Summary
Rational
Female genital mutilation (FGM) is prevalent in northern Ghana, as the practice is seen as a passage rite to women adulthood and thus undertaken just before marriage.
Objectives
We determined the changes in trend of FGM in deliveries at the Navrongo War Memorial hospital, and compared the outcomes and FGM status.
Design
Retrospective extraction and analysis of delivery data at the hospital from 1st January 1996 to 31st December 2003.
Results
Of the 5071 deliveries, about 29% (1466/5071) were associated with FGM. The highest prevalence (95% CI) of 61.5% (50.9, 71.2) was in women aged 40 years and above, and the lowest of 14.4% (11.7, 17.0) was in women below 20 years. The all-age prevalence of FGM showed a significant decline (p-value for linear trend < 0.01) from 35.2% in 1996 to 21.1% in 2003. About 6% (89/1466) of mothers with FGM had stillbirths compared with about 3% (123/3605) of mothers without FGM. Again FGM was associated with 8.2% (120/1466) caesarean section rate compared with 6.7% (241/3605) in mothers without FGM. Mean birth weight and frequency of low birth weights were not significantly associated with FGM status.
Conclusion
Although there is a high rate of FGM among mothers in the district and is associated with a higher proportion of stillbirths and caesarean sections, practice has shown a significant decline in the district in recent years due to the prevailing campaigns and intervention studies. There is therefore the need to sustain the ongoing intervention efforts.
Keywords: Delivery, outcomes, female genital mutilation
Introduction
The practice of female genital mutilation (FGM) regrettably persists in many parts of the developing world where it is firmly anchored in culture and traditions1,2. The procedure involves partial or total removal of the external female genitalia or other injury to the female genital organs for cultural, religious or other non-therapeutic reasons. The practice is coded into four types3, the most common type (II) account for up to 80% of all cases, while the most extreme type (III) constitutes about 15% of the total procedures. The health consequences vary according to the type and severity of the procedure performed4. Immediate complications include severe pain, shock, hemorrhage, urine retention, and injury to adjacent tissue or death. Long-term consequences include urinary incontinence, painful sexual intercourse, sexual dysfunction and difficulties with childbirth5. In recent times, there have been concerns about the potential risk of transmission of the human immunodeficiency virus due to the practice3. In the longer term, many victims suffer feelings of incompleteness, anxiety and depression6.
In Ghana, FGM is prevalent in the northern parts of the country, where type II is documented to be the commonest form of the practice7–9. In this area, FGM is seen as a passage rite in adulthood and is therefore undertaken just before marriage. Though recent evidence indicates the practice is undergoing a major decline, accurate measurements of individuals' status, which is necessary for proper evaluation of intervention studies, poses practical and ethical challenges. This is due to denial among respondents and also due to the criminalization of the practice in recent years9,10. Moreover, no study to date, has described the relationship between the FGM practice and delivery outcomes in northern Ghana.
The main objective of this study was therefore to examine the changes in trend of the practice over the period among women delivering in the district hospital and compare the delivery outcomes and female genital mutilation status.
Methods
Study area: The study was conducted in the War Memorial Hospital (WMH) located in the Kassena-Nankana District (KND) of northern Ghana. The WMH is a district health facility that offers secondary clinical and public health services. The KND has a mixture of rural and urban settlements with majority of the inhabitants being subsistence farmers. The district has two major ethnic groups with two distinct languages but with homogeneous socio-cultural and economic institutions. The total population of the district is estimated to be 151 000 with about 15% being women of childbearing age. The total fertility rate is 4.5 and an estimated 4000 live births are recorded per year11.
Study design: This study examined all deliveries in the district hospital from 1st January 1996 to 31st December 2003. Delivery data is systematically documented using a structured delivery record book. This information included maternal age, type of delivery, genital mutilation status, previous obstetric history and delivery outcome among others. A questionnaire was designed and used to extract the necessary data for the study. Two steps were used: first, all records on deliveries in the labour and lying in wards were reviewed and the required information extracted. The second step was by confirming the extracted data using confidential patient records from the medical records department. Two physicians working initially in parallel did all the extractions, and completed the study questionnaire in order to minimize bias and errors. To ensure confidentiality, all data extraction and analysis were done anonymously and unlinked. The hospital administration granted permission for the conduct of the study.
Data analysis: All the extracted data were analysed using EPI info version 3.3.2 (2002) and Statatm version 8 softwares. The analysis focused first on the entire data set and then highlighted the relationship between maternal age, parity and female genital mutilation on birth outcomes: mean birth weight, low birth weight, stillbirths and caesarean section. We described the characteristics of the stated outcomes and looked for trends by doing multiple strata comparisons among the outcomes. The statistical tests used were student's t tests for all continuous variables and χ2 tests for categorical variables. Point estimates were computed and presented as means, proportions or percentages. Interval estimates are presented in 95% confidence intervals or ranges. Odds ratio and associated 95% confidence intervals were calculated to estimate the magnitude of risk associated with various maternal characteristics and specific birth outcomes. Chi square test for linear trend in prevalence of female genital mutilation was also computed. All statistical tests were two sided and an alpha level < =0.05 was considered significant.
Results
Baseline Characteristics: In all, 5071 maternal deliveries were analysed. The average maternal age was 25.8 years (range 14–55 years), 14.6% were in their teens and about 2.0% (99/5071) had advanced maternal age of 40 years and above. About 35% (1795/5071) of mothers were primiparous.
Overall prevalence of FGM was 29% (1466/5071). The highest prevalence (95%CI) of female genital mutilation of 61.5% (95% 50.9, 71.2) was in the age group 40 years and above, and the lowest prevalence (95%CI) of 14.4% (11.7, 17.0) was in the teenage mothers. Less than half, 47.3% (95% CI 45.9, 48.7) of the babies delivered were females, the total average weight at birth was 2.85 kg (95%CI 2.83, 2.86) and about 16% (814/5165) weighed less than 2.5 kilograms. Also about 7% (361/5071) of the total deliveries were by caesarean section (Table 1).
Table 1.
Baseline characteristics of the circumcised and uncircumcised mothers
| Characteristics | Genital mutilation (n=1466) |
No genital mutilation (n=3605) |
| Mean maternal age, years (SD) |
28.5(6.3) | 24.7(5.5) |
| Maternal age < 20 years (%) |
107 (7.3) | 634 (17.6) |
| Mean maternal parity (SD) |
3.6(2.0) | 2.3(1.5) |
| No of primiparous mothers (%) |
259 (17.7) | 1536 (42.6) |
| No. of Caesarean section (%) |
120(8.2) | 241(6.7) |
| Mean birth weight, gms (SD) |
2.87(0.5) | 2.84(0.48) |
| No. of babies < 2500 (%) |
230 (15.7) | 584(16.2) |
| No. of female babies (%) |
730(48.9) | 1711(46.6) |
| No. of live births (%) |
1402(93.9) | 3547(96.6) |
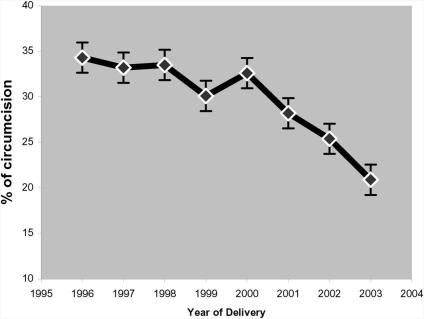
Trends in female genital mutilation: The results show a declining trend in prevalence of FGM among the mothers from 34.3% in 1996 to 20.9% in 2003 (Figure 1).
Figure 1.
Prevalence of FGM among mothers delivering at the WMH (1996–2003)
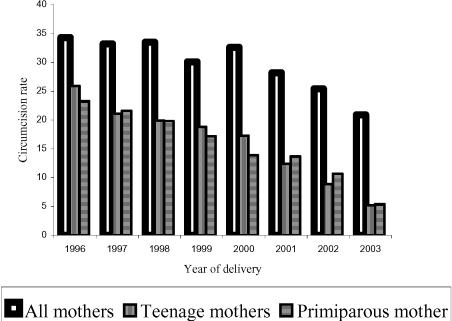
Compared with the prevalence of 1996, levels found in 2002 were not significantly different (p-value > 0.05) but the 2003 levels showed a statistically significant decline (p-value < 0.001). In 2003, of the total 858 hospital deliveries, only 179 (20.9%) were circumcised. Moreover, the prevalence of genital mutilation among mothers less than 20 years in 1996, 1999 and 2003 were 25.9%, 18.8% and 5.2% respectively (Figure 2), thus showing a significant decline over the period in this young maternal age group where most of female genital mutilation occurs in the district. During the same period, genital mutilation rate among mothers in the next higher age group, 20–29 years, were 35.2, 27.8 and 16.6 percent respectively, confirming the significant decline in the practice. While the all age group change in prevalence in mothers was 1.7% (34.3% to 32.6%) from 1996 to 2000 a period of four years, the change in prevalence significantly increased to 11.7% (32.6% to 20.9%) from 2000 to 2003 (p-value for linear trend < 0.01) in a shorter period of three-years. The same trend of prevalence was observed among primiparous mothers (Figure 2).
Figure 2.
Annual variations of genital mutilation rates in maternal age and parity groups.
Birth Outcomes and Female Genital Mutilation The babies delivered by circumcised mothers were on the average 30 grams (0.00, 0.06; p-value =0.04) heavier than babies of uncircumcised mothers (2870 vs. 2840 grams), this difference was however not statistically significant when maternal age was taken into consideration. When the analysis was stratified on maternal age the mean birth weight among circumcised and uncircumcised mothers aged less than 20years, 20–30 years and > 30 years were similar: 2642 vs. 2664; 2863 vs.2867; and 2956 vs.2951 grams respectively. The same trend was observed if stratified on maternal parity categorized as one, two or more than two. The respective birth weights among circumcised and uncircumcised mothers were 2664 vs. 2707; 2855 vs.2902; 2742 vs.2939 grams.
The frequency of low birth weight among circumcised mothers was 15.4% compared with 15.9% among non-circumcised mothers (Table 2). On the other hand, female genital mutilation was significantly associated with higher stillbirth rate (6.1% vs. 3.4%: p-value <0.001). Babies from circumcised mothers were approximately twice as likely to be stillborn compared with the non-circumcised mothers [OR (95% CI) = 1.8 (1.4, 2.4); p value < 0.01]. This finding changes only in the age group of lowest prevalence if the data is stratified on maternal age or parity (Table 2). The caesarean section rate in circumcised mothers compared with non-circumcised mothers was higher but not statistically significant: 8.2% (120/1466) vs. 6.7% (241/3605 p-value = 0.06). The respective comparative caesarean section rates among the maternal age and parity groups were also similar (Table 2).
Table 2.
Comparison of delivery outcomes and female genital mutilation status
| Characteristics | FGM n (%) | No FGM, n (%) | OR [95% CI] | p-value |
| Caesarean births | ||||
| Total Caesarean births | 120 (8.2) | 241(6.7) | 1.24 [0.98,1.57] | 0.068 |
| Maternal age < 20 | 09 (8.4) | 37(5.8) | 1.48 [0.61,3.25] | 0.421 |
| Maternal age >20 | 111 (8.2) | 204(6.9) | 1.21 [0.94,1.55] | 0.142 |
| Maternal parity =1 | 24 (9.3) | 103(6.7) | 1.42 [0.87,2.31 | 0.175 |
| Maternal parity >1 | 96 (8.0) | 138(6.7) | 1.21 [0.91,1.60] | 0.191 |
| Still births | ||||
| Total stillbirths | 91 (6.1) | 125 (3.4) | 1.84 [1.38,2.45] | 0.000 |
| Maternal age < 20 | 9 (8.3) | 27 (4.2) | 2.06 [0.83,4.68] | 0.108 |
| Maternal age >20- | 82 (5.9) | 98 (3.2) | 1.87 [1.37,2.56] | 0.000 |
| Maternal parity =1 | 18 (6.8) | 57 (3.6) | 1.94 [1.08,3.44] | 0.025 |
| Maternal party >1 | 73 (5.9) | 68 (3.2) | 1.89 [1.33,2.69] | 0.000 |
| Low birthweight | ||||
| Total low birthweight | 230 (15.4) | 584 (15.9) | 0.96 [0.81,1.14] | 0.686 |
| Maternal age < 20 | 30 (27.5) | 177 (27.4) | 1.01 [0.62,1.62] | 0.928 |
| Maternal age > 20 | 200 (14.5) | 407 (13.5) | 1.09 [0.9,1.31] | 0.403 |
| Maternal parity =1 | 70 (26.5) | 360 (23.0) | 1.21 [0.89,1.64] | 0.243 |
| Maternal party >1 | 160 (13.1) | 244 (11.6) | 1.14 [0.92,1.42] | 0.240 |
Discussion
Efforts to eliminate female genital mutilation (FGM) have often been unsuccessful because opponents of the practice have ignored its social and economic contexts. In some cases, external intervention strengthened the resolve of communities to continue their genital cutting rituals as a way of resisting what they perceive as cultural imperialism2,12,13. Thus FGM continues to abuse and violate international standards of rights of women as innate sexual beings14.
In the Kassena-Nankana district of northern Ghana where this study was conducted, female genital mutilation has been part of the passage rite in adulthood for decades7,8. Recent evidence suggests a major decline, attributable to the culturally appropriate community intervention embarked upon by the Navrongo Health Research Centre9. This assertion is confirmed by our results which showed a significant decline in the practice from 1996 (34.3%) to 2003 (20.9%). The decline is of recent occurrence because while there was 1.7 % decline in prevalence from 1996 to 2000, this significantly increased to over 10% from 2000 to 2003 predominantly in the teenage and primiparous mothers (Figure 2). The period of major decline coincided with the period of the promulgation of the law banning the practice in 1994 and the experimental intervention introduced by the Navrongo Health Research Center9. The decline may therefore be attributed to the combined effect of these two interventions. However, given the lack of effectiveness of enforcement of the law against such cultural practice, it is unclear whether the major decline is significantly associated with the legal approach.
Again the study provides evidence that despite the overall lack of consistency in self-reporting status among women in the study area in recent times 9,, significant changes in the practice seem to be occurring in the Kassena-Nankana District. The substantial decline is therefore likely to be due to the experimental intervention introduced by the Research Centre, which utilized culturally appropriate health promotion and communication, coupled with the growing public informational campaigns against the practice.
On the effect of female genital mutilation on foetal outcomes, the study showed that female genital mutilation was significantly associated with higher stillbirth rate in contrast with some earlier reports15,16. Babies from circumcised mothers were about two times more likely to be stillborn when compared with babies of uncircumcised mothers. Stillbirth rate among teenage circumcised mothers was also more than two times higher when compared with teenage non-circumcised mothers.
There is no doubt that in some cases, without surgical intervention before childbirth among circumcised mothers, prolonged labour may occur, causing life-threatening complications for both mother and infant17,18. Moreover, the conditions under which female genital mutilation is generally performed can lead to potentially fatal complications even in the less extensive forms. The type II and III are particularly likely to cause long-term health problems including birth complications, lowered fertility and reduction in a woman's ability to experience sexual pleasure. This not withstanding other suboptimal factors may be contributing to the high perinatal death among circumcised women in the study area which may need other longitudinal studies to clarify.
We showed in this study that the average weight at birth and the frequency of low birth weight among circumcised mothers and uncircumcised mothers were similar.
Given the reported complications and related consequences of the practice, it is often asked why the practice is still continuing. Reasons may be that it is unclear how frequently such problems occur, for little clinical data exist and what is available comes from small studies or is based on self-reports. Moreover, in societies where few women remain uncircumcised, problems arising from female genital mutilation are likely to be seen as a normal part of a woman's life and may not even be associated with genital mutilation. While the most important reasons may probably lie in the social and economic conditions of women's lives in many of such settings2, it is appropriate to document the adverse effects associated with the practice in our endemic setting as we have shown in this study.
This study is limited because it is based on secondary data and in a setting where significant number of deliveries is conducted at home. Also, as a retrospective study, some important obstetric outcomes and indications were not consistently documented and therefore could not be used for further sub-analysis. However, as a maiden study to document the trend in FGM status in our setting, the results do provide significant evidence for the prevailing situation and forms the basis for which future studies can be designed, conducted and compared.
Conclusion
We conclude that, the high rate of female genital mutilation among mothers in the Kassena-Nankana district is associated with a higher proportion of stillbirth, and caesarean sections though this procedure is not statistically significant. These not withstanding, other factors may be contributing to these high rates among circumcised women in the study area which need longitudinal studies to clarify. The practice has, however, shown a significant and dramatic decline in the district in the last few years due to the prevailing community interventions and campaigns which must to be sustained.
Acknowledgement
We are most indebted to the mothers and babies whose records were used. We also wish to thank all the staff and management of the Navrongo District Hospital and the Navrongo Health Research Centre (NHRC) for their invaluable assistance. We are particularly grateful to Mr. Raymond Aborigo of NHRC for the initial editing and proof reading of the manuscript.
References
- 1.Althaus FA. Female Circumcision: Rite of Passage Or Violation of Rights? Int Fam Plan Perspec- Special report. 1994:23. [Google Scholar]
- 2.Dorkenoo E. Combating female genital mutilation: an agenda for the next decade. Women's Studies Quarterly. 1999:1–2. [PubMed] [Google Scholar]
- 3.World Health Organization, author. Female Genital Mutilation: Report of a WHO Technical Working Group, Geneva. 1996
- 4.Morison L, et al. The long-term reproductive health consequences of female genital cutting in rural Gambia: a community-based survey. Trop Med Int Hlth. 2001;6:643–653. doi: 10.1046/j.1365-3156.2001.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones H, Diop N, Askew I, et al. Female genital cutting practices in Burkina Faso and Mali and their negative health outcomes. Stud Fam Plann. 1999;30:219–230. doi: 10.1111/j.1728-4465.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahran M. Medical dangers of female circumcision. IPPF Med Bull. 1981;15:1–3. [PubMed] [Google Scholar]
- 7.Adongo P, Akweongo P, Binka FN, et al. Female genital mutilation: Sociocultural factors that Influence the Practice in Kassena-Nankana District, Ghana. Afr J Reprod Health. 1998;2:25–36. [Google Scholar]
- 8.Mbacke C, Adongo P, Akweongo P, et al. Prevalence and Correlates of Female Genital Mutilation in the Kassena-Nanakana District of Northern Ghana. Afr J Reprod Hlth. 1998;2:13–24. [Google Scholar]
- 9.Jackson EF, Akweongo P, Sakeah E, et al. Inconsistent Reporting of Female Genital Cutting Status in Northern Ghana: Explanatory factors and analytical consequences. Stud Fam Plann. 2003;34:200–209. doi: 10.1111/j.1728-4465.2003.00200.x. [DOI] [PubMed] [Google Scholar]
- 10.Snow RC, Slanger TE, Okonofua FE, et al. Female genital cutting in southern urban and periurban Nigeria: Self-reported validity, social determinants and secular decline. Trop Med Int Hlth. 2002;7:91–100. doi: 10.1046/j.1365-3156.2002.00829.x. [DOI] [PubMed] [Google Scholar]
- 11.Debpuur C, Phillips J, Jackson EF, et al. The Impact of the Navrongo project on contraceptive knowledge and use of, reproductive preferences, and Fertility. Stud Fam Plann. 2002;33:141–164. doi: 10.1111/j.1728-4465.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- 12.Gruenbaum E. The cultural debate over female circumcision: the Sudanese are arguing this one out for themselves. Med Anthropol Q. 1996;10:455–475. doi: 10.1525/maq.1996.10.4.02a00030. [DOI] [PubMed] [Google Scholar]
- 13.Female genital mutilation: is it crime or culture? The Economist. 1999 [Google Scholar]
- 14.Cook RJ, Dicken BM, Fathalla MF. Female genital cutting (mutilation/circumcision): ethical and legal dimensions. Int J Gynaecol Obstet. 2002;79:281–287. doi: 10.1016/s0020-7292(02)00277-1. [DOI] [PubMed] [Google Scholar]
- 15.Essen B, Bodker B, Sjoberg NO, et al. Is there an association between female circumcision and perinatal death? Bull WHO. 2002;80:629–632. [PMC free article] [PubMed] [Google Scholar]
- 16.Hakim LY. Impact of female genital mutilation on maternal and neonatal outcomes during parturition. East Afr Med J. 2001;78:255–258. [PubMed] [Google Scholar]
- 17.Slanger T, Snow R, Okonofua F. The impact of female genital mutilation on first delivery in southwest Nigeria. Stud Fam Plann. 2002;33:173–184. doi: 10.1111/j.1728-4465.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsen U, Okonofua FE. Female circumcision and obstetrics complications. Int J Gynaecol Obstet. 2002;77:255–326. doi: 10.1016/s0020-7292(02)00028-0. [DOI] [PubMed] [Google Scholar]