Abstract
Physiological responses (i.e., EEG, heart period, respiratory sinus arrhythmia (RSA)) were monitored in 5-month-old infants during the replacement of an adult’s smiling (SF) with a blank face (BF) in a face-to-face setting. Affect, while the infant looked at and away from the adult’s face during both conditions, was analyzed. Infants displayed neutral and some positive affect while looking at both SF and BF. RSA was quantified continuously during both conditions. RSA increased during BF relative to SF. EEG was quantified only while the infants were looking at the adult’s face during both conditions. An increase in theta over multiple scalp areas (AF3,4; F7,8; FC3; T6) was observed during BF relative to SF. The data suggest that infant attention to BF and SF reflect different psychophysiological processes that can be indexed by RSA and scalp-recorded theta.
1. Introduction
When a young infant is face to face with a non-engaging adult displaying a static facial expression (i.e., still face), there is a tendency to look away from the adult and to express less positive affect (Tronick et al., 1978). The predominant emotional response of 2- to 6-month-old infants to this still-face presentation includes increased neutral affect with little protest or engagement (Fogel, 1982; Gusella et al., 1988; Mayes & Carter, 1990; Toda & Fogel, 1993; Tronick et al., 1978). Nevertheless, both positive and negative affective displays have been observed in infants during still-face (Bazhenova, 1980; Braungart-Rieker et al., 1998; Fogel, 1982; Lamb et al., 1987; Tronick et al., 1978; Weinberg & Tronick, 1996). Mayes and Carter (1990) reported that 23% of the 3- to 4-month-old infants tested showed either some positive affect or no negative affect during the still-face presentation demonstrating ability to maintain engagement with a non-engaging adult. Ability to express positive affect during the still-face increases during the first year of life (Cohen & Tronick, 1987). It is plausible to speculate that if the infant engages the non-engaging adult, the infant might be recruiting rudimentary internal capacities to maintain attention and to evoke reciprocity with the non-engaging partner. What neurophysiological processes might support the infant’s engagement with a non-engaging adult?
1.1. The physiological substrate of social engagement: the Social Engagement System
The Polyvagal Theory (Porges, 1995, 2001) proposes that behaviors of social engagement are fostered by calm visceral states and safe contexts. Based on this theory, the initiation and maintenance of engagement with a non-engaging adult should be paralleled by physiological processes that enhance visceral calmness and inhibit visceral agitation. According to Porges, corticobulbar pathways regulate social engagement via an integrated Social Engagement System that is functional at birth (SES, Porges, 2003). The SES includes two major components: visceromotor (i.e., myelinated portion of cranial nerve X (vagus) which regulates heart rate) and somatomotor (i.e., special visceral efferent pathways from cranial nerves IX, X, and XI, which regulate the striatal muscles of the face, head and neck). The visceromotor component regulates a visceral state, and the somatomotor component regulates affective displays (e.g., facial and vocal expressions, eye contact). According to the theory, only during states when the function of the visceromotor component is not compromised (i.e., the vagal influence on the heart is not withdrawn), are neural mechanisms available to regulate the somatomotor component of the SES. The visceromotor component can be indexed via respiratory sinus arrhythmia (RSA, see method section below). The somatomotor component can be indexed via observable behaviors (i.e., looking, displaying affect). The source nuclei of the visceromotor component are integrated with the source nuclei of the somatomotor component in the brain stem.
1.2. Ascending input from the source nuclei of SES in the medulla to upper cholinergic brain structures and attention
The Nucleus Tractus Salitarii (NTS), the sensory center of the visceromotor component of the SES, relays visceral ascending activity to the upper brain cholinergic systems: septum and basal forebrain structures (e.g., Meinert Nucleus). These systems have been implicated in attention and cognition (e.g., McGaughy et al., 2002; Sarter & Bruno, 1997; Sarter et al., 2001; Wenk, 1997) as well as in emotional and motivational processes (for a review, see Berntson et al., 2003). For example, the prefrontal cortex, which receives cholinergic modulation from the basal forebrain, has been linked to both emotion and attention regulation (Carmichael & Price, 1995; LeDoux, 1996; Selemon & Goldman-Rakic, 1985). Research with adults has associated voluntary attention with activity of the dorso-lateral prefrontal cortex and the anterior cingulate gyrus (Posner & Dahaene, 1994), and sustained attention with a reciprocal relationship between these anterior structures and the basal forebrain (Gill et al., 2000; Sarter et al, 2001; Zaborsky et al., 1999). Gill and colleagues (Gill et al., 2000) speculate that specific circuits between the frontal cortex and the basal forebrain regions could amplify selective attention processing in posterior sensory cortical areas. In addition, the medial septum diagonal band, the primary source of cholinergic input to hippocampus (Colgin et al., 2003; Lewis et al., 1967), has been hypothesized to support information selection (Vinogradova et al., 1993). In infants, as in adults, the ascending input from the source nuclei of SES in the medulla to upper cholinergic brain structures might modulate selective attention processing, and might support maintenance of attention in a face-to-face setting with an unresponsive adult. What neurophysiological processes might index such cholinergic modulation of attention?
1.3. Cholinergic theta activity and selective attention
Animal research has demonstrated that hippocampus and cortical brain areas generate rhythmic oscillations at theta frequencies when exposed to pharmacological conditions that mimic endogenous ascending cholinergic inputs (rat, Lukatch, & MacIver, 1997; rabbit, Vinogradova, 1995). Theta activity (5–10 Hz) in animals has been linked to attention (Vinogradova, 1995), voluntary movements (for review see Bland, 1986; Robinson & Wishaw, 1974), arousal (Green & Arduini, 1954), and exploration and orientation (Basar, 1998a, 1998b; Kemp & Kaada, 1975). For example, Vinogradova related theta activity to selective attention by demonstrating that a cholinergically-induced persistent theta-rhythm greatly increased the filtering of hippocampal input signals, thus preventing interference with the ongoing process of information processing and registration. Anticholinergic drugs abolished this finely tuned filtering function.
1.4. Human EEG theta activity and behavioral processes
Recent studies have shown that human cortical EEG (recorded from the cortical surface) indicates the presence of rhythmical slow activity similar to theta activity observed in animals (Caplan et al., 2000; for a review, see Kirk & Mackay, 2003). Theta power in the human scalp EEG synchronizes with increasing task demands, similar to animal theta. Also, similar behavioral processes have been linked to both the intracranial theta and the scalp-recorded theta (e.g. attention, memory, exploratory behavior). Despite this coincidence between cortical surface and scalp recordings, the functional meaning of theta synchronization in human EEG is not yet known. The neural mechanisms mediating the EEG measures on cortical surface and scalp might differ, since the scalp-recorded theta oscillation reflects a dynamic interaction of various synaptic and cellular mechanisms. Overall increases in the amplitude of scalp-recorded theta in humans are associated with increased emotional activation of both positive and negative valence (e.g., frustration in children and adults, for a review, see Panksepp et al., 1995; feeding in infants, Lehtonen et al., 2002; Paul et al., 1996; play in infants, Stroganova & Posikera, 1993). Topographically specific increases in theta amplitude might index the contribution of specific cortical network in behavior (see Kirk & MacKay, 2003). For example, increases in frontal midline theta were observed during effortful attention and concentration (Gevins et al., 1997; Inanaga, 1998; Nakashima & Sato, 1993).
Consistent with adult research, infant research has demonstrated that theta activity may be widely distributed over the scalp (Futagi et al., 1998; Stroganova et al., 1998), or may predominantly appear over specific scalp areas (Futagi et al., 1998; Nikitina et al., 1987; Stroganova & Posikera, 1993) indicating the contribution of different cortical networks in different infant behavior. For example, Stroganova and Posikera (1993) have observed increases in theta predominantly over parietal areas during tactile stimulation (e.g., tickling, kissing), and over both anterior and posterior scalp areas during complex social stimulation (e.g., “gonna-get-you” game). Important for this study, researchers have linked theta oscillations, and properties of theta in both adults and infants, to attention modulation (Asada et al., 1999; Ishii et al., 1999; Kahana et al., 2001; Smith et al., 1999; Stroganova et al., 1998).
Because the purpose of this study is to evaluate a possible neurophysiological processes that might support infant engagement with a non-engaging adult (i.e., looking at a non-engaging adult), theta activity would be a good candidate variable. Increases in theta over frontal areas of the brain might be expected during infant engagement with a non-engaging adult. Though the source of scalp-recorded theta remains unknown, there are indications that the source of frontally recorded theta may be linked to the anterior cingulate cortex (Gevins et al., 1997; Pizzagalli et al., 2003). Supporting this speculation, electrical stimulation of human anterior cingulate cortex elicits a 3–8 Hz EEG rhythm in frontomedial recordings (Brazier, 1968).
1.5. Human EEG theta and cardiac autonomic activities during attention tasks
The literature review above suggests that activity of the upper neural networks (indexed via theta) and activity of the visceromotor component of the SES (i.e., indexed via RSA) might support infant engagement with a non-engaging adult. Cholinergic theta might be modulated by the ascending cholinergic afferent input from the NTS, and thus might be related to the cholinergic modulation of the vagal efferent pathways regulating heart rate (i.e., RSA). The literature suggests a possible association between scalp-recorded theta and vagal control of the heart. Kubota and colleagues (Kubota et al., 2001) reported that increases in frontal midline theta were paralleled by increases in heart rate variability during attention task relative to the baseline condition. If modulation of internally driven attention in infants depends on both the functional state of upper brain structures sensitive to cholinergic modulation (indexed via theta) and the control of visceral state via cholinergic vagal efferents (indexed via RSA), then parallel changes in topographically specific theta and vagal control of the heart rate might be observed during an infant’s engagement with the non-engaging adult.
1.6. Study design
The current study was designed to investigate whether the young infant’s ability to maintain social engagement with a non-engaging adult is related to neurophysiological state indexed by (a) RSA and (b) scalp-recorded theta activity. EEG responses during still-face have not been studied and few researchers have directly studied autonomic responses (e.g., Bazhenova et al., 2001; Moore & Calkins, 2004; Weinberg & Tronick, 1996) during the still-face procedure.
In this study the classic still-face paradigm was modified to facilitate the evaluation of physiological processes. Specifically, the adult behavior during the ‘normal interaction’ condition in the classic still-face paradigm is not standardized and often includes talking to the infant. When the experimental condition follows the ‘normal interaction’ condition (as in the classic still-face paradigm or in the modification by Striano and colleagues (Striano & Liszkowski, 2005; Rochat et al., 2002) the disruption of infant-directed speech, a potent stimulus, might elicit distinct EEG responses that would confound the interpretation of the physiological data. For example, EEG studies indicate that speech elicits the appearance of frontal theta in infants (Nikitina et al., 1993). Thus, any differences in the infant’s physiological responses that are observed contrasting the still-face condition (i.e., happy, neutral or sad) and the ‘normal interaction’ condition that would include infant-directed verbal stimulation might be attributed to the absence of verbal stimulation during the still-face episode.
In addition, the importance of verbal stimulation as a contributing feature of the still-face phenomenon has been documented in behavioral research. Researchers assessed infant behavior in experiments where the partner used happy, sad or neutral expressions in a succession of still-face episodes interspersed by ‘normal interactions’ that included speech (D’Entremont & Muir, 1997; Rochat et al., 2002). The difference in infant smiling and gazing between the happy still face and the neutral or sad still-face conditions was less pronounced relative to the difference between the ‘normal interaction’ (i.e., with speech) and the still-face conditions regardless of the emotional expression.
Behavioral researchers have compared infant responses to an adult’s happy and neutral or sad facial expressions following a ‘normal interaction’ episode (D’Entremont & Muir, 1997; Rochat et al., 2002). These studies report that the still-face phenomenon is observed independently of the facial expression used in the still-face condition. It remains unknown if an infant can differentiate between face-to-face settings with a smiling versus not smiling adult when the two conditions follow each other, and interference of a ‘normal interaction’ condition is excluded.
This study utilizes an experimental design that enables a contrast between the condition during which the experimenter was smiling while looking at the infant with the condition during which the experimenter was only looking at the infant. Throughout the protocol, differences between conditions were minimized and adult speech, gesturing and touch were not allowed. Our hypotheses evaluated (a) whether infants increase cardiac vagal tone in a face-to-face setting with a non-engaging adult relative to an engaging adult; and (b) whether infants increase theta activity during looking at a non-engaging adult relative to looking at an engaging adult.
2. Method
2.1. Participants
The sample consisted of 16 infants (7 male, 9 female) recruited from families participating in the Moscow “Infant Early Education Program”. The infants were full-term (gestational age > 38 weeks) without medical or neurological complications in their clinical records and in current status. All birth weights were greater than 2500 grams (M = 3,359; SD = 416). The mean age of infants was 20 weeks (M = 19.7; SD = .89). Each infant scored within the normal range on the Mental and Psychomotor scales of the Bayley Scales of Infant Development (Bayley, 1993). Parents of participants were predominantly middle class based on education and financial resources. All infants were Caucasian. The study was approved by the IRBs of the University of Maryland at College Park and the University of Illinois at Chicago. Same three subjects were excluded from the EEG and ECG analyses due to recording artifacts.
2.2. Experimental design and procedures
The data reported are part of a larger longitudinal study evaluating the prognostic value of autonomic and EEG measures monitored during early infancy. The larger sample includes an additional 20 infants with very low birth weight. Experimental procedures were explained to the parent(s) upon their arrival at the laboratory and informed consent was obtained. The experimental session was conducted in an electrically shielded experimental chamber.
Because this research focuses on the infant’s ability to maintain engagement with a non-engaging adult, attempts to ameliorate the negative consequences of the blank face were made. The infant was held on the mother’s lap. Mothers were instructed not to talk to infants during EEG recording. The electrode placement for EEG, EOG and ECG recordings took approximately 20 minutes. During this 20 minute period, an experimenter sat in front of the infant and kept the infant involved in play. Experimental procedures began immediately after the electrode placement. Following the experimental procedures, developmental level was assessed with the Bayley Scales of Infant Development (Bayley, 1993). Testing was conducted only when the infant was in a calm and alert state. The experimental session was videotaped from a camera placed behind a black curtain placed directly in front of the infant. A continuous time record was encoded onto the videotape and synchronized with the EEG, EOG and ECG recordings. EEG, EOG and ECG were monitored during the whole experimental session.
A female experimenter conducted the experimental procedures. The experimenter sat on a couch facing the infant, who was seated on the parent’s lap approximately 50 cm from the experimenter. The experimenter’s face was at infant eye level. The experimental session consisted of two trials: Smiling Face (SF) and Blank Face (BF). During SF the experimenter smiled while looking at the infant. The experimenter did not move her head, or use touch or speech to engage the infant. The length of the SF trial was 30 s. During BF the experimenter stopped smiling and expressed a blank face while looking at the infant. The previous research has reported that infants increase gaze aversion during the blank face condition. Since the EEG data in this study were sampled during the infant looking at the adult, in was important to equalize the EEG data obtained for the Smiling Face and Blank Face conditions. In this study the length of the Blank Face episode was increased. The length of the BF trial varied from 50 s to 130 s (M = 89.3; SD = 30.4). BF was terminated, if the infant became distressed.
2.3. Behavioral recordings and coding
Two coders continuously coded each of the three general dimensions (looking, affect, motor) during each of the two experimental trials using separate viewing of the videotape for each behavioral dimension. Coders used the same onset time to start the coding of each SF and BF trial. The behavioral and electrophysiological recordings were synchronized. Within each dimension, the specific behavioral categories were defined as suggested by Mayes and Carter (1990) with the slight modification of adding the category ‘none of the above’ to include facial displays with unclear affective valence.
Looking
Two categories were coded with mutually exclusive codes within the dimension of looking. Looking was judged as ‘Look at’ when the infant’s gaze was directed toward the adult’s face. Conversely, any gaze away from the adult’s face was defined as ‘Look away’. Because, the adult’s face was not on the videotape, the category ‘Looking at’ was defined as any look by the infant in the direction of the adult’s head and above the adult’s shoulder, which was visible on the videotape. Correspondingly, a look towards the adult but at her shoulder or lower was coded as ‘Look away’.
Affect
Four categories were coded with mutually exclusive codes within the dimension of affect: (a) Positive affect was defined as any combination of a smile, laugh, babbling, or cooing. (b) Negative affect was defined as any combination of a frown, fussing, whimpering, whining, squirming, insistent grunting, or crying. (c) Neutral affect was defined as little or no change in facial expression and no vocal activity. (d) None of the above was defined as a change in facial expression or vocalization that was not clearly defined in any of the three categories. For example, “grimacing” as described by Gusella and colleagues (Gusella et al.,1988) would be coded as none of the above.
Motor
Within the dimension of motor activity, three categories were coded with mutually exclusive codes: (a) Quiet motor was defined as the infant sitting still and quietly with no or slight movement of fingers and hands. (b) Mild motor was defined as the infant moving his/her arms and/or head. (c) Gross motor was defined as the infant arching his/her back, or moving his/her trunk. This coding was consistent with the previous research (Bazhenova et al., 2001).
Cross-dimensional behavioral categories
From the primary categories described above, six combined categories of affect and looking behavior were defined: positive, negative or neutral affect while looking at the adult, and positive, negative, or neutral affect while looking away. These cross-dimensional categories were defined as the time in seconds the infant displayed both the specific affect and looking behavior.
2.4. Data Analysis
2.4.1. Behavioral data
Categories within each of the three dimensions were expressed initially as total duration in seconds for the session. Because the BF trials were of differing durations, depending upon each infant’s ability to remain in an organized state, duration of observed behaviors was converted to the percentage of time of total trial length for both SF and BF. A similar conversion was used to record the duration for the cross-dimensional categories.
Two coders, unaware of the study goals, coded the behavioral data for both SF and BF. Reliability among behavioral measures was established across a random subset of participants (20% of data). Agreements when coders designated that a behavior did not occur were not taken into account. Agreements were defined as both coders scoring the same behavior code in the same 1s interval. Cohen’s kappa (Cohen, 1960; Cicchetti & Feinstein, 1990) was calculated for each behavioral category (i.e., looking, affect and motor) with mutually exclusive codes. Mean kappas for looking were .76, for affect .71 and for motor .78.
2.4.2. Cardiovascular data
Three disposable Ag/AgCl ECG electrodes were placed on the infant's chest. Data were collected continuously during SF and BF. The ECG signal was amplified and output to a Vagal Tone Monitor (VTM, Delta-Biometrics, Inc.) for R-wave detection. The VTM timed the interval between heart beats (i.e.., heart period) to the nearest ms and output the data to a PC where the data were stored as a file. Heart period patterns are composed typically of rhythmic activity superimposed on a complex baseline trend. Relevant to the current study was the quantification of the rapid heart period oscillations of neural origin mediated by the myelinated vagus that originates in the medulla. Vagal influences originating in the nucleus ambiguus and traveling through myelinated vagal pathways to the sino-atrial node have a characteristic respiratory rhythm (Richter & Spyer, 1990) known as respiratory sinus arrhythmia (RSA). The greater the vagal influence through the myelinated pathways, the greater the amplitude of the rhythmic changes in heart period at frequencies associated with spontaneous breathing. Thus, the amplitude of RSA provides an accurate measure of cardiac vagal tone (Katona & Jih, 1975; Porges et al., 1982). Uncorrected for respiratory depth and respiratory frequency, RSA may be used to index within-subject changes in tonic vagal modulation of heart rate if the central respiratory drive is not expected to change (Denver et al., 2005; Houtveen et al., 2002).
ECG data were edited off-line and the amplitude of RSA was quantified with MXedit Software (Delta Biometrics Inc; for a detailed description, see Porges, 1985). Editing consisted of visual detection of outlier points followed by integer division or summation. Heart periods were sampled every 250 ms. In the current study RSA was defined in a frequency band consistent with the spontaneous breathing frequency of infants (i.e., from .24 to 1.04 Hz or approximately 15 to 60 breaths per minute). Amplitude of RSA was calculated by summing the variances across the band of frequencies associated with spontaneous respiration. The natural logarithm of the extracted variance for each successive 10 s epoch defined the vagal tone index (i.e., RSA).
2.4.3. EEG data
A set of 12 Ag-AgCl disc electrodes, attached individually with the specially designed elastic strips, was used to record EEG signals. Electrodes were placed at anterior frontal (AF3, 4), frontal-central (FC3, 4), lateral frontal (F7, 8), posterior temporal (T5, 6), parietal-occipital (PO3, 4), and occipital (O1,2) positions according to the Extended International Electrode (10–20) Placement-System (Guidelines Thirteen for standard electrode position nomenclature, 1994). Linked ears served as the reference. Recommended procedures regarding EEG data collection were followed (Pivik et al., 1993). Specifically, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel was placed in each site. Electrode impedance was below 5 kΩ. In order to control eye movement artifacts, the vertical EOG (electrodes below and above the left eye) and horizontal EOG (electrodes at the outer canthi of both eyes) were recorded bipolarly. EEG was recorded on a Nihon Kohden 4217 G electroencephalograph using a time constant of 0.1 s and a high frequency cut-off of 30 Hz. The data were stored on a TEAC XR-510 instrumentation tape recorder and digitized off-line at 128 Hz.
The digitized EEG data were visually inspected off-line for eye movements and motor artifacts (blinks and eye, head or body movements). Eye blink artifacts were identified by both the waveform (sharp negative shift of potential followed by the slow wave of inverse polarity) and occasionally high amplitude (> 150 μV) via visual inspection of EEG and EOG. The EEG epochs were compared to horizontal EOG channel epochs. The EEG epochs that corresponded to contaminated EOG epochs were excluded from the further analysis. This visual artifact rejection procedure is more conservative than computerized artifact correction algorithms, and is considered the most appropriate for infant EEG (Bell, 2002; Somsen & Van Beek, 1998). On average, less than 15% of the data were rejected.
Artifact-free EEG data corresponding to periods of at least 3 s of uninterrupted looking at the adult’s face during SF and BF were sampled and used for the analyses. The video-records were used to analyze infant looking behavior during SF and BF. Phases of uninterrupted looking at the adult’s face were indexed in the EEG data. Infants looked less at the adult during BF relative to the SF (see behavioral data in the result section). For SF, 16–25 s (median = 22 s) of artifact-free EEG were analyzed for each infant. For BF, 12–45 s (median = 25 s) of artifact-free EEG were analyzed for each infant. There was no statistically significant difference in mean length of EEG time period sampled for SF and BF [F(1,11)=2.017; p < .15]. Prior to the time series analyses, the raw data were subjected to time domain demeaning and linear detrending procedures. Epochs of 2.5 s EEG data were processed with a Winograd Fourier transform (Hamming window, 80% overlap; no-zero-padding) to generate the amplitude spectrum with discrete narrow bands of .4 Hz between .8 and 14.8 Hz. The valid EEG epochs were evenly distributed across the Blank Face episodes. Individual averaged EEG spectra were calculated for each experimental condition.
The correct identification of theta band in 5-month-old infants and differentiation of theta and alpha frequency bands is important for this study, since the EEG hypothesis is limited to theta activity during internally driven social engagement at this age. Previous research using both functional topography approach and factor analysis demonstrated four rhythmic components in infant EEG. The scalp topography and functional reactivity of these rhythmic components are similar to delta, theta and alpha (central (mu) and occipital (alpha)) EEG rhythms in adults (Nikitina et al.,1987; Orekhova et al., 1999, 2001; Stroganova et al., 1999b). Specifically, activity in the 3.6–5.6 Hz band in infant EEG resembles theta in adults and differs from alpha in several aspects. First, similar to adult theta this activity increases under memory and attentional load. These properties of infant theta are similar to the adult theta (Kugler & Laub, 1971; Orekhova et al., 1999; Stroganova et al., 1998). Second, changes within the 3.6–5.6 Hz frequency range in infant EEG are most pronounced over parietal, temporal and frontal scalp areas. Such cortical topography characterizes theta in human adults and other mammals (Basar-Eroglu & Demiralp, 2001; Klimesh, 1996). In contrast, increases in alpha activity are typically observed over the occipital brain areas (Stroganova et al., 1999). Third, in infants, as in adults, the 3.6–5.6 Hz frequency band is adjacent to the lower boundary of the alpha band (6.0–9.0 Hz). Finally, the 3.6–5.6 Hz activity does not react specifically to changes in visual input, and thus can not be considered as alpha rhythm.
Adult EEG research provides strong evidence for functional differences between narrow-bands within the traditional theta band (Da Silva, 1999; Klimesch, 1996). Consistent with adult findings, infant EEG research has demonstrated two functionally different narrow frequency bands within theta rhythm (Orekhova et al., 1999). Changes in a narrow band may remain undetected when mean amplitude of the entire theta band is used in the analyses. An absence of the amplitude changes within the broad theta band may reflect the sum of an increase in the predominant amplitude in a narrow frequency band and an unchanged or even suppressed activity in other narrow frequency bands.
The choice of defining a variable as the sum of spectral densities within a frequency band can be misleading, as it is always possible to generate a value for a frequency band even if there is no spectral peak within that band. As a safeguard against this possibility spectral analysis was used to confirm the presence of a peak in the infant theta band (i.e., 3.6–5.6 HZ) during the BF condition. Although the transition from the SF to BF condition leads to the increase in spectral amplitude in a number of frequency bands, the condition-related changes were most pronounced in the infant theta band (see the result section below).
EEG data were broken into narrow bands of .4 Hz to decrease probability of false negative results. A log10 transformation was applied to the spectral data to improve normality of the EEG amplitude distribution.
2.4.5. Statistical analysis
Data analyses were conducted in five stages. First, behavior was analyzed to confirm that infants maintain social engagement with a non-engaging adult. Second, looking, affect and motor behaviors during BF and SF were evaluated. Third, RSA and heart period during BF and SF were evaluated. Fourth, spectral analyses were conducted to confirm a spectral peak in theta frequency range during BF. Fifth, analyses explored the spatial distribution of theta responses during BF.
For each behavioral dimension (i.e., Looking, Affect and Motor) a repeated measures analysis of variance (ANOVA) with CONDITION (SF versus BF) and BEHAVIORAL CATEGORY as within subject factors was performed. The number of levels for the BEHAVIORAL CATEGORY factor varied. There were 3 levels for the motor dimension: Quiet, Mild and Gross; 4 levels for the affect dimension: Neutral, Positive, Negative and None of the above; and 2 levels for the looking dimension: Look at and Look away. In addition, repeated measures ANOVAs with one within subject factor (CONDITION) were performed for heart period and RSA. Repeated measures ANOVAs with two within subject factors CONDITION (2 levels) and ELECTRODE (12 levels) were performed for spectral amplitude of the 4.8 Hz frequency band. The Greenhouse-Geisser correction was applied, when necessary, to all analyses of variance with repeated measures. The corrected p values, based on the Greenhouse-Geisser correction and the original degrees of freedom, are reported.
3. Results
3.1. Behavioral Data
Descriptive analyses evaluated the percent of time infants were: 1) in a quiet motor state, 2) looking at BF, and 3) expressing either positive or negative affect while looking at and away from the adult. Based on previous research infants were expected to express some positive affect while looking at BF. Though infants were expected to express some negative affect during BF, it was unknown if they would express negative affect while looking at the non-engaging adult. The descriptive analyses (see Table 1) revealed that infants were looking at the adult for about 91% of the SF session length, and for about 43% of the BF session length. During SF, all infants expressed affect only while looking at the adult. The affect for these infants was primarily positive with only one infant expressing negative affect. During BF infants expressed positive affect only while looking at the adult (about 20% of the session length). Eight infants expressed none, 4 infants expressed negligible (less than 1% of the session length) and only one infant expressed a larger amount of negative affect while looking at the adult (37% of the session length). However, infants demonstrated negative affect while looking away from the adult during BF (about 9% of the session length). Thus, infants demonstrated social engagement with a non-engaging adult expressing some positive and negligible amounts of negative affect while looking at her. This observation is important for this study, since EEG data were sampled only while the infant was looking at the adult.
Table 1.
Means, Confidence Intervals and Planned Comparisons for behavioral measures for SF and BF
| Dimension | Variable | SF M / CI(95%) | BF M / C (95%) | F(1,15) | p |
|---|---|---|---|---|---|
| Motor | Quiet Motor | 53 / 43–62 | 16 / 10–22 | 40.03 | < .001 |
| Mild Motor | 44 / 36–51 | 70 / 59–80 | 17.92 | < .001 | |
| Gross Motor | 3 / 0–7 | 14 / 6–22 | 7.01 | < .05 | |
|
| |||||
| Looking | Look At | 93 / 87–97 | 39 / 26–52 | 64.57 | < .001 |
| Look Away | 7 / 1–11 | 61 / 46–72 | 63.43 | < .001 | |
|
| |||||
| Affect | Positive | 48 / 36–58 | 21 / 13–29 | 21.65 | < .001 |
| Negative | 0 / 0–1 | 19 / 6–33 | 9.36 | < .001 | |
| Neutral | 50 / 38–60 | 48 / 35–60 | .02 | ns | |
| None of the above | 2 / 0–4 | 11 / 5–32 | 7.95 | < .05 | |
|
| |||||
| Combined Variables | |||||
|
| |||||
| Positive while looking at | 45 / 34–56 | 17 / 9–24 | 25.08 | < .001 | |
| Negative while looking at | 0 / 0–1 | 5 / 0–10 | 3.35 | ns | |
| Neutral while looking at | 45 / 35–54 | 15 / 10–21 | 30.41 | < .001 | |
| None of the above while looking at | 1 / 0–2 | 2 / 0–3 | 1.85 | ns | |
| Positive while looking away | 3 / 0–5 | 4 / 0–7 | 2.75 | ns | |
| Negative while looking away | 0 / na | 14 / 2–25 | 6.47 | < .05 | |
| Neutral while looking away | 4 / 0–8 | 32 / 19–45 | 17.65 | < .001 | |
| None of above while looking away | 1 / 0–2 | 9 / 3–15 | 8.80 | < .01 | |
To examine the Blank Face effect on behavior, infant behavior during the SF and BF trials was analyzed. For each general behavioral dimension the interaction between CONDITION (SF and BF) and BEHAVIORAL DIMENSION (e.g., Motor: 3 levels; Looking: 2 levels; Affect: 4 levels) using repeated measures ANOVA was explored. Interactions were significant for Motor [F2, 28 = 23.08, p < .001], Affect [F3,42 = 7.82, p < .001], and Looking behaviors [F1,14 = 81.90, p < .001]. Specifically, as confirmed by planned comparisons (see Table 1), the transition from SF to BF, was characterized by changes in each behavioral dimension: (1) Motor behavior: Quiet Motor decreased, Mild Motor increased, Gross Motor increased, (2) Affect displays: Positive Affect decreased, Negative Affect increased, (3) Looking behavior: ‘Look at’ decreased, and ‘Look away’ increased. Thus, the blank face effect was observed as decreased positive affect, increased negative affect, gaze aversion (i.e., looking away), and motor activity (as evidenced by decreases in Quiet and increases in Mild and Gross motor activity). This response to blank face that follows the smiling face is similar to the well known response to still face (that can be neutral, sad or smiling) that follows interaction with the adult.
To evaluate if positive affect displays during BF reflected a social engagement strategy (i.e., directed towards the adult) the relations of positive and negative affective displays to looking behavior were analyzed. Negative affect displays were analyzed because it has been speculated (Weinberg & Tronick, 1996) that infants use negative affect to signal negative evaluation of the disruption of interaction with the adult. If this is correct, these two types of affective signaling, while looking at the adult, might reflect different types of internally driven social engagement. An additional repeated measure ANOVA was performed on the four combined behavioral categories measured during BF (i.e., positive or negative while looking at, and positive or negative while looking away) as dependent variables, and two within subject factors (2 levels of each): LOOKING (Look at, Look away) and AFFECT (Positive, Negative). There was a significant LOOKING x AFFECT interaction (F1,15 = 14.82, p < .002). This effect was due to the remarkable prevalence of positive affect displays while infants were looking at the adult compared to looking away (F1,15 = 11.32, p < .005). There was no significant difference in negative affect displays under these two looking conditions during BF. However, as described above only 1 infant displayed negative affect while looking at the adult, and 4 infants displayed negative affect while looking away during BF.
Thus, positive, not negative, affective displays during BF were predominantly directed at the unresponsive adult. This finding suggests that even though the disruption of the interaction elicited a decrease in the infant’s positive engagement, infants still demonstrated positive engagement for a pronounced proportion of the BF trial.
3.2. RSA and Heart period data
As illustrated in Fig. 1, RSA amplitude increased [F(1, 12)=5.48, p = .037] and heart period decreased [F(1,12)=10.48, p < .01] during BF relative to SF.
Figure 1.
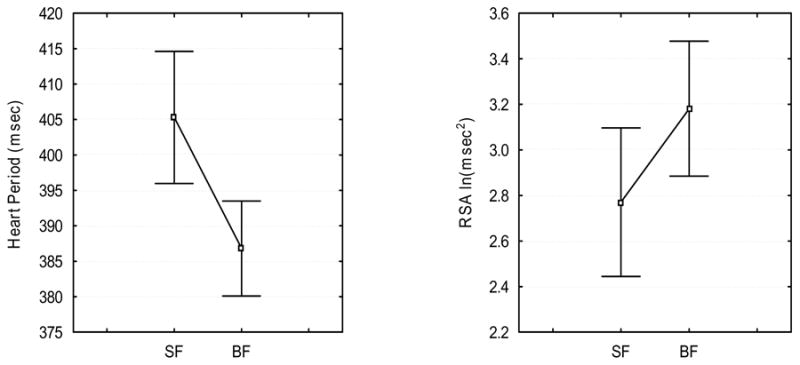
Mean RSA and heart period during Smiling Face (SF) and Blank Face (BF). Vertical bars denote .95 confidence intervals.
3.3. EEG data
3.3.1. Confirmation of the spectral peak in the theta range during BF
Grand average EEG spectra (spectrum averaged across all 13 infants) during both SF and BF conditions is illustrated in Fig. 2. Noticeable in the Fig. 2 are the spectral peaks in the 4.8 Hz frequency at anterior recording sites (AF 3,4; FC3,4; F 7) only during the BF condition. Paired sample t-tests were used to compare absolute amplitude values for two conditions at each frequency band within .8 Hz – 14.8 Hz range. The absolute amplitudes of EEG spectra were higher during BF than during SF condition. The significant differences were seen at frequency bands related to the delta, theta and alpha rhythms (see Fig. 2). However, these differences were greater and more consistent for the theta range. Thus, although the transition from SF to BF increased spectral amplitude in several frequency bands, the condition-related changes were most pronounced in the infant theta band (e.g., 4.8 Hz). To simplify the EEG analyses, to avoid false negative results and to decrease the number of multiple comparisons, only the spectral amplitude at 4.8 Hz in which the presence of peak was confirmed was selected for future analyses.
Figure 2.
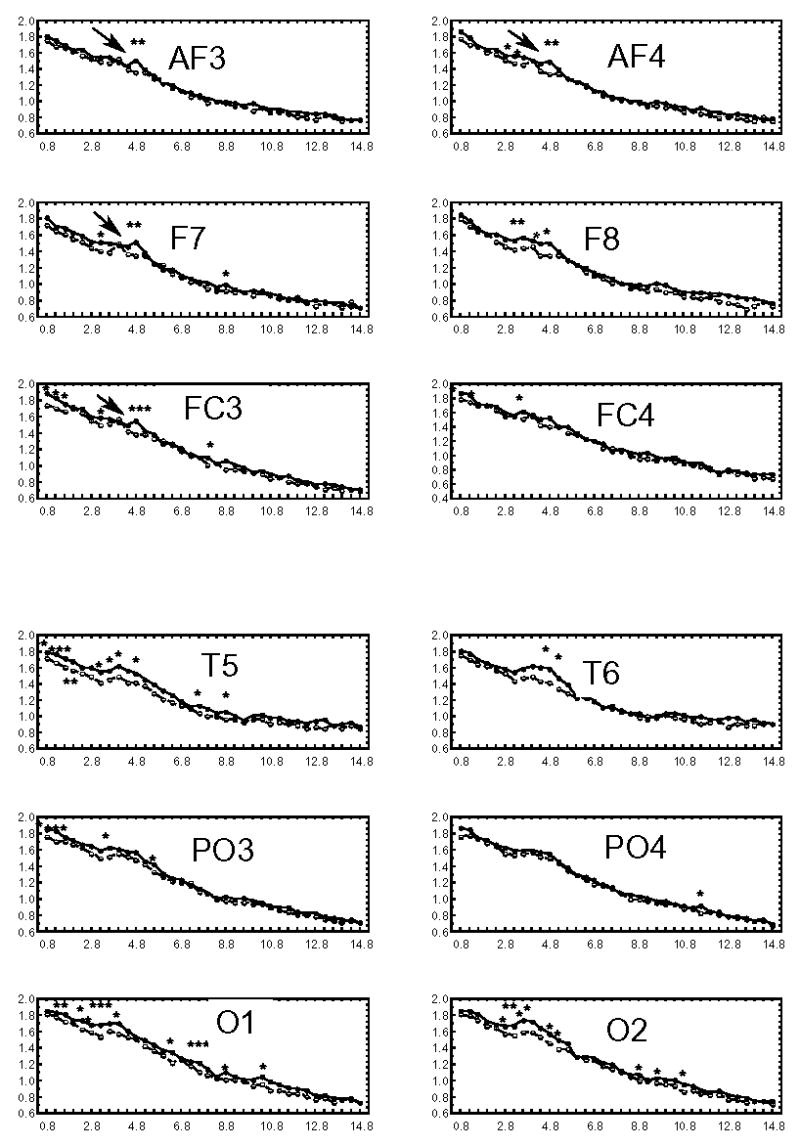
Grand average EEG amplitude spectra during SF (broken line) and BF (solid line) in 5-month-old infants. A distinct spectral peak (marked with an arrow) at 4.8 Hz in theta frequency band (3.6–5.2 Hz) over frontal scalp areas (AF3, AF4, F7, FC3) is observed during BF. * p<.5, ** p<.01, ***p<.005
3.3.2. EEG theta activity
Fig. 3 illustrates the spectral amplitude in the 4.8 Hz frequency band during BF and SF conditions for each electrode. Repeated measures ANOVAs revealed main effects of CONDITION [F (1,11) = 20.54, p < .001] and ELECTRODE [F(1,11) = 3.84, p < .000], and a CONDITION X ELECTRODE interaction [F(1,11) = 2.09, p < .025]. Post hoc Bonferroni tests identified significant contributions to the CONDITION effect from several electrode locations (AF3 (p<.013), AF4 (p< .006), F7 (p < .0007), F8 (p< .016), FC3 (p < .005), and T6 (p< .000001). There were no significant between condition differences in the 4.8Hz band at left temporal (T5, p < .15), right central frontal (FC4, p < .11 ), left and right parietal (PO3, p < 1.00; PO4, p < 1.00), and left and right occipital (O1, p < 1.00, O2, p < .13) electrodes. Overall, theta amplitude (in 4.8 Hz band) increased during BF relative to SF at the most frontal and right temporal electrode sites.
Figure 3.
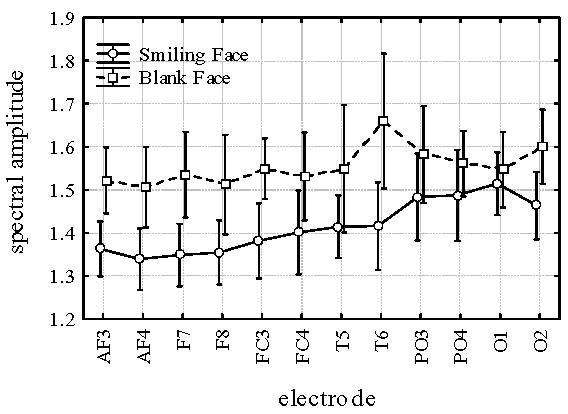
Theta (4.8 Hz) amplitudes at different electrodes during Smiling Face and Blank Face. Vertical bars denote 0.95 confidence intervals.
4. Discussion
4.1. Summary of findings
RSA, heart period and EEG rhythmical activity were evaluated in 5-month-old infants during two face-to-face settings (i.e., SF and BF) with an adult experimenter. During SF, adult smiling is assumed to support infant engagement (i.e., looking at the adult’s face and positive signaling). Thus, during BF, the external support for infant engagement is reduced when the adult stops smiling and does not respond to the infant’s affective displays. Infants demonstrated an ability to maintain engagement with the adult during both SF and BF displaying positive affect predominantly while looking at the adult. However, during SF, infants displayed positive affect while continuously looking at the adult, while during BF, infants remained mostly neutral and increased gaze aversion. The physiological data revealed that differences in infant engagement with the adult during these conditions were paralleled by different physiological processes. These data suggest that not only were the infants sensitive to changes in adult facial expression, but also that different neurophysiological mechanisms might support infant looking at the smiling versus neutral faces. First, increases in RSA were observed during BF relative to SF. Second, widespread increases in theta activity in the 4.8 Hz narrow band were observed over the majority of anterior and right posterior temporal scalp areas during infant engagement with a non-engaging adult (i.e., while infants were looking at the adult’s blank face).
4.2. RSA during BF
A description of the neurophysiological systems that support the infant’s ability to maintain social engagement with a non-engaging adult is the main focus of the present study. According to the Polyvagal Theory (Porges, 1995, 2001) regulation of visceral state via the myelinated vagus promotes engagement with the environment (e.g., eye contact, smiling, and vocalization) (Porges, 2003). While increased vagal activity promotes a visceral state supportive of social engagement, withdrawal of these vagal influences facilitates the disruption of engagement. Within this theoretical framework, infants exhibited a response during BF (i.e., small increases in RSA) consistent with a state supportive for sustaining engagement. Moreover, increases in RSA indicate increased activation of the visceromotor component of the Social Engagement System that provides ascending input to the anterior and temporal brain areas via the basal forebrain cholinergic system. In adults the cholinergic input to the frontoparietal cortex may influence the allocation of attention to emotional information contained in the facial cues (Bentley et al., 2003). This study suggests that there might be similar functional significance of the visceromotor component of the Social Engagement System in infant attention to the blank face, as increases in RSA were observed when the infant was challenged with sudden absence of facial cues.
Interestingly, changes in RSA and heart period were in the opposite direction during BF relative to SF. Decreases in heart period (increases in heart rate) during BF were paralleled by increases in motor activity. Increases in motor activity are associated with increased cardiac output, thus requiring decreased RSA and increased heart rate (Hatfield et al., 1998). However, in this study increases in metabolic demands were not paralleled by decreases in RSA. Thus, changes in RSA were not exclusively influenced by changes in motor activity during BF relative to SF. It is plausible, that the changes in engagement demands (i.e., transition from sustaining engagement with the smiling face to sustaining engagement with the blank face), and changes in motor activity influenced RSA in opposite directions. The competition of these two factors resulted in small increases in RSA during BF relative to SF. The absence of RSA withdrawal during BF indicated that the majority of infants in this study maintained control over their visceral state despite the change in experimental condition.
4.3. Theta during BF
Increases in theta paralleled increases in vagal activity (indexed via RSA) during BF relative to SF. Infants demonstrated theta changes at multiple electrode sites, while attending to the blank face compared to the smiling face. As illustrated in Fig. 3, the increases in theta during the BF condition were observed over right posterior temporal scalp area (T6) and over anterior scalp areas (AF3,4; F7,8; FC3).
4.4. RSA, theta and positive affect
To evaluate the potential associations between behavioral and physiological variables, we examined the correlations between changes in RSA (BF-SF) and the infant’s positive affect during BF (r = .65, p < .05); and between changes in theta and the infant’s positive affect during BF (changes in theta at T6 and affect, r = −.69, p < .05; ns correlations between changes in theta and affect at other locations). We also examined the correlation between changes in RSA and changes in theta (BF-SF) over frontal scalp areas (averaged changes in spectral amplitude at FP1 and FP2). This correlation was not significant but was in the predicted direction (r = .44, p <.13). Thus, it appears that the more positive infants demonstrated greater increases in RSA (i.e., were better able to maintain visceral state control), and the less positive infants demonstrated increases in theta over the right temporal brain area during BF. It is important to note that these analyses have very low power (.56) due to the small sample size. Further research is needed to examine these relationships.
4.5. Hemispheric differences in temporal theta and face processing
Recent research using positron emission tomography (Sergent et al., 1992), functional magnetic resonance imaging (Kanwisher et al., 1997) and event related potentials (Bentin et al., 1996) has reported involvement of the temporal brain areas in facial processing. These studies suggest that neurons in particular parts of the human temporal cortex (i.e., fusiform gyrus, superior temporal sulcus) are activated only by faces or are activated more by faces than by objects. In addition, infant research has demonstrated that by 4–9 months of age, hemispheric differences in face processing emerge (for a review, see de Schonen & Mathivet, 1989). The right hemispheric asymmetry in face processing has been reported in event related potential studies of infant face processing (de Haan & Nelson, 1999). Moreover, research of Deruelle and de Schonen (1991, 1995) has demonstrated that in infants the right hemisphere better detects changes in the spatial position of features (e.g., eye size or eye tilt) while the left tends to be better at detecting changes in the features (e.g., a different eye). Together these results suggest that by 5 months of age the right hemisphere might be more proficient than the left in facial expression recognition. Therefore, the right hemisphere compared to the left hemisphere might be more reactive to changes in a partner’s facial expressivity during a face-to-face interaction.
In the current study, an increase in theta was observed over the right temporal brain area in infants, while they looked at the blank face. This observation suggests greater right hemisphere involvement in face processing during BF. In addition, the right temporal theta synchronization during attention to the blank face in 5-month-old infants might reflect an activation of brain structures involved in modulation of attention to blank face. Right temporal theta might represent the interplay between temporal cortical areas and the hippocampus. Because hippocampal theta depends on ascending cholinergic input from the NTS, it might mediate the cholinergic influences, within temporal cortex, on allocation of anticipatory attention to facial emotional information recently demonstrated by Bentley and colleagues (2003).
4.6. Frontal theta synchronization and intentional engagement
It is tempting to speculate that positivity displayed by the infants during BF may represent an effort by the infant to evoke reciprocity (i.e., the infant very likely expects a reciprocal response of smiling from the social partner). Individuals who maintain positive affect in response to rapid shifts in the social cues of a partner (e.g., neutral face) may have positive developmental consequences in spite of the initial failure to elicit reciprocity. As noted by Walter (1953), the first failure may be the beginning of the first learning. Displaying positive affect during BF may be viewed as an active engagement to reduce the negative or neutral cues being presented, and/or as an attempt to re-instate interaction. The neurophysiological responses support this interpretation. For example, the pronounced EEG responses indicate that infants are sensitive to the change in facial signaling. Moreover, the increased activation over frontal areas of the brain, where large effects were observed, implies the activity of the anterior attention network that might support emerging executive functions and rudimentary intentional capacities.
The increases in theta at the frontal recording sites during the BF condition do not directly indicate that the source of observed theta response is within the prefrontal cortex, as no conclusive interpretation can be made solely from scalp recordings. Thus, the data on the topographical amplitude distribution should be considered with caution, especially given the small number of electrodes and linked ear reference2 used in this study (Lehmann, 1987). However, several arguments support the suggestion that the observed frontal theta activity reflects frontal cortex activation. For example, research with adults indicates that scalp recordings of frontal theta are likely to originate from the frontal cortex and not merely volume conduction from hippocampus ( source-localization techniques, Ishii et al, 1999; Asada et al, 1999; intracortical EEG studies, Caplan et al., 2001; Kahana et al., 2001; see Kirk & Mackay, 2003). Based on the literature with human infants and adults in which activation of the frontal cortex, indexed by theta, is related to internally controlled sustained attention (Ishii et al., 1999; Orekhova et al., 1999; Smith et al., 1999), we suggest that the increased frontal theta observed while a 5-month-old infant attends to a blank face similarly reflects an activation of anterior areas subserving executive aspects of attention regulation.
4.7. Physiological consequences of the short-term ‘failure’ to evoke social reciprocity
Frontal cortex plays a central role in the neural regulation of goal oriented behavior in older children and adults. It is tempting to speculate that in 5-month-old infants, mild to moderate excitation of the neural circuits mediating intentional engagement potentiates functional activity of the frontal cortex. This may occur even though these structures are neuroanatomically immature (Chugani, 1996) and “silent” in other tasks requiring frontally-mediated executive control (Stroganova et al, 1999a). Based on this speculation we would like to consider the possible functional significance of experiential input of the short-term ‘failure’ to evoke reciprocity in early social interactions for the development of the neuroanatomical structures within frontal cortex. Short- term failure to evoke reciprocity challenges an infant with a new context, where learned behavioral strategies and responses do not work and new behavioral and self-regulatory skills are needed. In this sense, short-term interactive mismatch might provide a unique experience that both triggers and fosters development of frontal cortex function. As suggested by Vygotsky (1980), during environmental interactions the infant might not only be acting upon the environment but also changing his/her own biology.
Whereas contingent behavior from a caregiver is vital for infant social development (e.g., Ainsworth et al., 1974; Bowlby, 1969), short-term experiential input of interactive mismatch during interaction with adults might play an important role in the infant’s mental development (i.e., triggering and fostering development of frontal cortex function) if the infant remains viscerally calm during interactive mismatch. This conclusion should not be interpreted as a developmental benefit of ignoring infants, but only as an indication that central mechanisms that guide goal-oriented behavior might be potentiated during naturally occurring interactive mismatches. Interactive mismatches have been reported in 70% of natural mother-infant interactions (Tronick & Gianino, 1986). Interestingly, Miller (1991) hypothesized that the theta rhythm is likely to be generated in hippocampus-neocortical circuitry when information important to the species is to be gathered from the environment. According to Miller, socially significant novel stimuli would be the most potent trigger of this activity in humans. Infant behavior during interactive mismatches might be considered an active behavioral state characterized by search for the relevant social cues (e.g., search for changes in the adult’s facial expression). If so, short-term maintenance of social engagement with a non-engaging adult might be adaptive in that it fosters the development of executive control and perhaps provides vital exercise for the developing brain.
In summary, the study provides data that demonstrate that both vagal activity and theta are involved in maintenance of social engagement behaviors when adult regulatory input, aimed to induce, organize and support an infant's affective state, is absent. Parallel increases in RSA and scalp recorded theta were observed.
Acknowledgments
The data were presented at the meeting for the International Conference on Infant Studies, May 2004. This research was supported, in part, by National Institutes of Health grants (HD22628 and MH60625) awarded to S.W. Porges, and by a Fogarty International Research Collaboration Award 5R03TW00693 to T.A. Stroganova as a supplement to NIH grant HD22628 awarded to S.W.Porges. Jane Doussard-Roosevelt is now at the Center for Scientific Review, NIH. This article was written in a personal capacity and does not necessarily represent the opinions or reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the Federal Government. The authors wish to thank the children and their families who participated.
Footnotes
There is no agreement in the literature on how using linked ears as an electrode reference influences the distribution of scalp potentials. It has been reported that linked ears may have a significant effect (Lehmann, 1987). In contrast, there have been convincing arguments that in practice there is little evidence of this effect (Burges & Gruzelier, 1997). For example, da Silva (Da Silva, 1999) demonstrated that the linked ears reference could lead to an artificial spreading of oscillations over scalp areas. However, even if this occurred, it would reduce the likelihood of finding a real topographic effect. Thus, it could not explain the current findings.
Olga V. Bazhenova, University of Illinois at Chicago. Address: Brain-Body Center (MC747), UIC 1747 W Roosevelt, Chicago Il, 60608 USA; e-mail obazhenova@gmail.com. Phone: 312-996-3167.
Tatiana Stroganova, Brain Research Institute RAMS. Address: Brain Research Institute, per.Obukha, 5, Moscow 103064, Russia.
Irina Posikera, Brain Research Institute RAMS. Address: Brain Research Institute, per.Obukha, 5 Moscow 103064, Russia.
Jane Doussard-Roosevelt, Department of Human Development, University of Maryland, College Park, College Park, MD 20742
Stephen W. Porges, University of Illinois at Chicago. Address: Brain-Body Center (MC747), UIC 1747 W Roosevelt, Chicago Il, 60608 USA.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth MDS, Bell SM, Stayton DJ. Infant-mother attachment and social development: Socialization as a product of reciprocal responsiveness to signals. In: Richards MPM, editor. The Integration of a Child into a Social World. Cambridge University Press; London: 1974. pp. 99–135. [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulatedcortex in humans. Neurosci Let. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Basar E. Brain Function and Oscillations. I. Brain Oscillations - Principles and Approaches. Springer; Berlin: 1998a. [Google Scholar]
- Basar E. Brain Function and Oscillations. II. Integrative Brain Function. Neurophysiology and Cognitive Processes. Springer; Berlin: 1998b. [Google Scholar]
- Basar-Eroglu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int J of Psychophysiol. 2001;39:167–195. doi: 10.1016/s0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Bayley N. The Bayley II Scales of Infant Development. Psychological Corporation; New York: 1993. [Google Scholar]
- Bazhenova OV. Experimental’noe issledovanie potrebnosti v obschenii [Experimental Investigation of the Infant Need for Communication] In: Leont’ev AN, Artem’eva E, editors. Problems of Medical Psychology. Moscow University Publishing House; Moscow: 1980. pp. 38–47. [Google Scholar]
- Bazhenova OV, Plonskaia O, Porges SW. Infant vagal reactivity and affect adjustment during interaction challenges. Ch Dev. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Garwood MM, Powers BP, Notaro PC. Infant affect and affect-regulation during the still-face paradigm with mothers and fathers: The role of infant characteristics and parental behavior. Dev Psychol. 1998;34:1428–1437. doi: 10.1037//0012-1649.34.6.1428. [DOI] [PubMed] [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cognitive Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergicenhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Basic Books; New York: 1969. p. 428. [Google Scholar]
- Brazier MA. Electrical activity recorded simultaneously from the scalp and deep structures of the human brain. J Nerv Ment Dis. 1968;147:31–39. doi: 10.1097/00005053-196807000-00003. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Kahana MJ, Sekuler R, Kirschen MP, Madsen JR. Taskdependence of human theta: the case for multiple cognitive functions. Neurocomputing. 2000;32–33:659–665. [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, Feinstein AR. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–549. doi: 10.1016/0895-4356(90)90158-l. [DOI] [PubMed] [Google Scholar]
- Cohen JF. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Cohen J, Tronick EZ. Mother-infant face-to-face interaction: the sequence of dyadic states at 3, 6, and 9 months. Dev Psychol. 1987;23:68–77. [Google Scholar]
- Colgin L, Kramar E, Gall CM, Lynch G. Septal modulation of excitatory transmission in hippocampus. J Neurophysiol. 2003;90:2358–2366. doi: 10.1152/jn.00262.2003. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. In: Johnson M, editor. Brain Development and Cognition. Oxford: Blackwell Publishers; 1996. pp. 125–145. [DOI] [PubMed] [Google Scholar]
- Da Silva LFH. EEG analysis: Theory and Practice. In: Niedermeyer E, da Silva LF, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Williams & Wilkins; Baltimore: 1999. pp. 1135–1163. [Google Scholar]
- De Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- D’Entremomt B, Muir D. Five-month-olds attention and affective responses to still-faced emotional expressions. Infant Behav Dev. 1997;20:563–568. [Google Scholar]
- Denver JW, Reed ShF, Porges SW. Methodological Issues in the Quantification of RSA. Biol Psychol Biol Psycho. 2005 doi: 10.1016/j.biopsycho.2005.09.00. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruelle C, de Schonen S. Hemispheric asymmetries in visual pattern processing in infancy. Brain Cognition. 1991;16:151–179. doi: 10.1016/0278-2626(91)90004-r. [DOI] [PubMed] [Google Scholar]
- Deruelle C, de Schonen S. Pattern processing in infancy: Hemispheric differences in the processing of the shape and location of visual components. Infant Behav Dev. 1995;18:123–132. [Google Scholar]
- de Schonen S, Mathivet E. First come, first served: A scenario about the development of hemispheric specialization in face recognition during infancy. Eur B Cog Psychol. 1989;9:3–44. [Google Scholar]
- Fogel A. Early adult-infant interaction: Expectable sequences of behavior. J Pediatr Psychol. 1982;7:1–22. doi: 10.1093/jpepsy/7.1.1. [DOI] [PubMed] [Google Scholar]
- Futagi Y, Ishihara T, Tsuda K, Suzuki Y, Goto M. Theta rhythms associated with sucking, crying, gazing and handling in infants. Electroen Clin Neuro. 1998;106:392–399. doi: 10.1016/s0013-4694(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: Evidence of cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–554. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Guideline thirteen: Guidelines for standard electrode position nomenclature. Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- Gusella JL, Muir D, Tronick EZ. The effect of manipulating maternal behavior during an interaction on three- and six-month-olds' affect and attention. Ch Dev. 1988;59:1111–24. doi: 10.1111/j.1467-8624.1988.tb03264.x. [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Santa Maria DL, Porges SW, Potts JT, Spalding T, Byrne EA. Respiratory sinus arrhythmia during exercise in aerobically trained and untrained men. Med Sci Sport Exer. 1998;30:206–214. doi: 10.1097/00005768-199802000-00006. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, De Gues EJC. Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39:427–436. doi: 10.1017.S0048577202394022. [DOI] [PubMed] [Google Scholar]
- Inanaga K. Frontal midline theta rhythm and mental activity. Psychiat Clin Neuros. 1998;52:555–566. doi: 10.1046/j.1440-1819.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott &, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kemp IR, Kaada BR. The relation of hippocampal theta activity to arousal, attentive behavior and somatomotor movements in unrestrained cats. Brain Res. 1975;95:323–342. doi: 10.1016/0006-8993(75)90110-9. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronizing and integrating activity in distributed mnemonic networks. Cortex. 2003;39:993–1008. doi: 10.1016/s0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillation and EEG synchronisation. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cognitive Brain Res. 2001;11:281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Kugler J, Laub M. ‘Puppet show’ theta rhythm. ElectroenClin Neuro. 1971;31:532–533. [Google Scholar]
- Lamb ME, Morrison DC, Malkin CM. The development of infant social expectations in face-to-face interaction – a longitudinal study. Merill Palmer Quart. 1987;33:241–254. [Google Scholar]
- LeDoux JE. The Emotional Brain. Simon and Schuster; New York: 1996. p. 384. [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Methods of Analysis of Brain Electrical and Magnetic Signals. Handbook of Electroencephalography and Clinical Neurophysiology. Vol. 1. Elsevier; Amsterdam: 1987. pp. 309–354. [Google Scholar]
- Lehtonen J, Kononen M, Purhonen M, Partanen J, Saarikoski S. The effects of feeding on the electroencephalogram in 3-and 6-month-old infants. Psychophysiology. 2002;39:73–79. doi: 10.1017/S0048577202000239. [DOI] [PubMed] [Google Scholar]
- Lewis PR, Shute CC, Silver AA. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967;191:215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukatch HS, MacIver MB. Physiology, pharmacology, and topography of cholinergic neocortical oscillations in vitro. J Neurophysiol. 1997;77:2427–2445. doi: 10.1152/jn.1997.77.5.2427. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Carter AS. Emerging social regulatory capacities as seen in the still- face situation. Ch Dev. 1990;61:754–63. [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother–infant interaction. Dev Psychol. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. Cortico-hippocampal Interplay and Representation of Contexts in the Brain. Springer-Verlag; New York: 1991. p. 267. [Google Scholar]
- Nakashima K, Sato H. Relationship between frontal midline theta activity in EEG and concentration. J Hum Ergology. 1993;22:63–67. [PubMed] [Google Scholar]
- Nikitina GM, Stroganova TA, Posikera IN. Central organization of emotional reactions of infants during the first year of life. In: Trojan S, Stastny F, editors. Ontogenesis of the Brain. Universitas Carolina; Praha: 1987. pp. 223–228. [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. Int J Psychophysiol. 1999;32:151–172. doi: 10.1016/s0167-8760(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin Neurophysiol. 2001;112:740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Bird L. On the brain and personality substrates of psychopathy. Behav Brain Sci. 1995;8:568–600. [Google Scholar]
- Paul K, Dittrichova J, Papousek H. Infant feeding behavior: Development in patterns and motivation. Dev Psychobiol. 1996;29:563–576. doi: 10.1002/(SICI)1098-2302(199611)29:7<563::AID-DEV2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Copolla R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- Porges SW. Respiratory Sinus Arrhythmia: An index of vagal tone. In: Orlebeke JF, Mulder G, Van Dornen LJPE, editors. Psychophysiology of Cardiovascular Control: Models, Methods, and Data. Plenum; New York: 1985. pp. 437–450. [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory Psychophysiology. 1995;32:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic substrates of a social nervous system. Int J Physiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contribution to social behavior. Physiol Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges S, McCabe P, Yongue B. Respiratory-heart-rate interactions: Psychophysiological implications for pathophysiology and behavior. In: Cacioppo JJ, Petty R, editors. Perspectives in Cardiovascular Psychophysiology. Guilford; New York: 1982. pp. 223–264. [Google Scholar]
- Posner MI, Dahaene S. Attentional networks. Trends Cogn Sci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Cardiorespiratory control. In: Loewy AD, Spyder KM, editors. Central Regulation of Autonomic Function. Oxford University Press; New York: 1990. pp. 189–207. [Google Scholar]
- Robinson TE, Wishaw IQ. Effect of posterior hypothalamic lesions on voluntary behavior and hippocampal electroencephalogram in the rat. J CompPhysiol Psych. 1974;86:768–786. doi: 10.1037/h0036397. [DOI] [PubMed] [Google Scholar]
- Rochat P, Striano T, Blatt L. Differential effects of happy, neutral, and still-faces on 2-, 4-, and 6-month-old infants. Infant Child Dev. 2002;11:289–303. [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Ohata S, MacDonald B. Functional neuroanatomy of face and object processing: A positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Smith ME, McEvoy LK, Gevins A. Neurophysiological indices of strategy development and skill acquisition. Cognitive Brain Res. 1999;7:389–404. doi: 10.1016/s0926-6410(98)00043-3. [DOI] [PubMed] [Google Scholar]
- Somsen R, Van Beek B. Ocular artifacts in children’s EEG: Selection better than correction. Biol Psychol. 1998;48:281–300. doi: 10.1016/s0301-0511(98)00041-6. [DOI] [PubMed] [Google Scholar]
- Striano T, Liszkowski U. Sensitivity of the context of facial expression in the still- face at 3-, 6-, and 9-months of age. Infant Behav Dev. 2005;28:10–19. [Google Scholar]
- Stroganova TA, Posikera IN. Functional organization of behavioral states in wakefulness during infancy (EEG study) In: Adrianov OS, editor. Mozg I Povedenie Mladentsa [Brain and Behavior in Infancy] IPRAN Press; Moscow: 1993. pp. 78–166. [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. Externally and internally controlled attention in infants: EEG Study. Int J Psychophysiol. 1998;30:339–351. doi: 10.1016/s0167-8760(98)00026-9. [DOI] [PubMed] [Google Scholar]
- Stroganova T, Bazhenova O, Posikeira I, Doussard-Roosevelt J, Porges S. Cortical and autonomic regulation of sustained visual attention in 5-month-old infants. Psychophysiology. 1999a;36:S112. [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clin Neurophysiol. 1999b;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Toda S, Fogel A. Infant response to the still-face situation at three and six months. Developmental Psychology. 1993;29:532–538. [Google Scholar]
- Tronick EZ, Gianino AF. Interactive mismatch and repair: Challenges to the coping infants. Zero to Three. 1986;6:1–6. [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant's response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Ch Psychiat. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Expression, control, and probable functional significance of the neuronal theta-rhythm. Prog Neurobiol. 1995;45:523–583. doi: 10.1016/0301-0082(94)00051-i. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS, Brazhnik ES, Stafekhina VS, Kitchigina VF. Theta-rhythm, acetylcholine and activity of the hippocampal neurons in rabbit: II Septal input. Neuroscience. 1993;53:971–979. doi: 10.1016/0306-4522(93)90482-u. [DOI] [PubMed] [Google Scholar]
- Vygotsky L. Mind and Society: The Development of Higher Psychological Processes. Harvard University Press; Harvard: 1980. p. 176. [Google Scholar]
- Walter WG. The Living Brain. Norton; New York: 1953. p. 311. [Google Scholar]
- Weinberg MK, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Ch Dev. 1996;67:905–14. [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: One hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]


