Summary
We present a case of disseminated invasive aspergillosis in a young male adult with progressive complex gastrointestinal, non-specific peripheral neural and respiratory symptoms and subsequent acute haemorrhagic conjunctivitis and generalized dermatitis.
The case emphasizes the difficulty in diagnosing systemic mycosis and a high index of suspicion is recommended particularly in patients on prolonged antibiotics and systemic steroids.
Keywords: Invasive aspergillosis, systemic mycosis
Introduction
Systemic mycosis is an uncommon disease which most often affects immunosupressed patients. The incidence of systemic mycoses at postmortem in different studies varies from 0.98% to 10.41,2. There is an overall tendency towards an increase in the number of cases world-wide, however, cases in which mycosis is the immediate cause of death have decreased2. Aspergillosis is caused by Aspergillus fumigatum, flavus and less commonly by A. terreus, nidulans and niger. The most common portal of entry is the respiratory system. Aspergillus fumigatum is the most prevalent fungal pathogen responsible for fatal invasive aspergillosis. In an immunosupressed host, the infection may present as allergic sinusitis, bronchitis or localized pulmonary infection superimposed on underlying chronic lung disease. It is rarely a primary cutaneous lesion in immunocompetent patients4,5.
The major predisposing factors for development or progression to invasive aspergillosis are: deficiency of agglutinating surface surfactant proteins and C3, C5 complement factors, inhibition of anticonidial macrophageal activity, thrombocytopenia and neutropenia, low count of CD4 + T lymphocytes or failure to produce IL (interleukin)- 12, INF (interferon) or TNF (tumour necrosis factor), prolonged antibiotic or steroids therapy6–10. In the presence of severe granulocytopenia and in patients with cerebral abscesses mortality is as high as 100%11.
Case Report
A 20 year old student was transferred from a Polyclinic to the Korle Bu Teaching Hospital (KBTH) with a 2 week history of fever, ascending stiffness and generalized body pain. He also complained of headache, chest pain with productive cough, progressive abdominal pain, vomiting and diarrhea with mucus and frank blood. There was a 5 day history of difficulty in breathing, sore eyes and mouth. There was no previous history of chronic disease or admission to a hospital. He had sickle cell AS trait. During the present illness he had been treated at a private hospital and herbal centre for malaria, typhoid fever and measles. Examination revealed an ill looking febrile young male adult with mouth sores and reddish conjunctiva with purulent discharge. He had tachypnoea with respiratory rate of 36 per minute, chest wall tenderness and generalized tenderness of the abdomen. He was conscious and alert, neck was supple and Kernig's was negative with normal muscle tone and power. An initial diagnosis of enteric fever and drug reaction were made and treatment with IV fluids, metoclopramide 10mg/8hrs, IV ranitidine 50mg/8hrs, IV ciprofloxacin 200mg/12hrs, IV tramadol 50mg/8hrs, quinine 20mg/kg in 10% dextrose, was started.
The initial haemorglobin level was 14.8 gm/dl and a high white blood cell count of 15.1K/uL with 95% granulocytes. There was elevated anion gap (27.4 to 21.5mmol/L), liver transaminases and bilirubin were also high (GOT (AST) 135 U/L, GPT (ALT) 147 U/L, Alkaline Phos 273 U/L, GGT 305 U/L). Urine examination showed haematuria and granular casts. Culture of urine did not show bacterial growth. The stool showed white blood cells only and no Salmonella or Shigella were isolated. Culture of blood showed no bacterial growth. HIV test was negative. Despite treatment, the patient did not improve, but continued to have repeated episodes of loose watery or bloody stool, haematemesis, progressive fever, palor and weight loss and a hyperaemic rash, which started from the face and later became generalized and hyperemic conjunctivae with purulent discharge. Upper GI endoscopy (day 9) showed haemorrhagic oesophagitis and multiple whitish patches, haemorrhagic gastritis with multiple erosions, blood with clots in the stomach and bulb of the duodenum. Oral Nystatin was started (100.000 units/4 times daily for 5 days).
Colonoscopy (day 14) was limited only to rectosigmoid colon due to severely inflamed, oedematous and haemorrhagic mucosa. A diagnosis of acute inflammatory bowel disease suggestive of Crohn's colitis or ulcerative colitis was made and treatment with systemic corticosteroids (Hydrocortisone/Prednisolone) were started and administered for 9 days prior to death on the basis of the clinical findings of severe colonic haemorrhagic inflammation.
Histological examination of rectal and sigmoid mucosa revealed marked infiltration of the lamina propria by acute and chronic inflammatory cells with replacement of normal mucosal architecture with only few glands present exhibiting marked degree of dysplastic changes of the glandular epithelium. Conclusive diagnosis of inflammatory bowel disease could not be made due to limited biopsy, however marked dysplasia (high grade) suggested possibility of it, with possible further progression into carcinoma in situ. During admission patient was transfused with a total 14 units of whole blood. However, his condition worsened and he died on the 22nd day of admission.
At postmortem the body was that of the young male adult dehydrated, pale and jaundiced with oral ulcers and harmorrhagic conjunctivae. The heart was normal.
Cut sections of both lungs revealed multiple well circumscribed necrotic areas ranging in size from 1 to 2.5cm across. The oesophagus showed a severely inflamed mucosa with multiple ulcers. The stomach contained 400ml of blood clots, mucosa was haemorrhagic with large fundal ulcers ranging in diameter from 2 to 5 cm. The small intestine and colon contained watery stool, and showed haemorhagic mucosa with multiple ulcers, most prominent and confluent in the small intestine. The liver was normal in size and was mildly congested but not septic. Both kidneys were oedematous with pale, yellow subcapsular surface, pale cortex and congested medulla consistent with shock. The brain was heavy (1370gm) and oedematous; the meninges showed focal yellowish cloudy areas and severely congested vessels. Cut sections of the brain showed a large poorly circumscribed necrotic area in the right ceberal hemisphere.
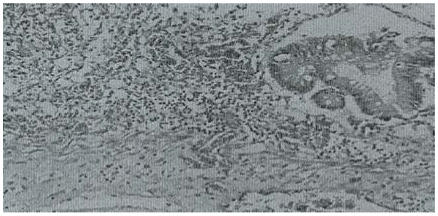
Histological examination of the small intestines and colon showed extensive mucosal ulceration, bacterial overgrowth, severe vascular congestion and infiltration of the lamina propria by mixed inflammatory cells. Many vessels showed fibrin masses admixed with fungal hyphae that had also penetrated the walls. There were a few colonic glands lined by stratified epithelium with loss of polarity and normal mitotic figures (Figure 1).
Figure 1.
Colon with ulceration, mixed inflammatory infiltrate in the wall and fungal mycelia. H & E stain. X 100
A review of the histological slides from the rectosigmoid mucosa taken from the patient on admission did not show any evidence of fungal hyphae.
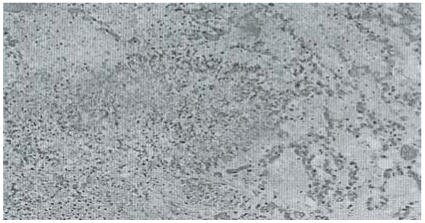
The lungs showed vascular congestion and oedema with multiple circumscribed nodules composed of masses of fibrinoid necrosis admixed with fungal colonies (Figure 2).
Figure 2.
Abscess in the lung with mycelia growth. H & E stain. X100
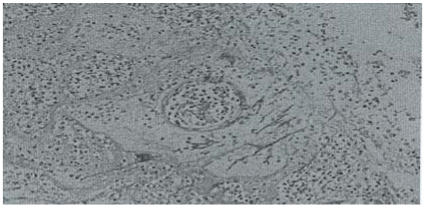
Similar lesions were noted in the white matter of the cerebral hemisphere with vascular invasion of the cerebral and leptomeningeal vessels as well (Figure 3).
Figure 3.
Cerebral abscesses with fungal growth. H & E stain. X 100
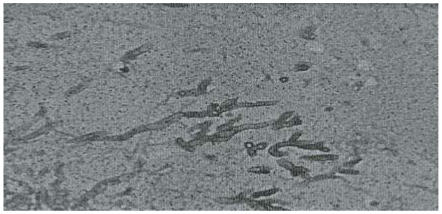
Section of the kidneys showed marked vascular congestion, focal tubular necrosis with hylaline and pigmented casts consistent with shock (hypo-volaemic and endotoxic). Periodic acid Schiff (PAS) stain of the fungus demonstrated the distinct dichotomous acute angle branching of septate hyphae with orientation of the branches in similar direction (Figure 4). The postmortem diagnosis made was disseminated invasive aspergillosis and endotoxic shock.
Figure 4.
Aspergillus species. PAS stain X 400
Discussion
In the past aspergillus was considered as a weak pathogen and cases of disease in immunocompetent host were regarded as scientific curiosity. More recently with increase in the number of immunosupressed patients, aspergillus became the most common pathogenic mould worldwide. The average incidence of invasive aspergillosis at autopsy is 0.19%10. It is estimated to be 5 to 25% in patients with acute leukemia and 0.5 to 5% after cytotoxic treatment of blood disease12,13. It is increasingly being reported in AIDS patients (1–12%) and also a common complication of chronic granulomatous disease (25–40%)9. The most common symptoms of invasive aspergillosis are non-specific and include: fever, chest pain, cough, malaise, weight loss and dyspnoea. However, approximately 41% of patients with invasive aspergillosis have no respiratory symptoms and corticosteroids treated patient frequently do not have elevated temperature14,15.
Invasive aspegillosis still remains a big clinical challenge and diagnosis relies largely on histopathological evidence of mycelial growth in tissue. Unfortunately, for many such cases where histology is most needed, this diagnosis becomes available only at autopsy16,17.
The culture or microscopic examination of sputum and bronchoalveolar lavage specimens can be used despite the observation that a positive test may only mean colonization of the airways by airborne conidia and not invasive aspergillosis18. For high risk groups a positive test is presumptive of invasive aspergillosis19. A recently developed enzyme-linked immunosorbent assay (ELISA) test is very specific for aspergillus but only helpful for diagnosis of allergic bronchoalveolar aspergillosis20.
Predisposing factors should be taken into account and if such are present empirical therapy started immediately, due to rapid progression of the disease and its severity.
In this case the predisposing factors were obscure. Prolonged antibiotic use before presentation at the polyclinic cannot be excluded since he was being treated for “typhoid” at the private hospital. Most likely the aspergillosis was present before hospital admission. The continued use of antibiotics and subsequent use of steroids only worsened the disease course. The few obscure pointers to mycotic disease were the white patches in the oesophagus seen at endoscopy, which necessitated treatment with nystatin for presumed candidiasis, and the negative culture of stools and blood. In this case, the premortem histological slides showed absence of fungal hyphae in the colonic mucosal samples examined. The reason for the negative histology finding in the colonic specimen may have been that
the severe colitis did not allow extensive sampling
the small intestine was predominantly involved
the fungal hyphae were deeper within the wall of the intestine
The vascular invasion by the fungal hyphae seen in the autopsy samples of bowel most likely led to infarction of the mucosa with extensive ulceration and would explain the severe haemorrhagic colitis seen on colonoscopy.
A high index of suspicion, vigorous search for fungi by cultures and empirical treatment for highly suspicious cases remain the mainstay of management of these cases.
References
- 1.Kedzeirska J, Wegryn J, Kedziersa A, Pietzyk A. Bacterial and fungal bloodstream infections in patients at the university hospital in Cracov. Med Dosw Mikrobiol. 2003;55(3):259–270. [PubMed] [Google Scholar]
- 2.Kochy S, Hohne FM, Tietz HJ. Incidence of systemic mycoses in autopsy material. Mycoses. 2004 Feb;47(1–2):40–46. doi: 10.1046/j.0933-7407.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 3.Romano C, Miracco C. Primary cutaneous aspergillosis in an immunocompetnet patient. Mycoses. 2003 Feb;46(1–2):56–59. doi: 10.1046/j.1439-0507.2003.00836.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornet M, Mallat H, Somme D, et al. Fulminant invasive aspergillosis in immunocompetent patients-Two case report. Clin Microbiol Infect. 2003 Dec;9(12):1224–1227. doi: 10.1111/j.1469-0691.2003.00792.x. [DOI] [PubMed] [Google Scholar]
- 5.Karim M, Alam M, Shah AA, Ahmed R, Sheik H. Chronic invasive aspergillosis in apparently immunocompetent hosts. Clinc Infect Dis. 24:723–733. doi: 10.1093/clind/24.4.723. [DOI] [PubMed] [Google Scholar]
- 6.Braedel S, Radsak M, Einsele H, Latge JP, Michan A, et al. Aspergi: Ilus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br J Haematol. 2004 May;125(3):392–399. doi: 10.1111/j.1365-2141.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 7.Philippe B, Ibrahim-Granet O, Prevost MC, et al. Killing of aspergillus fumigatus by alveolar macrophages in mediated by reactive oxidant intermediates. Infect Immun. 2003 Jun;71(6):3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, et al. Phagocytosis and intercellular fate of aspergillus fumigatus conidia in alveolar macrophages. Infect Immun. 2003 Feb;71(2):891–903. doi: 10.1128/IAI.71.2.891-903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microb Rev. 1999 Apr;12(2):310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaideeswar P, Presed S, Deshande JR, Pandit SP. Invasive pulmonary aspergillosis: A study of 39 cases at autopsy. J Postgrad Med. 2004 Jan–Mar;50(1):21–26. [PubMed] [Google Scholar]
- 11.E-Medicine. 2001. Aspergillosis. Technical information. [Google Scholar]
- 12.Denning DW. Issues in the management of invasive aspergillosis. Ann Med Intern. 1995;146:106–110. [PubMed] [Google Scholar]
- 13.Denning DW. Therepeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–614. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 14.Ribaud P, Chastang C, Latge JP, Baffroy L. Survival and prognostic factors of invasive aspergillosis after allopenic bone marrow transplantation. Clin Infect Dis. 1999;28:322–330. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 15.Saugier-Veber P, Devergie A, Sulahian A. Epidemiology and diagnosis of invasive pulmonary aspergillosis in bone marrow transplantation patient: result of a five-year retrospective study. Bone Marrow. 1993;12:121–124. [PubMed] [Google Scholar]
- 16.Groll AH, Shah PM, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996 Jul;33(1):23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 17.Boon AP, O'Brien D, Adams DH. Ten years review of invasive aspergillosis detected at necroscopy. J Clin Path. 1991;44:452–454. doi: 10.1136/jcp.44.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher BD, Armstrong D, Yu B, Gold JW. Invasive aspergillosis. Progress in early diagnosis and treatment. Am J Med. 1981 Oct;71(4):571–577. doi: 10.1016/0002-9343(81)90208-4. [DOI] [PubMed] [Google Scholar]
- 19.Treger TR, Visscher DW, Bartlett MS, Smith JW. Diagnosis of pulmonary infection caused by Aspergillus: usefulness of respiratory cultures. J Infect Dis. 1985 Sep;152(3):572–576. doi: 10.1093/infdis/152.3.572. [DOI] [PubMed] [Google Scholar]
- 20.Raghuwanshi SK, Kumar M, Kavishwar A, Chaturvedi AK, Murthy PS, Shukla Immunocolonization of secretary proteins of Aspergillus fumigatus using polyclonal antibodies in a murine model. Mycoses. 2005 Sep;48(5):313–320. doi: 10.1111/j.1439-0507.2005.01141.x. [DOI] [PubMed] [Google Scholar]