birch, Susan A. Cotter, Sean P. Donahue, Donald F. Everett, Raymond T. Kraker
Amblyopia Treatment Study Steering Committee: Roy W. Beck, Eileen E. Birch, Stephen P. Christiansen, Susan A. Cotter, Sean P. Donahue, Donald F. Everett, Allison R. Edwards, Richard W. Hertle, Rhonda Hodde, Jonathan M. Holmes, Don W. Lyon, Noelle S. Matta, Brian G. Mohney, Pamela S. Moke, Graham E. Quinn, Michael X. Repka, Nicholas Sala, Mitchell M. Scheiman, David K. Wallace, David R. Weakley
Data and Safety Monitoring Committee: William Barlow (Chair), Edward G. Buckley, Barry Davis, Velma Dobson, John L. Keltner, Hana Osman, Earl A. Palmer, Dale L. Phelps, Stephen W. Poff, Richard A. Saunders
LensCrafters provided spectacles to some patients at a reduced cost.
Footnotes
Supported through a cooperative agreement from the National Eye Institute EY11751
There are no conflicts of interest.
References
- Pediatric Eye Disease Investigator Group The clinical profile of moderate amblyopia in children younger than 7 years. Arch Ophthalmol. 2002;120:281–7. [PubMed] [Google Scholar]
- Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 1981;18:47–56. doi: 10.3928/0191-3913-19810901-12. [DOI] [PubMed] [Google Scholar]
- Clarke WN, Noel LP. Prognostic indicators for avoiding occlusion therapy in anisometropic amblyopia. American Orthoptic Journal. 1990;40:57–63. [Google Scholar]
- Moseley MJ, Fielder AR, Irwin M, et al. Effectiveness of occlusion therapy in ametropic amblyopia: a pilot study. Br J Ophthalmol. 1997;81:956–61. doi: 10.1136/bjo.81.11.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholtz I, FitzGerald D. Efficacy of treatment modalities in refractive amblyopia. J Am Optom Assoc. 1999;70:399–404. [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR, et al. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004;88:1552–6. doi: 10.1136/bjo.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley MJ, Neufeld M, McCarry B, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmic Physiol Opt. 2002;22:296–9. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- Helveston EM, von Noorden GK. Microtropia. A newly defined entity. Arch Ophthalmol. 1967;78:272–81. doi: 10.1001/archopht.1967.00980030274003. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthamol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group A randomized trial to evaluate two hours of daily patching for amblyopia in children. Ophthalmology. 2005;(Nov) doi: 10.1016/j.ophtha.2006.01.069. to be submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern KD, Manny RE. Visual acuity of the preschool child: a review. Am J Optom Physiol Opt. 1986;63:319–45. doi: 10.1097/00006324-198605000-00003. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–87. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–11. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–85. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- von Noorden GK, Campos EC. Binocular Vision and Ocular Motility. Mosby; St. Louis: 2001. pp. 340–4. [Google Scholar]
- Parkes LC. An investigation of the impact of occlusion therapy on children with amblyopia, its effect on their families, and compliance with treatment. Br Orthopt J. 2001;58:30–7. [Google Scholar]
- Awan M, Proudlock FA, Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Invest Ophthalmol Vis Sci. 2005;46:1435–39. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- Nucci P, Alfarano R, Piantanida A, et al. Compliance in antiamblyopia occlusion therapy. Acta Ophthalmol (Copenh) 1992;70:128–31. doi: 10.1111/j.1755-3768.1992.tb02104.x. [DOI] [PubMed] [Google Scholar]