Abstract
The normal diurnal cortisol cycle has a peak in the morning, decreasing rapidly over the day, with low levels during the night, then rising rapidly again to the morning peak. A pattern of flatter daytime slopes has been associated with more rapid cancer progression in both animals and humans. We studied the relationship between the daytime slopes and other daytime cortisol responses to both pharmacological and psychosocial challenges of hypothalamic-pituitary-adrenal (HPA) axis function as well as DHEA in a sample of 99 women with metastatic breast cancer, in hopes of elucidating the dysregulatory process.
We found that the different components of HPA regulation: the daytime cortisol slope, the rise in cortisol from waking to 30 minutes later, and cortisol response to various challenges, including dexamethasone (DEX) suppression, corticotrophin releasing factor (CRF) activation, and the Trier Social Stress Task, were at best modestly associated. Escape from suppression stimulated by 1 mg of dexamethasone administered the night before was moderately but significantly associated with flatter daytime cortisol slopes (r=0..28 to .30 at different times of the post dexamethasone administration day, all p<.01) . Daytime cortisol slopes were also moderately but significant associated with the rise in cortisol from waking to 30 minutes after awakening (r=.29, p=.004, N=96), but not with waking cortisol level (r=−0.13, p=.19). However, we could not detect any association between daytime cortisol slope and activation of cortisol secretion by either CRF infusion or the Trier Social Stress Task. The CRF activation test (following 1.5 mg of dexamethasone to assure that the effect was due to exogenous CRF) produced ACTH levels that were correlated (r=0.66 p<.0001, N = 74) with serum cortisol levels, indicating adrenal responsiveness to ACTH stimulation. Daytime cortisol slopes were significantly correlated with the slope of DHEA (r=.21, p=.04, N=95). Our general findings suggest that flatter daytime cortisol slopes among metastatic breast cancer patients may be related to disrupted feedback inhibition rather than hypersensitivity in response to stimulation.
Keywords: Cortisol, HPA, stress, dexamethasone, CRF, metastatic breast cancer
Introduction
Stress Sensitivity in Metastatic Breast Cancer: Analysis of Hypothalamic-Pituitary-Adrenal Axis Function
The diagnosis of breast cancer constitutes a series of stressors, including existential threat, changes in social and vocational roles, the need to make important treatment decisions, and serious side effects associated with treatment. Although most women diagnosed with breast cancer will not develop a psychiatric diagnosis, the vast majority will experience the disease as a major stressor, and 10% have severe maladjustment as long as six years later (Omne-Ponten et al., 1994). Thus, breast cancer causes substantial psychological stress, which is likely to increase physiological consequences as well. Recent work in stress response physiology has emphasized the cumulative effect of stressors on physiological response systems, referred to as “allostatic load” (McEwen, 1998). Allostatic load has been defined as the price that the body has to pay for maintaining allostasis, or stability through physiologic change. Recently, it has been suggested that increased allostatic load, which may occur through cumulative buildup of stress over a lifespan, may significantly hinder the functioning of different physiologic systems (Seeman et al., 1997;McEwen, 1998). Exposure to frequent or severe stress over a period of time may lead to increased sensitization of the hypothalamic-pituitary-adrenal (HPA) axis resulting in pronounced stress response (Rosmond et al., 1998;Heim et al., 2000). Abnormalities in the trajectory of the daytime slope of cortisol have been observed in about 10% of normal populations (Stone et al., 2001), and at higher levels among subjects undergoing psychological stress (e.g., depression, unemployment, post-traumatic stress disorder) (Irwin et al., 1988;Ockenfels et al., 1995;Yehuda et al., 1996) and some types of physical stress (e.g., shift work and excessive workloads) (Caplan et al., 1979;Shinkai et al., 1993). However, chronic stress may also result in hypocortisolism (Heim et al., 2000;Fries et al., 2005) along with flatter daytime slope, with exaggerated stress sensitivity and proliferation of glucocorticoid receptors on peripheral lymphocytes and the CNS (Heim et al., 2000). Recent studies of children show that those who are under stress and those who are less outgoing and energetic show flatter daytime cortisol slopes (Adam and Gunnar, 2001). There is evidence that a substantial minority of ‘normal’ individuals have flatter daytime cortisol slopes, and that it is a trait-like measure (Smyth et al., 1997;Stone et al., 2001) (i.e., it does not change over long periods of time) A recent study showed that mothers with flatter daytime cortisol slopes had more children at home, poorer relationships with them, and more hours of maternal employment (Adam and Gunnar, 2001). Health problems associated with aberrations in the trajectory of the daytime cortisol slope include PTSD, chronic fatigue syndrome, and fibromyalgia/pain hypersensitivity (Yehuda et al., 1993;Yehuda et al., 1995;Heim et al., 1998;Bower et al., 2005;Bower et al., 2005;Griffin et al., 2005).
Two other daytime cortisol activation measures considered to be important indicators of HPA regulation are the awakening level and waking rise (change in cortisol from waking to a set time, usually 30-45 minutes after waking). There is still considerable lack of agreement about what the waking rise in cortisol means. One study suggests that a strong waking rise in cortisol may indicate the general health of the HPA (Pruessner et al., 1997). However, a number of studies have shown that daily stress and overcommitment at work are positively associated with waking rise in cortisol (Kunz-Ebrecht et al., 2004). Furthermore, there is evidence that the waking rise is lower on weekends than weekdays (Kunz-Ebrecht et al., 2004). However, while waking response is higher with perceived stress, it may be lower among those with ‘burnout’ (Pruessner et al., 1999;Gunnar and Vazquez, 2000). In a preliminary study from this project (Kraemer et al., 2006), the test-retest reliability of waking rise from one day to another is quite low, suggesting that this behaves more as a “state” than a “trait” response. Such low reliability would make it difficult to document trait correlates of the waking rise in cortisol.
These findings in relation to stress, trauma and depression are salient to the chronic stress of breast cancer as well. One study found a blunted ACTH response to stress among metastatic breast cancer patients, and an inverse relationship between stress-induced ACTH and basal cortisol levels, suggesting that the blunted response was a result of hypercortisolemia (van der Pompe et al., 1996). However, low plasma cortisol levels have been found among recently diagnosed breast cancer patients with a history of depression or PTSD (Luecken et al., 2004). Decreased peak levels occurring at abnormal times in the cortisol profile may be caused by general HPA hyporesponsiveness (McEwen, 1998) or increased metabolic clearance of cortisol related to the increased use of metabolic substrates that has been observed with neoplastic growth (van der Pompe et al., 1996).
Flatter daytime slopes of cortisol have been associated with fatigue in breast cancer patients (Bower et al., 2005;Bower et al., 2005), and are predictive of earlier mortality with metastatic breast cancer (Sephton et al., 2000), independent of other known prognostic factors. This latter observation provided evidence that abnormalities in daytime slopes of cortisol were related to host resistance to cancer progression. We found that only one-third of a sample of 104 women with metastatic breast cancer exhibited the normal pattern with high levels in the morning, rapidly decreasing throughout the day. The remainder exhibited either flatter trajectories, or even increases at noon, 1700h, or 2100h, with considerable variability in the sample of the trajectory. Flatter slopes predicted earlier mortality up to seven years later, and were associated with loss of a spouse and reported sleep disruptions. In a related study we observed that women with metastatic breast cancer were more likely to have flatter slopes than a comparison sample without disease (Abercrombie et al., 2004). The sources of such dysregulation could be failure of feedback inhibition, excessive responsiveness to activation from stress throughout the day, alterations in cortisol clearance, or sleep disruption. For these reasons, it is important to examine whether flatter cortisol response is associated with failure of feedback inhibition (to administration of dexamethasone), hyper-responsiveness to stimulation (either by CRF or acute stress), or is associated with disruption in other diurnal rhythms, such as dehydroepiandrosterone (DHEA).
Recent research shows how circadian dysregulation might underlie associations between stress and cancer (Sephton and Spiegel, 2003). Two separate lines of investigation have demonstrated that stress (e.g., chronic/work-related stress, depression) can disrupt circadian corticosteroid rhythms (Chrousos and Gold, 1998), favoring tumor initiation and progression (Filipski et al., 2002;Sephton and Spiegel, 2003). Nighttime shift work, which disrupts circadian endocrine rhythms, is a risk factor for breast and colorectal cancer (Schernhammer et al., 2003). Mice with circadian gene mutations are prone to tumor development and early death (Fu et al., 2002;Fu and Lee, 2003). Cancer patients as well as tumor-bearing animals show disruption in rhythms of cortisol, melatonin (Blask et al., 2005), prolactin, TSH, GH, LH, FSH, temperature, circulating proteins, and cell trafficking and proliferation cycles. Advanced cases demonstrate the greatest circadian disruption (Mormont and Levi, 1997). In murine studies, tumor progression and mortality are accelerated after elimination of circadian rhythms (Filipski et al., 2002). Two clinical studies have shown that circadian (cortisol and rest-activity) cycles predict long-term cancer survival (Mormont et al., 2000;Sephton et al., 2000).
The association between daytime cortisol rhythm and DHEA might be important in understanding the relationship between daytime cortisol slope and cancer survival. DHEA is produced in large quantities by the adrenal cortex, shows some daytime variation, and is considered an ‘anti-glucocorticoid’ hormone. There is evidence that higher levels of the adrenal androgens dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) are associated with higher breast cancer risk, especially in post-menopausal women, possibly because of conversion by aromatase to estrogen-like steroids that can stimulate tumor growth, (Massobrio et al., 1994;Secreto and Zumoff, 1994;Dorgan et al., 1997;Kaaks et al., 2005), although one study found lower levels of DHEA-S in women with metastatic breast cancer, especially those with visceral metastases (Lissoni et al., 1998). It may also act as a partial estrogen receptor agonist blocking estrogen receptors, which would have a protective effect in pre-menopausal women.
The present study was designed to analyze the pathophysiology of certain disruptions in daytime cortisol patterns (cortisol slope, waking cortisol, and rise 30 minutes after waking) among women with metastatic breast cancer. In this study we examined the specific hypotheses that a flatter daytime cortisol slope would be related to 1) lack of suppression of cortisol in response to 0.5 mg of dexamethasone; 2) hyperactive and protracted ACTH and cortisol response to CRF challenge; 3) higher cortisol response to the Trier Social Stress Task (TSST) and higher afternoon and evening cortisol levels following the TSST, 4) DHEA diurnal slope and a lower DHEA/cortisol ratio. In addition, we conducted exploratory analyses examining relationships among other measures of daytime HPA function.
Methods
Sample
One hundred eleven women with metastatic breast cancer were eligible and consented to participation from a total pool of 221. Referrals came from oncologists at Stanford and in the Bay Area, responses to newspaper advertisements, and word of mouth among subjects and patients. Of these referrals, 25 were not eligible, 83 were eligible but not interested, and 2 died before the study began. Of the 111 eligible who consented, 8 dropouts provided no data and four additional subjects who reported steroid use during baseline assessment were excluded, leaving a study sample of 99. Inclusion criteria were documented metastatic or recurrent breast cancer; age 55 or older, a Karnofsky rating of at least 70%; residence within the Greater San Francisco Bay Area; and proficiency in English. Exclusion criteria were: active cancers within the past 10 years other than breast cancer, basal cell or squamous cell carcinomas of the skin, or in situ cancer of the cervix; positive supraclavicular lymph nodes as the only metastatic lesion at the time of initial diagnosis; a concurrent medical condition likely to influence short term survival, utilization of a corticosteroid within the preceding month, or a history of major psychiatric illness for which patient was hospitalized or medicated, with the exception of depression or anxiety.
Participants began collection of baseline saliva and questionnaires a median of 3 days after consenting to participate. Our goal was then to collect measures in the following order with a minimum of one week between each major physiological assessment: 1) Three-day at home collection of baseline saliva samples (Cortisol, DHEA), in which day-3 sample collection was to determine response to dexamethasone suppression; and baseline questionnaires (including demographics, medical history, medical data from oncologist); 2) the Trier Social Stress Task (TSST) was conducted a median of 18 days later; 3) Blood samples for measuring baseline ACTH and the CRF Challenge test were collected a median of 14 days later. Due to scheduling issues (e.g., participant treatments that included Decadron or Steroids), the time interval between tests could not be identical across subjects.
Not all measures were obtained on all 111 subjects who qualified. Eight dropped out of the study before providing any data. One provided no biologic data and another subject provided inadequate saliva samples for the cortisol assay. Eight others did not participate in the TSST, leaving 93 with those measures. Of these, 17 did not provide blood samples for immunophenotyping, largely because of limited available veins due to nodal dissections and the need to preserve remaining venous access. Table 1 documents current levels of chemotherapy, radiation, hormonal treatments, and antidepressant use in this population. Antidepressant use included, 18.2% on SSRIs, 11.1% on SNRIs, 2.0% on tricyclic antidepressants, 1.0% on buproprion, 1.0% on an SNRI/tricyclic combination, 2.0% on an SNRI and buproprion, 1.0% on an SSRI and tricyclic, 2.0% on an SSRI/buproprion combination, and 1.0% on hypericum.
Table 1.
Descriptive Statistics for Sociodemographic, Medical, and Treatment Variables in Metastatic Breast Cancer Patients (N=99)
| N | Percentile | |||
|---|---|---|---|---|
| Measure | 25th | Median (50th) or % |
75th | |
| Demographic | ||||
| Age at Baseline (years) | 99 | 47 | 54 | 62 |
| Age at Initial Diagnosis | 93 | 42 | 47 | 53 |
| Age at Metastasis/Recurrence | 93 | 45 | 51 | 58 |
| Ethnicity (% Caucasian) | 98 | 85.7 | ||
| Education | 99 | |||
| High School Diploma or Below | 2.0 | |||
| Trade School or Some College | 34.3 | |||
| Bachelor's Degree | 20.2 | |||
| Some Graduate School or Master's | 39.4 | |||
| Ph.D., M.D. or J.D. | 4.0 | |||
| Income (%) | 89 | |||
| < $20,000 | 5.6 | |||
| $20,000-$39,999 | 20.2 | |||
| $40,000-$59,999 | 12.4 | |||
| $60,000-$79,999 | 18.0 | |||
| $80,000-$99,999 | 13.5 | |||
| $100,000 and Above | 30.3 | |||
| Marital Status (%) | 99 | |||
| Single/ Never Married | 7.1 | |||
| Married/ Living as Married | 67.7 | |||
| Separated, Divorced, or Widowed | 25.3 | |||
| Employment Status (%) | 99 | |||
| Full Time | 22.2 | |||
| Part Time | 12.1 | |||
| Retired/Not Employed | 65.7 | |||
| Medical Status, Treatment for Cancer | ||||
| Disease-Free Interval | 93 | 14 | 37 | 84 |
| Time DX to Study Entry (Months) | 93 | 30 | 77 | 116 |
| Time Metastasis to Study Entry (Months) | 93 | 7 | 13 | 36 |
| Estrogen Receptor Status (% Negative) | 92 | 29.3 | ||
| Progesterone Receptor Status (% Negative) | 89 | 31.5 | ||
| Site of Metastasis/ Recurrence | 93 | |||
| Chestwall | 35.5 | |||
| Bone | 22.6 | |||
| Viscera | 41.9 | |||
| Treatment (% Yes) | ||||
| Surgery | 93 | 93.8 | ||
| Reconstruction | 93 | 25.8 | ||
| Chemotherapy Within 2 months of baseline | 98 | 14.3 | ||
| Radiation Within 2 months of baseline | 98 | 12.2 | ||
| Hormone Therapy Within 2 months of baseline | 98 | 29.6 | ||
| Reported Antidepressant Use at Baseline | 97 | 40.2 | ||
| Ever had Diabetes? | 98 | 3.1 | ||
| Ever had a Stroke? | 98 | 1.0 | ||
| Ever had High Blood Pressure? | 98 | 17.3 | ||
| Ever had Heart Disease? | 97 | 3.1 | ||
| Do you smoke? | 99 | 5,1 | ||
Measures
Demographics
We obtained demographics and medical history from a brief, self-report measure. Medical data were also obtained using a brief questionnaire in which treating oncologists reported patients' diagnosis, treatments, and hormone receptor status.
Physiological Measures
Cortisol
Salivary cortisol has been found to be a reliable tool for investigations of hypothalamic-pituitary-adrenal axis activity (Kirschbaum and Hellhammer, 1994). It is a non-invasive approach that reflects unbound plasma cortisol levels regardless of salivation rate (Ockenfels et al., 1995). Salivary cortisol is known to be responsive to anxiety or stress in normal subjects (Shinkai et al., 1993;Yehuda et al., 1996). Saliva samples were obtained on two consecutive days, at five intervals throughout each day (at waking, 30 minutes later, 1200h, 1700h and 2100h) in their homes. The assessments at waking and 30 minutes later were obtained to examine the pattern of morning increase noted to index normal HPA activation in the morning (Pruessner et al., 1997). In a preliminary study, we documented the test-retest reliability of daytime cortisol slopes and waking rise obtained in this protocol (Kraemer et al., 2006). In the current study, daytime cortisol slope was calculated by regressing cortisol values on time from awakening on all points (1200h, 1700h, and 2100h but not wake + 30) for 2 days. Waking cortisol was the mean of waking levels for 2 days. Waking rise was the 2-day mean of the difference between the cortisol levels at waking and 30 minutes post-waking. Recovery at 2100h on the day of the TSST was the difference score between the average log cortisol level at 2100h during the 2-day baseline and the 2100h cortisol level following the TSST. Recovery at wake the day following the TSST was computed similarly. Spearman correlations (and percentiles rather than means and standard deviations in the tables) were utilized because of the non-normal distributions and outliers.
Each participant was scheduled to obtain saliva samples on each of two baseline saliva collection days. One mg of dexamethasone was administered after the evening saliva sample was obtained on the second day, and similar samples throughout the day were collected on day 3 to determine response to dexamethasone suppression. The Trier Social Stress Task (TSST) (Kirschbaum et al., 1993) was administered at least a week after this baseline sampling. Saliva samples throughout the day were obtained on the day of, during, and the day following the stress task. Patients were offered wrist-worn timers to prompt them at scheduled saliva collection times. Saliva collection swabs (Sarstedt, Inc., Newton, NC) were stored in medication event monitoring system bottles so that exact saliva collection times could be recorded electronically. During home-based collections, participants were instructed to refrigerate completed samples. They were advised not to eat, drink, smoke, brush teeth, or use mouthwash for 30 minutes prior to before saliva collections, and not to collect saliva if they had any mouth wound. They were asked not to drink alcohol during the hours prior to, or during any days when saliva samples were collected.
Samples were stored at −70C prior to laboratory centrifugation and assays for salivary cortisol using luminescence immunoassay (LIA) reagents provided by Immuno-Biological Laboratories, Inc. Hamburg, Germany. Assay sensitivity was 0.015 μg/dl. Intra assay variation on three saliva pools of the low, medium, and high controls were averaged 2.8%, 10.5%, and 4.8% respectively. The mean values of the low, medium, and high controls were .054 μg/dl, .228 μg/dl, and .863 μg/dl. The inter-assay coefficients of variation for the low, medium, and high controls were 10.9%, 10.5%, and 5.5%, respectively. The range of sensitivity is from .07 to 3.12 ug/dl, and the average intra- and inter-assay coefficients of variation are 5.4% and 7.3% respectively. Values from matched serum and saliva samples were strongly correlated (r (17)=0.94, p<.0001). External controls were included in each batch representing low and high cortisol levels, and all samples were assayed in duplicate. Those duplicates between .07 and .18 ug/dl with a coefficient of variation greater than 15% were reassayed, and those between .18 and 3.12 ug/dl were reassayed if the CV was greater than 10%. Those below .07 ug/dl were not reassayed, and those above 3.12 ug/dl and outside of the standard range were reassayed using dilutions.
ACTH
The ACTH Double Antibody IRMA, by DiaSorin Corporation was used. External controls were included in each batch representing low and high ACTH levels. All samples were assayed in duplicate. The minimal detectable dose is approximately 1.5 pg/ml. The ACTH antiserum used in the test is highly specific for ACTH, with an extremely low cross reactivity to other hormones. CVs are low and uniform. The intra-assay reliability for low controls was 4.18 and for high controls was 3.54. The inter-assay reliability for low control is 5.74 and for high control is 3.16. The linear range of with 2 S.D. is 7.0 to 1128 pg/ml of ACTH. Sample values that represent extreme scores were retested using two and fourfold dilutions.
DHEA
DHEA was analyzed using a Salivary DHEA ELISA Kit, by Salimetrics. It is an enzyme immunoassay (no radioactivity). The average intra-assay coefficient of variation is 4.9% (1.0% for high and 5.9% for low DHEA levels). The minimal concentration of DHEA that can be distinguished from 0 is 10 pg/mL. The correlation between serum and salivary DHEA was determined by assaying 40 matched samples using the Diagnostic Systems Laboratories serum DHEA radioimmunoassay and the Salimetrics Salivary DHEA EIA. The serum-saliva correlation was, r (38) = 0.883, p < .001. All samples were assayed in duplicate. Sample values that represented extreme scores were retested dilutions.
HPA Suppression
Dexamethasone Suppression Test
To assess feedback sensitivity of the HPA axis, the dexamethasone suppression test (DST) was conducted by giving 1 mg. of dexamethasone at 2200h and then obtaining saliva samples for cortisol measurement at waking, wake plus 30, 1200h, 1700h, and 2100h the next day.
HPA Activation
Trier Social Stress Task (TSST)
A modified version of the TSST (Kirschbaum et al., 1993) was used in this study. The TSST is a standardized social and cognitive stressor composed of 5 minutes of anticipatory stress, 5 minutes of public speaking in the form of a job interview, and 5 minutes of mental arithmetic done in front of a panel of 2 evaluators. Upon arrival, participants were fitted with physiological recording equipment. After a 5 min baseline and a calibration procedure for the respiration belts, participants were told that they would have 5 minutes to prepare a speech for a job interview. The 5 min speech task was immediately followed by a mental math task. Evaluators provided no facial feedback in response to the participants. Participants were then instructed to sit quietly for the next hour, without moving or speaking much. Debriefing occurred the next-day following saliva collection.
On the day of the stress task, samples were obtained at waking, 30 minutes later, 1200h, and at 2100h. The stress task began in the late afternoon between 1600h and 1900h because past research has shown that HPA effect sizes for stress tasks are greater for late afternoon sessions due to the lower background daytime level of cortisol (Dickerson and Kemeny, 2004). Saliva samples during the stress task were collected 10 times: 1) when participants first arrived at the GCRC before autonomic hook up, 2) after electrode hook-up and before respiration calibration, 3) After 5 min of baseline quiet sitting and 5 min. of paced breathing, right before stress task instructions, 4) after 5 min speech preparation, 5) after the speech (5 min) and math (5 min) stressors, and at 10, 20, 30, 45, and 60 minutes. Saliva was sampled 5 times the following day on the same time schedule as the two baseline days.
CRF Challenge
The CRF challenge was utilized to assess the possibility that flattening of daytime slope is due to hyper responsiveness of the adrenal cortex to stimulation. To assess optimal pituitary responsiveness, the CRF stimulation test was applied in the afternoon, a time when endogenous pituitary-adrenal activity is low (Linton and Lowry, 1989). Patients were pretreated with 1.5 mg of dexamethasone at 2200h the night before to be sure that response the next day was due purely to the exogenously administered CRF. On arrival at the General Clinical Research Center (GCRC), patients were asked to recline from 1530h – 1800h. At 1530h, an indwelling heparinized catheter was inserted into a forearm vein (on the side contra lateral to the breast cancer), and at 1600h 1 micrograms/kg of CRF was administered by intravenous bolus injection. Specimens of blood were obtained at −15, 0, 15, 30, 45, 60, 75, 90, 105, and 120 minutes for determination of plasma ACTH and cortisol levels. Blood was collected in EDTA/Trasylol-containing tubes, placed immediately on ice, and centrifuged at 3000 rpm at 4 degrees C for 10 minutes. Plasma was separated and stored at −80 degrees C until it is assayed. Saliva was collected in Salivettes and stored at −80 degrees C until assayed.
Results
The demographic and clinical characteristics of this sample are presented in Table 1. Descriptive statistics describing the pertinent physiological measures are presented in Table 2. Spearman correlations between daytime cortisol measures (slope, waking level, and waking rise at 30 min) and demographic and medical variables are in Table 3. Daytime cortisol slope was not significantly correlated with most demographic variables such as age, medical variables such as disease-free interval and treatment variables such as chemotherapy. The only demographic or medical condition variable that was correlated with daytime cortisol slope was income (Spearman r =−0.28, p=.008, N=86). This suggests that the stress associated with lower income may be associated with flatter daytime slopes.
Table 2.
Descriptive Statistics for Baseline Biological Measures in Metastatic Breast Cancer Patients (N=99)
| Measures | N | 25th | Median: 50th | 75th |
|---|---|---|---|---|
| Percentile | Percentile | Percentile | ||
| Log Cortisol Slope (2-day Mean) | 96 | −0.21 | −0.16 | −0.11 |
| Hypothesis 1. | ||||
| 1200h and 1700h Cortisol on Dex Day (Mean) | 93 | 0.01 | 0.01 | 0.02 |
| Hypothesis 2. | ||||
| Dex-CRF ACTH (pg/ml) (Mean) | 74 | 9.68 | 15.85 | 23.27 |
| Dex-CRF Serum Cortisol (Mean) | 74 | 4.85 | 6.49 | 11.32 |
| Hypothesis 3. | ||||
| TSST Log Cortisol AUC | 87 | −2.68 | −2.07 | −1.43 |
| TSST Log Cortisol Recovery 2100h | 87 | −0.58 | 0.00 | 0.85 |
| TSST Log Cortisol Recovery Wake | 88 | −0.39 | −0.09 | 0.26 |
| Hypothesis 4. | ||||
| DHEA Slope (pg/ml-hr) (2-day Mean) | 95 | −16.11 | −7.14 | −3.61 |
| DHEA/Cortisol Ratio at 2100h (2-day Mean) | 97 | 573.88 | 1130.34 | 2919.09 |
| Exploratory. | ||||
| Waking Cortisol (2-day Mean) | 97 | 0.43 | 0.53 | 0.71 |
| 30 min. Waking Rise in Cortisol (2-day Mean) | 97 | −0.04 | 0.21 | 0.46 |
| TSST Log Cortisol Recovery Waking Rise at 30 min. | 88 | −0.40 | −0.01 | 0.34 |
| DHEA Wake + 30 (2-day Mean) | 96 | 94.60 | 168.76 | 327.45 |
| DHEA 2100h (2-day Mean) | 97 | 32.59 | 57.35 | 93.18 |
| DHEA/Cortisol Ratio at Wake + 30 (2-day Mean) | 96 | 132.60 | 244.47 | 515.81 |
Note. In bold are variables that were specifically tested in a priori hypotheses in this paper. Other variables are presented for hypothesis generation.
Table 3.
Spearman Correlations Among Demographic and Medical Variables and Diurnal log Cortisol Slope, Waking log Cortisol Level, and Waking log Cortisol Rise at 30 minutes in Metastatic Breast Cancer Patients (N=99).
| Diurnal log Cortisol Slope |
Waking log Cortisol Level |
Waking log Cortisol Rise |
|
|---|---|---|---|
| Measure | (N) | (N) | (N) |
| Demographic | |||
| Age at Baseline (years) | 0.13 (96) | 0.23* (97) | 0.05 (97) |
| Age at Initial Diagnosis | 0.14 (92) | 0.25* (92) | 0.03 (92) |
| Age at Metastasis/Recurrence | 0.10 (92) | 0.26* (92) | 0.05 (92) |
| Ethnicity (% Caucasian) | 0.08 (95) | 0.20 (96) | −0.05 (96) |
| Education | 0.14 (96) | 0.08 (97) | −0.07 (97) |
| High School Diploma or Below | |||
| Trade School or Some College | |||
| Bachelor's Degree | |||
| Some Graduate School or Master's | |||
| Ph.D., M.D. or J.D. | |||
| Income (%) | −0.28* (86) | 0.15 (87) | −0.14 (87) |
| < $20,000 | |||
| $20,000-$39,999 | |||
| $40,000-$59,999 | |||
| $60,000-$79,999 | |||
| $80,000-$99,999 | |||
| $100,000 and above | |||
| Marital Status (%) | −0.00 (96) | 0.14 (97) | 0.01 (97) |
| Married/ Living as Married | |||
| Single, Separated, Divorced, or Widowed | |||
| Employment Status (%) | 0.01 (96) | 0.01 (97) | 0.00 (97) |
| Full Time | |||
| Part Time | |||
| Retired/Not Employed | |||
| Medical Status, Treatment for Cancer | |||
| Disease-Free Interval | −0.12 (92) | −0.02 (92) | 0.14 (92) |
| Time DX to Study Entry (Months) | −0.07 (92) | 0.01 (92) | 0.07 (92) |
| Time Metastasis to Study Entry (Months) | 0.05 (92) | 0.07 (92) | −0.15 (92) |
| Estrogen Receptor Status (% Negative) | 0.13 (91) | −0.14 (91) | 0.09 (91) |
| Progesterone Receptor Status (% Negative) | 0.06 (88) | −0.18 (88) | 0.22* (88) |
| Site of Metastasis/ Recurrence | 0.01 (92) | 0.03 (92) | −0.07 (92) |
| Chestwall | |||
| Bone | |||
| Viscera | |||
| Treatment (% Yes) | |||
| Surgery | −0.09 (92) | −0.09 (92) | 0.15 (92) |
| Reconstruction | −0.20 (92) | 0.13 (92) | −0.04 (92) |
| Chemotherapy Within 2 months of baseline | 0.06 (96) | 0.16 (97) | 0.08 (97) |
| Radiation Within 2 months of baseline | 0.13 (96) | −0.04 (97) | 0.16 (97) |
| Hormone Therapy Within 2 months of baseline | 0.12 (96) | −0.04 (97) | 0.16 (97) |
| Reported Antidepressant Use at Baseline | −0.17 (98) | 0.23* (98) | −0.33* (98) |
| Ever had Diabetes? | 0.08 (95) | 0.11 (96) | −0.07 (96) |
| Ever had a Stroke? | −0.06 (95) | −0.08 (96) | −0.17 (96) |
| Ever had High Blood Pressure? | −0.05 (95) | 0.08 (96) | 0.06 (96) |
| Ever had Heart Disease? | 0.09 (94) | 0.06 (95) | 0.06 (95) |
| Do you smoke? | 0.22* (96) | −0.15 (97) | −0.03 (97) |
p<.05;
p<.01.
Spearman correlations were utilized due to non-normal distributions and numerous outliers in the physiological data
We performed preliminary analyses to remove redundant measures to reduce problems due to multiple testing. Cortisol measures were relatively independent of each other. Response to the DEX-CRF challenge has two physiologically related components: ACTH and cortisol. They were strongly correlated (r=0.66 p=.0001, N=74). This indicates adrenal response to ACTH, but both ACTH and cortisol responses to CRF infusion are of physiological significance, and their associations with other variables are likely to be similar.
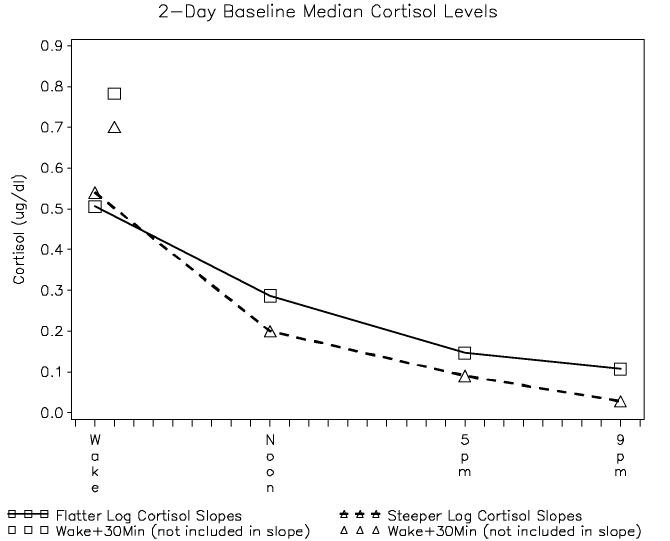
Daytime slope was significantly correlated with cortisol rise 30 minutes after waking (r=0.29, p=.004, N=96), but not with waking cortisol level (r=−0.13, p=.19, N=196). The association of steeper slope with waking level might be expected mathematically, since the starting point is higher, but the relationship of a flatter slope with a greater wake plus 30 minute rise suggests that a lack of feedback inhibition may be associated with both phenomena (Figure 1). Relatively flatter cortisol slopes were significantly associated with higher 2100h cortisol levels (r = 0.85, p < .0001).
Figure 1.
In our results, we present correlations between diurnal cortisol slope and waking cortisol levels, waking cortisol rise, and 2100 levels. We present here a figure to assist the reader in visualizing these associations by creating a median split in daytime cortisol slopes in women with metastatic breast cancer (N=99) and plotting the slope against the cortisol levels at each point in the day. Cortisol waking rise is plotted separately because it was not included in the calculation of the cortisol slopes used in this study. Relatively flatter cortisol slopes were significantly associated with higher waking rise and higher 2100 level, but not with waking level.
Consistent with reduced inhibition, a relatively flatter daytime cortisol slope was significantly associated with escape from cortisol suppression caused by 1 mg of dexamethasone administered the night before, (Spearman r=0.30, p=.005, N=88 at waking, r=0.29, p=.005, N=90 at wake plus 30 minutes; r=0.26, p=.01, N=90 at 1200h; r=0.29, p=.005, N=93 at 1700h; and r=0.28, p=.007, N=92 at 2100h), as can be seen in Figure 2. Such a result would be consistent with a failure of feedback inhibition.
Figure 2.
Daytime cortisol on the day after administration of 1 mg of dexamethasone at 2200h. Median splits are plotted as a visual aid to the reader to illustrate the significant correlation between flatter daytime cortisol slopes and less dexamethasone suppression (N=99).
Daytime Cortisol Slope and HPA Activation Tests
We could detect no significant relationships between daytime cortisol slope and activation of cortisol secretion by either administration of CRF or for social stress (the TSST). The CRF activation test (following 1.5 mg of dexamethasone to assure that the effect was due to exogenous CRF) produced ACTH levels that were highly correlated (r=0.66, p<.0001, N = 74) with serum cortisol levels, indicating that the adrenal was responsive to ACTH stimulation. The absence of detectable association between daytime cortisol slope weakens the hypothesis that there is hyper-responsiveness to stimulation.
TSST
We also could not detect any association between daytime cortisol slope and cortisol response during the TSST. As illustrated in Figure 3, these women with metastatic breast cancer have a blunted cortisol response to the TSST, with their peak levels (.1 ug/dl at the peak) at about the same level as the baseline 1700h daytime average (.1−.2 ug/dl) (in Figure 1). Again, this weakens the hypothesis that there is hyper-responsiveness to stimulation.
Figure 3.
Cortisol response during the Trier Social Stress Task for metastatic breast cancer patients can be seen in this figure to peak at 0.1 (ug/dl) which is similar to their late afternoon baseline daytime levels (0.1-0.2 ug/dl) in Figure 1. For visual comparison, we also offer the illustration of the TSST response of non-depressed women at risk for cardiovascular disease (CVD). These women's response was taken (with his permission) from C. Barr Taylor's (Taylor et al., In Press) study examining depressed and non-depressed men and women at risk for CVD. All data for both studies were obtained utilizing the same protocol, panel, staff, equipment, and data reduction.
In order to help the reader to visualize the blunting of the response to TSST, we have included in Figure 3 the response to the TSST of a group of non-depressed women at risk for cardiovascular disease taken from a study conducted by C. Barr Taylor (Taylor et al., In press). Taylor's depression study shared identical protocols, panel members, equipment, staff, and data reduction methods as one of the 4 studies in our program project grant. In response to a reviewer, we have conducted a statistical test of this difference between groups using a Hierarchical Linear model with Group (CVD non-depressed females (N=8) vs. Metastatic Breast Cancer (N=89), Time, and their interaction, assuming an autoregressive covariance structure. Baseline values were subtracted for each response in the both trajectories. We could not detect a main effect for group (F=3.11, p=0.08), but shape of the trajectories were significantly different from each other (Time x Group, F=2.22, p=0.04), despite the very small sample in the reference group.
There were no significant differences between the 2-day baseline cortisol slope and the cortisol slope on the day of or the day after the TSST. Also, those with flatter daytime cortisol slopes at the 2-day baseline had lower 2100h post TSST cortisol recovery levels relative to their baseline 2100h levels after the test (r=−0.46, p<.0001, N=86). Since the TSST did not activate the HPA after the test was concluded, this may simply reflect correlating two different measures of the same response.
DHEA
The slope of DHEA throughout the day, measured at waking plus 30 minutes and 2100h, was significantly correlated with the daytime slope of cortisol (r=0.21, p=.04, N=95). In examining the source of this association in exploratory analyses, flatter cortisol slopes were correlated with higher DHEA levels at 2100h (r=0.24, p=.02, N=96) but not at waking plus 30 minutes (r=0.11, p=.28, N=95). Daytime cortisol slopes were also correlated with DHEA/Cortisol ratios at 2100h (r=-0.48, p<.0001, N=96) but not at waking plus 30 (r=0.02, p=.83, N=95). This leaves open the possibility that some other disruption of diurnal rhythms plays some part in determining the daytime pattern of cortisol response.
Exploratory Analyses of Medical Variables with Daytime Cortisol
As Table 3 indicates, age was significantly positively correlated with waking cortisol (r=0.23, p=.02, N=97), as was age at diagnosis (r=0.25, p=.01, N=92), and age at recurrence (r=0.26, p =.01, N=92). Antidepressant medication use was associated with higher waking cortisol (r=0.21, p=.04, N=96) and lower waking cortisol rise (r=-0.32, p=.001, N=96). Those who were progesterone receptor positive had lower waking cortisol rise (r=0.22, p=.04, N=88). All of these correlations are low to moderate.
Discussion
We found that flatter daytime cortisol slope was associated with, a steeper rise in cortisol 30 minutes after waking and with nonsuppression by dexamethasone. Flatter daytime cortisol slopes were largely a function of elevations later in the day rather than lower waking cortisol levels. In our data, slope correlated r=0.52 with the 1700h sample, and r=0.85 with the 2100h sample, but was not significantly associated with lower waking cortisol levels (r=.13). We could find no significant relationship between daytime cortisol slope and stimulation by CRF or social stress on the TSST. Cortisol slope was modestly associated with DHEA slope and with higher 2100h levels of the DHEA/Cortisol ratio. Our data indicate that the flatter daytime cortisol slope does not seem due to excessive HPA responsiveness to stimulation, either by CRF or social stress. Rather this pattern seems to be related to a failure of feedback inhibition, either naturally in the morning when cortisol levels are already high, or after administration of dexamethasone. Flatter daytime cortisol slope was modestly but significantly associated with failure of HPA suppression by dexamethasone, but not with pharmacological or psychosocial stress-induced activation. This is consistent with rodent studies showing that sympathomedullary activation is more closely related to acute stress than HPA response (Dronjak et al., 2004). There is evidence in female rhesus monkeys that aging is associated with flattening of daytime cortisol slope, reduced feedback inhibition to dexamethasone, and that social isolation diminishes cortisol response to CRF (Gust et al., 2000). This suggests that a higher set point for HPA feedback inhibition may contribute to the disruption of daytime cortisol slope that has been shown in humans (Sephton et al., 2000) and animals (Filipski et al., 2002) to predict more rapid cancer progression. The daytime cortisol slope was significantly associated with the daytime slope of DHEA, suggesting an antiglucorticoid effect of DHEA (Reus et al., 1997). DHEA has also been shown to predict the development of postmenopausal breast cancer (Gordon et al., 1990).
The pattern linking a flatter daytime cortisol slope to relative non-suppression by DEX but not hyperactivation by ACTH has been observed throughout the life cycle, e.g. among children with ADHD (Kaneko et al., 1992), in patients diagnosed with schizophrenia (Kaneko et al., 1992), and in association with cognitive and physical frailty in the elderly (Carvalhaes-Neto et al., 2003). These findings are consistent with the idea that a more robust cortisol response to acute stress does not contribute to abnormalities of daytime cortisol slope, which may be due to prolonged unremitting stress.
Prolonged acute and chronic traumatic stress and PTSD have been found to be associated with hypersensitivity to DEX suppression and generally lower cortisol levels (Goenjian et al., 1996;Yehuda et al., 1996). The pattern among our patients with abnormal slopes was different – hyposensitivity to DEX and higher waking cortisol rise. There is evidence of low plasma cortisol levels among recently diagnosed breast cancer patients with a history of depression or PTSD (Luecken et al., 2004). Jezova and colleagues (Jezova et al., 2004) found that individuals high in anxiety actually produced less of a cortisol increase in response to stress than low anxious subjects. This may help to explain our observation of low cortisol response to the TSST among our metastatic breast cancer patients, despite a rise in the self-report of negative affect during the task (Giese-Davis et al., In press) The inability to mount a cortisol response may be experienced as anxiety (physiological helplessness, lack of glucose mobilization in the face of a stressor), which compounds stress and anxiety. Elevated prevalence of PTSD has been observed in women with breast cancer (Andrykowski et al., 1998), and the symptoms were particularly associated with a tendency to suppress emotion (Andrykowski and Cordova, 1998). Those with chronic medical illnesses, depression or PTSD may be depleted in their ability to mount a physiological stress response and mobilize glucose (Burke et al., 2005), leading to feelings of fatigue and helplessness, which could compound the stress and related anxiety (Anderson et al., 2003;Bower et al., 2004;Bower et al., 2005). A syndrome of stress sensitivity, pain and fatigue associated with hypocortisolism has recently been described (Fries et al., 2005) resulting from prior stress-related HPA hyperactivity. While perceived stress among teachers has been related to higher waking cortisol rise and burnout to lower waking cortisol rise and hypersensitivity to dexamethasone (0.5 mg) suppression (Pruessner et al., 1999), the subgroup with both high perceived stress and burnout showed a combination of lower cortisol in the morning and lack of dexamethasone suppression, coupled with low self-esteem, high external locus of control, and the most somatic complaints. These latter findings are consistent with ours among metastatic breast cancer patients. Those with flatter daytime cortisol slopes had higher waking cortisol rise and resistance to dexamethasone suppression. In exploratory analyses of our other daytime cortisol variables, higher waking cortisol was also associated with more advanced age in general. Progesterone negative (poorer prognosis) tumors were significantly associated with lower waking cortisol rise, indicating muted HPA activation in the natural setting.
Our findings suggest that flatter daytime cortisol slopes among metastatic breast cancer patients are related to disrupted feedback inhibition rather than hypersensitivity in response to stimulation. Those with flatter daytime cortisol slopes evidenced persistent elevation of cortisol levels in the face of what should have been strong inhibitory pressure: after 1 mg of dexamethasone the night before, and after waking when cortisol levels are high anyway. Thus weakened inhibition seems to play a role in our relatively flatter cortisol slopes during the day. This could reflect chronically elevated CRF or ACTH secretion in the face of feedback inhibition.
However, our finding of flatter slopes among women with higher waking rise is the opposite of that of Vedhara et al (Vedhara et al., 2006) that steeper slopes were related to waking rise in cortisol (r=−0.26, p=.022, p. 9) among newly diagnosed breast cancer patients. They also found that other various measures of cortisol function were relatively independent, as we did. The difference could be related to the earlier stage of disease in their sample. HPA hyporesponsiveness has been observed among people with a history of traumatic stressors, PTSD and some types of depressive symptoms. Thus our findings are consistent with changes related to some types of chronic burnout rather than acute hyperactivation. We could not demonstrate that acute activation of the HPA in these patients, either with administration of CRF or through social stress, was associated with a flatter daytime cortisol slopes. While those with relatively flatter slopes might be considered somewhat hypercortisolemic, since this pattern was associated with loss of feedback inhibition to dexamethasone and greater waking rise in cortisol, it seems unlikely that they would have had hyperactive feedback inhibition in response to the social stressor. In the TSST there was evidence of psychological stress and autonomic activation (Giese-Davis et al., In press) but little cortisol response. This could indicate either that the TSST was only modestly arousing (social stress being less important than disease-related anxiety), or that these women could no longer mount a cortisol response to an acute but artificially induced stressor. Further research is needed to explore factors mediating chronic vs. acute HPA response patterns, including interaction with depression, anxiety and PTSD symptoms and other circadian patterns, such as sleep.
Table 4.
Spearman Correlations among Baseline Biological Measures in Metastatic Breast Cancer Patients (N=99)
| Measures | Log Cortisol Slope (2-day Mean) |
Log Waking Cortisol (2-day Mean) |
30 min. Log Waking Rise in Cortisol (2-day Mean) |
|---|---|---|---|
| r (N) | r (N) | r (N) | |
| Log Cortisol Slope (2-day Mean) | --- | −0.13 (96) | 0.29** (96) |
| Hypothesis 1. | |||
| 1200h and 1700h Log Cortisol on Dex Day (Mean) | 0.27** (93) | −0.04 (93) | 0.22* (93) |
| Hypothesis 2. | |||
| Dex-CRF ACTH (pg/ml) (Mean) | −0.09 (73) | −0.04 (73) | −0.07 (73) |
| Dex-CRF Log Serum Cortisol (Mean) | 0.20 (73) | 0.10 (73) | −0.09 (73) |
| Hypothesis 3. | |||
| TSST Log Cortisol AUC | 0.03 (87) | 0.19 (87) | 0.02 (87) |
| TSST Log Cortisol Recovery 2100h | −0.46*** (86) | −0.08 (86) | −0.03 (86) |
| TSST Log Cortisol Recovery Wake | 0.14 (88) | −0.39*** (88) | 0.28** (88) |
| Hypothesis 4. | |||
| DHEA Slope (pg/ml-hr) (2-day Mean) | 0.21* (95) | 0.07 (95) | −0.22* (95) |
| DHEA/Cortisol Ratio at 2100h (2-day Mean) | −0.48*** (96) | −0.12 (96) | −0.11 (96) |
| Exploratory. | |||
| Log Waking Cortisol (2-day Mean) | --- | --- | −0.54*** (97) |
| 30 min. Log Waking Rise in Cortisol (2-day Mean) | --- | −0.54*** (97) | --- |
| TSST Log Cortisol Recovery Waking Rise at 30 min. | −0.11 (88) | 0.36*** (88) | −0.55*** (88) |
| DHEA Wake + 30 (2-day Mean) | 0.11 (95) | −0.01 (96) | 0.22* (96) |
| DHEA 2100h (2-day Mean) | 0.24* (96) | −0.05 (96) | 0.09 (96) |
| DHEA/Cortisol Ratio at Wake + 30 (2-day Mean) | 0.02 (95) | −0.16 (96) | −0.10 (96) |
Note: A priori hypotheses are highlighted in bold
p<.05,
p<.01,
p<.001.
Acknowledgements
This study was supported by NIA/NCI Program Project AG18784. It was also supported in part by grant 5 M01 RR000070 from the National Center for Research Resources, National Institutes of Health. We would also like to thank Sandra Sephton, Ph.D., Seymour Levine, Ph.D., and Dirk Hellhammer, Ph.D. for consultation regarding cortisol measurement for this paper, Bita Nouriani, M.A. project director, Manijeh Parineh for recruitment, Eric Neri for data management, Mark Rothkopf for sample management, and Ben Varasteh and the General Clinical Research Center at Stanford for conducting cortisol assays, supported by grant NIH 5 M01 RR000070-44. Lastly, decisions about the cortisol collection protocol were carefully made through many discussions with the Program Project team including consultants and members of the Advisory Board. Those not mentioned so far who contributed to these decisions include Robert Carlson, M.D., Elissa Epel, Ph.D., Firdaus Dhabhar, Ph.D., Robert Sapolsky, M.D., Alan Schatzberg, M.D., Bruce McEwen, M.D., Charles Nemeroff, M.D., Owen Wolkowitz, M.D., Rachel Yehuda, Ph.D., and Jerry Yesavage, M.D. Our special thanks to Drs. Firdaus Dhabhar, Oxana Palesh, Robert Sapolsky and Owen Wolkowitz for careful critical review of the manuscript.
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, Cleeland CS. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003 Apr 25;:307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Cordova MJ. Factors associated with PTSD symptoms following treatment for breast cancer: A test of the Andersen model. Journal of Traumatic Stress. 1998;11:189–203. doi: 10.1023/A:1024490718043. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Cordova MJ, Studts JL, Miller TW. Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. Journal of Consulting and Clinical Psychology. 1998;66:586–590. doi: 10.1037//0022-006x.66.3.586. [DOI] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal Cortisol Rhythm and Fatigue in Breast Cancer Survivors. Psychoneuroendocrinology. 2004 doi: 10.1016/j.psyneuen.2004.06.003. in press. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Caplan R, Cobb S, French JJ. White collar work load and cortisol: disruption of a circadian rhythm by job stress? Journal of Psychosomatic Research. 1979;23:181–192. doi: 10.1016/0022-3999(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Carvalhaes-Neto N, Huayllas MK, Ramos LR, Cendoroglo MS, Kater CE. Cortisol, DHEAS and aging: resistance to cortisol suppression in frail institutionalized elderly. J Endocrinol Invest. 2003;26:17–22. doi: 10.1007/BF03345117. [DOI] [PubMed] [Google Scholar]
- Chrousos G, Gold P. Editorial: A healthy body in a healthy mind--and vice versa--The damaging power of uncontrollable stress. Journal of clinical endocrinology and metabolism. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorgan J, Stanczyk F, Longcope C, Stephenson HJ, Chang L, Miller R, Franz C, Falk R, Kahle L. Relationship of serum dehydroepiandrosterone (DHEA) DHEA sulfate, and 5-androstene-3 beta, 17 beta-diol to risk of breast cancer in postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 1997;6:177–181. [PubMed] [Google Scholar]
- Dronjak S, Jezova D, Kvetnansky R. Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci. 2004;1018:113–123. doi: 10.1196/annals.1296.013. [DOI] [PubMed] [Google Scholar]
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, Claustrat B, Hastings MH, Levi F. Host circadian clock as a control point in tumor progression. Journal of the National Cancer Institute. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Spiegel D. Stress Reactivity in Metastatic Breast Cancer Patients: Effects of Depression. Psychosomatic Medicine. doi: 10.1097/01.psy.0000238216.88515.e5. In press. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Gordon GB, Bush TL, Helzlsouer KJ, Miller SR, Comstock GW. Relationship of serum levels of dehydroepiandrosterone and dehydroepiandrosteron sulfate to the risk of developing postmenopausal breast cancer. Cancer Research. 1990;50:3859–3862. [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am J Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. 2000 doi: 10.1017/s0954579401003066. under review. [DOI] [PubMed] [Google Scholar]
- Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker J, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic Medicine. 1998;60:309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Irwin M, Daniels M, Risch SC, Bloom E, Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biol Psychiatry. 1988;24:173–178. doi: 10.1016/0006-3223(88)90272-7. [DOI] [PubMed] [Google Scholar]
- Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quiros JR, Roddam A, Thiebaut A, Tjonneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Yokoyama F, Hoshino Y, Takahagi K, Murata S, Watanabe M, Kumashiro H. Hypothalamic-pituitary-adrenal axis function in chronic schizophrenia: association with clinical features. Neuropsychobiology. 1992;25:1–7. doi: 10.1159/000118800. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Linton EA, Lowry PJ. Corticotropin releasing factor in man and its measurement: A review. Clin. Endocrinol. 1989;31:225–249. doi: 10.1111/j.1365-2265.1989.tb01246.x. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Rovelli F, Giani L, Mandala M, Meregalli S, Barni S, Confalonieri G, Bonfanti A. Dehydroepiandrosterone sulfate (DHEAS) secretion in early and advanced solid neoplasms: selective deficiency in metastatioc disease. International Journal of Biological Markers. 1998;13:154–157. doi: 10.1177/172460089801300306. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Dausch B, Gulla V, Hong R, Compas BE. Alterations in morning cortisol associated with PTSD in women with breast cancer. J Psychosom Res. 2004;56:13–15. doi: 10.1016/S0022-3999(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Massobrio M, Migliardi M, Cassoni P, Menzaghi C, Revelli A, Cenderelli G. Steroid gradients across the cancerous breast: an index of altered steroid metabolism in breast cancer? J Steroid Biochemistry & Molecular Biology. 1994;51:175–181. doi: 10.1016/0960-0760(94)90091-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6:3038–3045. [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Omne-Ponten M, Holmberg L, Sjoden PO. Psychosocial adjustment among women with breast cancer stages I and II: six-year follow-up of consecutive patients. J Clin Oncol. 1994;12:1778–1782. doi: 10.1200/JCO.1994.12.9.1778. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Reus VI, Wolkowitz OM, Frederick S. Antiglucocorticoid treatments in psychiatry. Psychoneuroendocrinology. 1997;22(Suppl 1):S121–124. doi: 10.1016/s0306-4530(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Dallman M, Bjorntorp P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. Journal of Clinical Endocrinology and Metabolism. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- Secreto G, Zumoff B. Abnormal production of androgens in women with breast cancer. Anticancer Research. 1994;14:2113–2117. [PubMed] [Google Scholar]
- Seeman T, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Sephton S, Spiegel D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Dhabhar FS, Classen C, Spiegel D. The diurnal cortisol slope as a predictor of immune reactivity to interpersonal stress. Brain, Behavior & Immunity. 2000;14:128. [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shinkai S, Watanabe S, Kurokawa Y, Torii J. Salivary cortisol for monitoring circadian rhythm variation in adrenal activity during shiftwork. Int Arch Occup Environ Health. 1993;64:499–502. doi: 10.1007/BF00381098. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Conrad A, Wilhelm FH, Neri E, DeLorenzo A, Kramer MA, Giese-Davis J, Roth WT, Oka R, Cooke JP, Kraemer HC, Spiegel D. Psychophysiological and cortisol responses to psychological stress in depressed and non-depressed older men and women with elevated CVD risk. Psychosomatic Medicine. doi: 10.1097/01.psy.0000222372.16274.92. In press. [DOI] [PubMed] [Google Scholar]
- van der Pompe G, Antoni MH, Heijnen CJ. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology. 1996;21:361–374. doi: 10.1016/0306-4530(96)00009-1. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Stra JT, Miles JN, Sanderman R, Ranchor AV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology. 2006;31:299–311. doi: 10.1016/j.psyneuen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr. Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]