Summary
Introduction
Buruli ulcer disease is endemic in many developing countries in Africa. It is caused by Mycobacterium ulcerans, a toxin-producing bacterium with predilection for the skin and its deeper tissues. The exact mode of transmission is unclear and the pathogenesis is also not well understood, necessitating further elucidation through animal studies.
Objective
The study assessed the infectivity of a Ghanaian Mycobacterium ulcerans isolate and the dose-response pattern in BALB/c mice.
Method
Ten standardized bacterial suspensions of different concentrations were prepared from the M. ulcerans isolate and inoculated into the foot-pads of the mice. Thereafter they were observed for clinical signs of Buruli ulcer, upon which they were serially euthanised and evaluated for pathological and microbiological changes.
Results
Irrespective of the inoculum dose, all the experimentally infected mice developed similar clinical lesions, from erythema to foot ulceration (3.1 to 6.7 weeks after inoculation). However, the higher the inoculum dose the earlier the onset of the lesions. After the development of foot ulceration, mice that had received between 1 to 4 doses developed gangrene (5.7 to 7.2 weeks after inoculation) and died within a week, while those that had received 5 to 10 doses lost their limbs spontaneously (5.6 to 6.1 weeks after inoculation), followed by sudden clinical recovery. Eight weeks after the spontaneous amputation the amputees relapsed with concomitant metastasis, anasarca and death. Acid-fast bacilli (AFBs) were detected in inoculated and non-inoculated limbs, tails, visceral organs, faecal pellets and caecal contents of the mice. The AFBs detected in the caecal samples were innumerable and unusually long. Though AFBs were consistently detected in lymph nodes they were never detected in blood samples.
Conclusion
The findings suggest that the progression and final outcome of an M. ulcerans infection maybe dose related. The unequivocal absence of AFBs in the blood, but their consistent presence in lymph nodes located in the lower limbs right up to the neck, suggests that the microbes are disseminated through the lymphatic system rather than through the blood.
Keywords: Mycobacterium ulcerans, Buruli ulcer, animal model, spontaneous amputation, metastasis
Introduction
Buruli ulcer disease (BU) is the third most common mycobacteriosis of immunocompetent hosts after tuberculosis and leprosy1. It is a poorly understood disease caused by a toxin-producing mycobacterium, Mycobacterium ulcerans, which has predilection for the skin and its deeper tissues2,3. The exact mode of transmission is unclear and the indolent progression of the disease often results in massive ulceration of the skin with impairment of body functions4. All people, irrespective of age, sex, race, health or socio-economic status are susceptible to the disease. Women and children living in sub-Saharan Africa, however, are the most commonly affected4.
Though the disease was first described in 1897 by Sir Albert Cook in the Buruli District of Uganda5 , and later definitively described by MacCallum in Bainsdale, Australia in 19486, BU has largely gone unnoticed until recently7. Increases in the incidence of BU have been reported in several African countries including Ghana, and it is estimated that the prevalence is as high as 150.8/100,000 population in Ghana8. Its prevalence is higher than that of tuberculosis and leprosy in some communities8. The disease is also endemic in countries outside Africa7.
Clinically, the disease could present as papules, nodules, plaques, oedema or ulcers, all mostly on the extremities4,9. Treatment with antimicrobial agents has presented varying degrees of success, though recent data suggest that rifampicin-streptomycin combination is effective10,11 in combination with surgery, the current treatment option12.
The complications associated with the disease, the long hospitalisation in the face of the scarcity of health resources, as well as the difficulty in diagnosis, the non-availability of effective treatment, the growing spread of the disease and its long term socio-economic impact have made it a disease of public health importance, especially in Africa4.
Since the 18th century, animal modelling has facilitated and enhanced the multifaceted elucidation of several human diseases13. Therefore in the face of the enigma that Buruli ulcer presents, it has been recommended that animal studies should be undertaken worldwide, to understand the pathogenesis and mode of transmission of the disease and to provide valuable new approaches for the diagnosis, treatment, management and general control of the disease4.
Though mice, rats, guinea pigs and the nine-banded armadillo are BU animal models, each of them has limitations in replicating the whole spectrum of features presented in humans, thus leaving a lot of gaps in knowledge on the pathogenesis, transmission, diagnosis, treatment and general control of Buruli ulcer2,3,14.
This paper presents clinical and microbiological findings from M. ulcerans infection in a Buruli ulcer mouse model study undertaken as part of preparatory experiments to identify another Buruli ulcer animal model and to investigate the susceptibility of Ghanaian M. ulcerans isolates to antibiotics and herbal medicines.
Materials and Methods
Bacterial Strain: The Mycobacterium ulcerans strain used for the investigation was provided by the Department of Bacteriology of the Noguchi Memorial Institute for Medical Research (NMIMR) as a primary isolate. The isolate had previously been confirmed as M. ulcerans by the polymerase chain reaction (PCR). The BU tissue was originally obtained from biopsy of a Buruli ulcer patient from Amasaman in the Ga District of the Greater Accra Region, Ghana.
Experimental Animals: Thirty-six, 6-week old specific pathogen free (SPF) BALB/c mice produced in the Animal Barrier Facility of the Department of Animal Experimentation of NMIMR were used for the study. The study was conducted in accordance with institutional guidelines on animal experimentation, which required among other safeguards, that moribund animals or those in undue pain be put to sleep, if alleviation of the pain would be detrimental to the study.
Preparation of M. ulcerans Inoculum
The primary isolate was subcultured on Löwenstein-Jensen (L-J) slants at 32°C for eight weeks, during which period they were examined for growth and contamination. The subcultured M. ulcerans was suspended in phosphate-buffered saline (PBS) and standardized by the McFarland nephelometric standards. Briefly, a drop of sterile PBS (0.01M, pH 7.0) was added to a test tube containing 15–20 sterilized glass beads to wet them. A loopful of M. ulcerans subculture was added to the beads and drops of the sterile PBS were added intermittently and vortexed to break up the M. ulcerans colonies and to adjust the turbidity of the suspension to that of a selected McFarland nephelometric standard. Since M. ulcerans in culture is very waxy and not easily dispersible in water, all manipulations were done on ice.
Smears of the resultant M. ulcerans suspensions were stained with Ziehl-Neelsen stain (ZN) for detection of acid-fast bacilli and checked for microbiological purity of the suspensions. Portions of the suspensions were also serially diluted ten-fold and two drops per dilution were inoculated on duplicates of L-J slants and incubated at 32°C for 8 weeks, to check for viability of the inoculum and determine the colony forming units.
Experimental Infection of BALB/c Mice
Thirty BALB/c mice were divided into 10 equal groups and inoculated with the 10 bacterial suspensions equivalent to 10 McFarland standards (1 to 10). Each standard was inoculated into three mice and each mouse was inoculated in the right hind footpad with 0.05 ml of the inoculum. Six SPF BALB/c mice were used as controls and because they were identical (inbred) to the experimental group in all respects, they were inoculated in the left hind footpad to prevent any inadvertent mix-up, in case they also developed post-inoculation lesions. Three of these control mice were inoculated with 0.05 ml sterile PBS and the remaining three with 0.05 ml sterile L-J-PBS. The L-J-PBS was prepared by incubating the sterile PBS on L-J slants for 72 hours at 32°C; to somewhat simulate the conditions under which M. ulcerans is cultured. This was to determine any possible effect of the L-J on the inoculum, in case it had been picked together with the isolate and therefore been inadvertently used in the preparation of the inoculum.
Post-Inoculation Management and Evaluation of BALB/c Mice
Animal Management: The experimental and control mice were maintained in a level two animal containment facility. The animals were kept in the same room but in two separate negative pressure air racks. The room was maintained at an ambient temperature and relative humidity ranging between 22–25°C, and 55–65% respectively. A 12-12 hour light-dark cycle was provided by means of an automatic lighting system. The mice were fed daily with autoclaved feed pellets and autoclaved water, ad libitum. The animal room and clean air racks were disinfected daily, while the cages and beddings were replaced with autoclaved ones twice weekly.
Clinical Evaluation: Both the experimental and control mice were observed daily for post-inoculation changes, such as erythema, footpad swelling, foot oedema, thigh oedema and ulcer. The inoculated and control limbs of the experimental animals were measured and pricked with a sterile needle weekly to assess the progression of the oedema and anaesthetic function respectively. The test for anaesthesia was conducted to ascertain the painless nature of the disease.
Gross/Histopathological and Microbiological Evaluation: Two out of every three experimental mice in each set were euthanised on developing either foot-thigh oedema or ulcer, while the infection was observed beyond foot ulceration in the third animal in the set. A few drops of blood were sampled monthly from the tails of the 30 experimental animals for detection of AFBs. Faecal samples were also collected monthly for detection of AFBs. Carcasses of the experimentally infected mice were examined for gross pathological changes and evidence of AFB in the inoculated limb (foot and its corresponding thigh). Their blood and lymph nodes (popliteal, inguinal, axillary, mandibular, cervical and suprascapular) were examined for presence of AFBs to determine mode of dissemination. The brain, heart, lung, liver, spleen, pancreas, kidney, caecum and contralateral limbs were also examined for presence of AFBs as evidence of systemic spread of the infection.
Briefly, each limb was separated into foot and thigh portions. Each of the portions was divided either saggitally or transversally (depending on the presentation of the lesion) into two parts. One portion was homogenised, while the other portion was fixed in 10% phosphate buffered formalin. Portions of the homogenised limbs were spread out into smears, stained with ZN stain and examined for the presence of AFBs. The rest of the limb homogenates were decontaminated by standard methods such as Petroff, modified Petroff, oxalic acid and modified oxalic acid methods, as well as that described by Douglas Walsh et al, (1999)15. All these different methods were employed to enhance the recovery of viable M. ulcerans, since the microbe is susceptible to decontamination methods16. The decontaminated limb homogenates were inoculated on L-J slants and incubated at 32°C for eight weeks for the isolation of viable M. ulcerans. The portions of the limbs, which were fixed in 10% phosphate buffered formalin, were decalcified and processed for histopathological examination. The limbs of the control mice were also fixed in 10% phosphate buffered formalin and processed similarly for histopathological examination (the gross and histopathological findings will be reported separately).
Statistical Analyses
The Statistical Package for the Social Sciences (SPSS), version 12.0.1 was used for data analysis. The case summaries procedure was used to determine the means and standard deviations of the sizes of the inoculated and control limbs and the times of the onset of the lesions for each dosage group. The data were partitioned into three categories (Group 1: McFarland 1,2,3; Group 2: McFarland 4,5,6; Group 3: McFarland 8,9,10), after deleting the McFarland 7 group (since 2 out of the set of 3 died in the first and second week of the study). The Kruskal-Wallis Test was used to determine if time of lesion onset varied with the inoculum dose and if size of oedema varied with the inoculum dose. Spearman's correlation and Eta/Eta2 procedure were used to determine if there were associations between dose, onset of lesion and size of oedema.
Results
Clinical Picture
All the mice inoculated with the M. ulcerans suspensions developed similar clinical lesions, which followed the same progression irrespective of the inoculum dose. The lesions progressed from erythema of the footpad to swelling of the footpad, through to development of foot oedema, to thigh oedema and ulcer of the foot (Figure 1). Though the animals developed similar lesions, the onset and progression of the lesions varied inversely and significantly with the inoculum dose. The observation was confirmed by the correlation determinations as follows: Erythema: Rho = -950, P<0.01; Swelling of footpad: Rho = -941, P<0.01; Foot oedema: Rho = -957, P<0.01; Thigh oedema: Rho = -860, P<0.01 and Ulcer: Rho = -935, P<0.01. The variations in the onset of the lesions in relation to the inoculum dose levels are presented in Table 1. The Eta2 coefficient showed that the inoculum dose accounted principally for the differences in the onset of the lesions: Erythema of footpad = 0.857 (85.7%); Swelling of footpad = 0.951 (95.1%); Foot oedema = 0.837 (83.7%); Thigh oedema = 0.912 (91.2%) and Ulcer = 0.843 (84.3%).
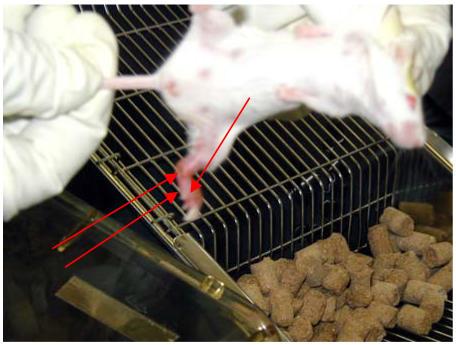
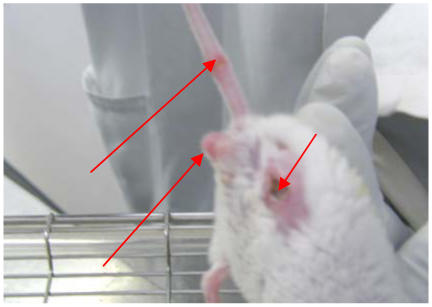
Figure 1.
Non-ulcerative and ulcerative oedema in a Mycobacterium ulcerans infected mouse [foot-thigh oedema =left arrows; ulcer = right arrow]
Table 1.
Onset of clinical lesions in Mycobacterium ulcerans infected mice in relation to inoculum dose. Results presented as mean +/- SD
| Inoculum Dose | MCF1 | MCF2 | MCF3 | MCF4 | MCF5 | MCF6 | MCF8 | MCF9 | MCF10 |
| Onset of Lesions (Days) | |||||||||
|
Clinical Lesion |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
Mean SD |
| Erythema | 19.00 | 18.00 | 14.00 | 12.67 | 11.33 | 8.00 | 6.67 | 6.33 | 6.33 |
| 1.00 | 1.00 | .00 | 0.58 | 0.58 | .00 | 0.58 | 0.58 | 0.58 | |
| Swelling of Foot-pad | 23.00 | 23.00 | 22.00 | 16.33 | 14.67 | 12.67 | 11.67 | 11.33 | 10.33 |
| 1.00 | 1.00 | 00 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | |
| Foot oedema | 29.50 | 21.50 | 30.00 | 19.50 | 17.00 | 14.00 | 13.00 | 13.00 | 12.50 |
| 0.71 | 0.71 | .00 | 0.71 | .00 | .00 | .00 | 00 | 0.71 | |
| Thigh oedema | 37.00 | 32.50 | 33.00 | 25.50 | 20.50 | 23.00 | 22.00 | 20.50 | 19.50 |
| 00 | 0.71 | .00 | 0.71 | 0.71 | .00 | 1.41 | 0.71 | 0.71 | |
| Foot Ulcer | 47.00 | 44.00 | 39.00 | 28.50 | 26.50 | 26.50 | 24.50 | 25.00 | 22.50 |
| .00 | 11.31 | 5.66 | 0.71 | 0.71 | 0.71 | 0.71 | 1.41 | 0.71 | |
| Gangrene | 51.00 | Nsm | 40.00 | 45.00 | |||||
| Spontaneous | 43.00 | Nsm | 49.00 | 45.00 | 39.00 | ||||
| Amputation | |||||||||
| Recurrence | 102.00 | 88.00 | |||||||
Key: Nsm - No similar manifestation
Though the sizes of the oedema were also inversely associated with the inoculum dose, the association was rather weak and not significant (Rho = -158 (P>0.05). The Eta2 coefficient also confirmed the weak relationship (Eta2 = 0.162 (16.2%)), in that only 16.2% of the variation in the size of the oedema could be accounted for by the inoculum dose.
Irrespective of the inoculum dose, the mice failed to withdraw their limbs when needle pricked; this tactile defect manifested after the onset of foot-thigh oedema or foot ulceration.
After the onset of foot ulceration, the manifestation of further lesions progressed along dose lines. The mice that had been inoculated with inoculum doses ranging between 1 and 4 developed foot gangrene, while those that had been inoculated with inoculum doses ranging between 5 and 10 developed ulcer of the thigh, with eventual loss of their limbs at the level of the femur, through spontaneous amputation. Two of the 4 amputees (hereafter referred to as mouse McFarland 8 (male) and mouse McFarland 9 (female) were maintained for further observation. Mouse McFarland 8 and mouse McFarland 9 lost their limbs 49 and 45 days respectively after inoculation. Mouse McFarland 8 and mouse McFarland 9 however presented smooth fur and resumed normal behaviour (feeding, drinking, moving and jumping about briskly in their cages) 4 and 6 days respectively after the spontaneous amputation. Their amputation sites healed, with the presentation of clean scars 2 weeks after the event. Mouse McFarland 8 and mouse McFarland 9 suffered relapses 54 and 43 days respectively, after the spontaneous amputation with concomitant metastasis. Mouse McFarland 8 presented Buruli lesions (in the form of congestion and swelling) on the tail, rump and scrotum and recurrence at the amputation site, which was evidenced by cyanosis (Figure 2) of the formerly clean-scarred area. Mouse McFarland 9 presented similar Buruli lesions on the tail and rump and recurrence at the amputation site, which had also become cyanotic. Mouse McFarland 9 and McFarland 8 became anasarcous and died 5 and 11 days respectively after the relapse. The mice in the control group did not develop any lesion during the period of observation.
Figure 2.
Recurrence of Buruli ulcer lesion at amputation site, evidenced by cyanosis (right arrow) and metastasis at level of tail and scrotum (Left Arrows) in an amputee
Microbiology
Mice that were sampled at the time of presentation of foot-thigh oedema were positive for AFBs only in the foot or the foot and adjoining thigh.
The limbs that were AFB positive were also culture positive for M. ulcerans. After the onset of ulcers, irrespective of the inoculum dose, AFBs were usually detected in the liver, spleen, lung, faecal pellets and lymph nodes (popliteal, inguinal, axillary, suprascapular, mandibular, cervical) (Figure 3).
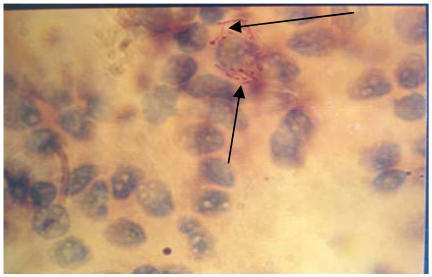
Figure 3.
Acid Fast Bacilli (Arrowed) in Lymph Node Smear (x 3300) (mag. 3300x)
Irrespective of the inoculum dose and lesion, AFBs were sometimes detected in caecal contents (Figure 4). AFBs were infrequently detected in contralateral (i.e. non-inoculated) limbs of mice that were sampled after the presentation of ulcers (which were accompanied by extensive necrosis).
Figure 4.
A Field of Acid Fast Bacilli (Atypical Mycobacterium) in Caecal Content Smear (mag. 3300x)
The AFBs detected in the caecal samples were innumerable and longer than the AFBs of the original inoculum and the other tissues sampled. AFBs were copiously present in the bone marrows of all the amputees. The tails and rumps of mouse McFarland 8 and mouse McFarland 9 were copiously positive for AFBs. The scrotum and testis of mouse McFarland 8 were also positive for AFBs. No AFBs were ever detected in any of the blood smears, brain, kidney or pancreas.
Discussion
The mouse presented a plethora of BU lesions, confirming its usefulness as a BU animal model. However, its manifestation of ulcer, the hallmark of BU, was not classical, thus subtracting from its suitability. Firstly, the mouse was too small to facilitate proper clinical evaluation of the ulcers. Secondly, the ulcers were short-lived and progressed rapidly to necrosis, resulting in the destruction of the limb. These findings give support to the assertion that better BU animal models need to be found15. These shortcomings notwithstanding, the mouse model study brought to the fore very plausible insights:
The mouse study showed that the higher the inoculum dose, the faster the progression of the disease, the more severe the lesions and final outcome and the worse the prognosis. It could therefore be inferred that the comparatively more frequent, extensive and debilitating BU lesions observed in women and children4,7,17,18,19 could be due to their exposure to larger inocula of M. ulcerans as a result of more frequent contact with the source(s) of the infection.
The detection of innumerable and unusually long AFBs in the caecal contents is both unexpected and interesting. Most probably, the mice ingested the microbe from their BU lesions through their routine grooming behaviour or the microbes disseminated to the gastrointestinal tract because it is a predilection site.
In most of the mouse foot-thigh oedematous samples, which were collected before the onset of ulceration, AFBs were not detected in the oedematous thighs, though AFBs were copiously present in the oedematous foot, suggesting that probably the thigh oedema is due to the systemic effect of toxin elicited by the microbes in the foot. This animal finding goes to support the speculation that the systemic effect of the toxin may be responsible for the faster progression and worse prognosis of patients who present with oedematous lesions4. It has already been established that the toxin elicited by M. ulcerans is a virulence factor20, however, further studies have to be conducted to determine if there is correlation between the onset and progression of the various lesions and the production of toxin, in order to elucidate the exact role that the toxin plays and when. This will facilitate appropriate management of the disease. Research efforts must also be intensified to produce anti-toxin to effectively manage the toxin-related aspects of the disease.
Four mice experienced spontaneous amputation of their limbs, 6 – 7 weeks after experimental infection, and two of them were maintained in continuation for observation. The two amputees remained healthy and active but relapsed 6 to 8 weeks after spontaneous amputation. It is particularly interesting that the two events (i.e. spontaneous amputation and relapse) had similar periods of ‘incubation’. This similarity suggests that the amputation must have resulted in massive reduction in microbial load and also probably the level of toxin. The animals however relapsed, probably because some of the microbes had already disseminated into other parts of their bodies before the spontaneous amputation took place. This finding supports Amofah's assertion that excision of pre-ulcerative lesions is effective in preventing deformities but less so in preventing recurrence of lesions.21 Our finding suggests that surgery, the current treatment option is quite appropriate, in that, with the extensive excision, a good measure of the microbes and probably toxin are removed, therefore if systemic antimicrobial therapies are administered to support the surgery, all or most of any remaining microbes would be destroyed and relapses could be reduced or even prevented altogether.
The microbes disseminated sparingly into visceral organs and lymph nodes and without the manifestation of any recognizable BU lesion. On the other hand, the microbes disseminated massively into locations at the extremities, such as the rump, tail, contralateral limb and scrotum, with resultant exteriorization of the infection. This finding goes to confirm the microbe's preference for sites of lower temperature4. In view of the microbe's preference for cooler body areas, the heat therapy being advocated for the treatment of BU22 and the traditional treatment of sores with hot water compress in Ghana should be upheld. However, the former has drawbacks7 and should be improved to make it user-friendlier, while the traditional heat therapy should not be discouraged, as is currently the case but investigated further. This study indicates that probably some of the new lesions seen on BU patients (either at the same or different sites) may not necessarily be new infections but rather the exteriorization of an ongoing dissemination from a previous infection. This brings into question the classification of some BU patients as having inactive infection4, since our study suggests that the microbe may actually be actively disseminating to predilection sites to exteriorize after incubation.
Scanty AFBs were consistently found in all lymph nodes located at the major lymph node locations (the limb, the pelvis, thorax and head), but never in the blood smears sampled during the course of the disease and at the conclusion of the study. This suggests that the microbes are most probably disseminated through the lymphatic system and not haematogenously as speculated4,23.
The animals at a stage did not respond to needle pricks, which is suggestive of peripheral anaesthesia. This may be one of the reasons why BU is not usually associated with pain. The pathogenesis of M. ulcerans infection with respect to nerve involvement may be better understood if BU is studied in the light of what is known about leprosy.
At the terminal stage of the disease, the mice became generally oedematous and necropsy revealed involvement of lungs, livers, spleens and hypertrophied lymph nodes, which were also AFB positive. The swelling of the lymph nodes could also have resulted in impaired lymphatic drainage, a contributory factor to the development of the oedema. These findings coincide with observations made in terminally ill BU patients who present with widespread oedema, impaired renal function and other evidence suggesting visceral organ involvement4. Further animal studies are required to throw more light on visceral organ involvement and the terminal presentation of the disease as a whole.
On the whole, though the findings of the mouse study cannot be extrapolated in every respect, they have still provided us with insights that could inform us on the way forward.
Acknowledgments
We acknowledge with gratitude the generosity and assistance of the staff of the Department of Bacteriology of the Noguchi Memorial Institute for Medical Research, particularly, Mrs. Dorothy Yeboah-Manu, Ms. Mariko Minamikawa and Dr. Kwasi Addo. The study was funded by the Noguchi Memorial Institute for Medical Research.
References
- 1.Meyers WM, Tignokpa N, Priuli GB, Portaels F. Mycobacterium ulcerans infection (Buruli ulcer): first reported patients in Togo. Br J Dermatol. 1996;134:1116–1121. [PubMed] [Google Scholar]
- 2.Kreig RE, Hockmeyer WT, Connor DH. Toxin of Mycobacterium ulcerans. Production and effects in guinea pig skin. Arch Dermatol. 1974;110:783–788. doi: 10.1001/archderm.110.5.783. [DOI] [PubMed] [Google Scholar]
- 3.Read JK, Heiggie CM, Meyers WM, Connors DH. Cytotoxic activity of Mycobacterium ulcerans. Infect Immun. 1974;9:1114–1122. doi: 10.1128/iai.9.6.1114-1122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation, author. Buruli ulcer. 2001 Fact Sheet No 199. Available online at: http://www.who.int/inf-fs/en/fact199.htm.
- 5.Meyers WM. Mycobacterial infections of the skin. In: Seifert G, editor. Tropical dermatology. Heidelberg: Springer-Verlag; 1994. [Google Scholar]
- 6.MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93–122. [PubMed] [Google Scholar]
- 7.Thangaraj HS, Evans MRW, Wansbrough-Jones MH. Mycobacterium ulcerans disease; Buruli ulcer. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:337–340. doi: 10.1016/s0035-9203(99)90104-9. [DOI] [PubMed] [Google Scholar]
- 8.Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoah K. Buruli ulcer in Ghana: Results of a national case search. Emerg Infect Dis. 2002;8:167–170. doi: 10.3201/eid0802.010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barneston RS. Skin diseases in the Tropics. Med J Aust. 1993;159:321–325. doi: 10.5694/j.1326-5377.1993.tb137869.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation, author. Buruli ulcer disease: Mycobacterium ulcerans infection. Wkly Epidemiol Rec. 2003;78:163–168. [PubMed] [Google Scholar]
- 11.Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horsfield C, Phillips R, Evans M, Ofori-Adjei D, Klustse E, Owusu-Boateng J, Amedofu GK, Awuah P, Ampadu E, Amofah G, Asiedu K, Wansbrough-Jones M. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005;8:3182–3186. doi: 10.1128/AAC.49.8.3182-3186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation, author. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer) Geneva: WHO; 2004. [Google Scholar]
- 13.Migaki G, Capen CC. Animal models in biomedical research. In: Fox JG, Cohen BJ, Loew FM, editors. Laboratory animal medicine. Academic Press, Inc.; 1984. [Google Scholar]
- 14.Pattyn SR, Royackers J. Treatment of experimental infection by Mycobacterium ulcerans and Mycobacterium balnei in mice. Ann Soc belg Med Trop. 1965;45:31–38. [PubMed] [Google Scholar]
- 15.Walsh DS, Meyers WM, Kreig RE, Walsh GP. Transmission of Mycobacterium ulcerans to the nine-banded armadillo. Am J TropMed Hyg. 1999;61(5):694–697. doi: 10.4269/ajtmh.1999.61.694. [DOI] [PubMed] [Google Scholar]
- 16.Palomino JC, Portaels F. Effects of decontamination methods and culture conditions on viability of Mycobacterium ulcerans in the BACTEC system. J Clin Microbiology. 1998;36:402–408. doi: 10.1128/jcm.36.2.402-408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mensah-Quianoo E. A study of the magnitude and determinants of Buruli ulcer disease in the Ga District of Ghana. 2004 Available online: http://www.who.int/gtb-buruli/archives/Yamoussoukro/abstracts/ernestina.htm.
- 18.Raghuanathan PL, Whitney EAS, Asamoa K, Stienstra Y, Taylor TH, Jr, Amofah GK, Ofori-Adjei D, Dobos K, Guarner J, Martin S, Pathak S, Klutse E, Etuaful S, van der Graaf WTA, van der Werf TS, King CH, Tappero JW, Ashford d. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): Results from a case-control study in Ghana. Clin Infectious Diseases. 2005;40:1445–1453. doi: 10.1086/429623. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PDR, Stinear T, Small PLC, Plushke G, Merrit RW, Portaels F, Huygen K, Hayman JA, Asiedu K. Buruli ulcer (M. ulcerans Infection): New insights, new hope for disease control. PLoS Med. 2005;2(5):e173. doi: 10.1371/journal.pmed.0020108. Available online at DOI: 10.1371/journal.pmed.0020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George K M, Chaterjee D, Geewananda G, Welty D, Hayman J, Lee R, Small PL. Mycolactone: a polykitide toxin from Mycobacterium ulcerans. Infect Immun. 1999;66:587–593. [Google Scholar]
- 21.Amofah GK. Effectiveness of excision of the pre-ulcerative Buruli lesions and wound dressing of Buruli ulcer in field stations in a rural district in Ghana. 2004 doi: 10.1177/004947559802800208. http://www.who.int/gtb-buruli/archives/Yamoussoukro/abstracts/amofah.htm. [DOI] [PubMed]
- 22.Glynn PJ. The use of surgery and local temperature elevation in Mycobacterium ulcerans infection. Australian and New Zealand Journal of Surgery. 1972;41:312–317. [PubMed] [Google Scholar]
- 23.Portaels F, Traore H, De Ridder K, Meyers WM. In vitro susceptibility of Mycobacterium ulcerans to Clarithromycin. Antimicrobial Agents and Chemotherapy. 1998;42:2070–2073. doi: 10.1128/aac.42.8.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]