Abstract
All cells respond to stress through the activation of primitive, evolutionarily conserved genetic programs that maintain homeostasis and assure cell survival. Stress adaptation, which is known in the literature by a myriad of terms, including tolerance, desensitization, conditioning, and reprogramming, is a common paradigm found throughout nature, in which a primary exposure of a cell or organism to a stressful stimulus (e.g., heat) results in an adaptive response by which a second exposure to the same stimulus produces a minimal response. More interesting is the phenomenon of cross-tolerance, by which a primary exposure to a stressful stimulus results in an adaptive response whereby the cell or organism is resistant to a subsequent stress that is different from the initial stress (i.e. exposure to heat stress leading to resistance to oxidant stress). The heat shock response is one of the more commonly described examples of stress adaptation and is characterized by the rapid expression of a unique group of proteins collectively known as heat shock proteins (also commonly referred to as stress proteins). The expression of heat shock proteins is well described in both whole lungs and in specific lung cells from a variety of species and in response to a variety of stressors. More importantly, in vitro data, as well as data from various animal models of acute lung injury, demonstrate that heat shock proteins, especially Hsp27, Hsp32, Hsp60, and Hsp70 have an important cytoprotective role during lung inflammation and injury.
Introduction
That which drug fails to cure, the scalpel can cure. That which the scalpel fails to cure, heat can cure. If the heat cannot cure, it must be determined to be incurable.
-Hippocrates
Ferruccio Ritossa unintentionally observed a novel hyperthermia-dependent puffing pattern in the giant chromosomes from the salivary glands of Drosophila melanogaster in 1962 (1). By chance occurrence, a colleague accidentally increased the temperature of one of the incubators in which he kept his specimens and the following morning Ritossa discovered a new puffing pattern that had not been there on the previous day. Realizing the mistake, Ritossa conducted additional, properly controlled experiments, and subsequently linked this new chromosomal puffing pattern with the expression of a specific group of proteins that he fittingly called heat shock proteins (1, 2). Notably, the editors of one of the more reputable scientific journals at the time rejected his manuscript, describing the findings as irrelevant and unimportant. Fortunately, investigations into this new area continued and since that time, there has been growing interest in what is now commonly referred to as the heat shock response.
The heat shock response is characterized by the rapid expression of a unique set of proteins collectively known as heat shock proteins (3–5). These highly conserved proteins have been identified in virtually all eukaryotic and prokaryotic species examined to date. While classically described as a response to thermal stress (hence the term heat shock response) (1, 6), heat shock proteins can be induced by a wide variety of non-thermal stressors and pharmacological agents (Table 1). For this reason, the terms stress response and stress proteins may be more appropriate, though these terms will be used interchangeably throughout the remainder of the present discussion. Whether induced by thermal or non-thermal stress, the stress response confers protection against subsequent and otherwise lethal hyperthermia, a phenomenon that is referred to as thermotolerance (6, 7). Perhaps more interesting from a clinical standpoint is the phenomenon of cross-tolerance, whereby induction of the stress response confers protection against non-thermal cytotoxic stimuli. For example, in vitro experiments have demonstrated that induction of the stress response protects endothelial cells against endotoxin-mediated apoptosis (8). Other examples include stress response-dependent protection against nitric oxide (9), peroxynitrite (10), and hydrogen peroxide (11). In vivo, induction of the stress response protects animals against endotoxemia/sepsis (12, 13), acute lung injury (14, 15), and ischemia-reperfusion (I/R) injury (15).
Table 1.
Inducers of the Stress response
| TYPE OF STRESS | AGENT | COMMENTS |
|---|---|---|
| ENVIRONMENTAL | Temperature | |
| Heavy metals
Ethanol Oxygen radicals |
Cadmium, zinc | |
| METABOLIC | Hyperosmolality
Glucose starvation Tunicamycin Calcium ionophores Amino acid analogs |
|
| CLINICAL | Ischemia/Reperfusion
Shock Anoxia Endotoxin |
Reperfusion seems to be the limiting factor |
| PHARMACOLOGIC | Sodium arsenite | Used extensively in vitro and in vivo |
| Herbimycin A | Tyrosine kinase inhibitor | |
| Geldanamycin | Tyrosine kinase inhibitor and HSP90 inhibitor | |
| Prostaglandin A1 | Other prostaglandins are also active | |
| Dexamethasone Aspirin | Lowers temperature threshold for HSP induction | |
| Non-steroidal anti-inflammatory drugs | Lowers temperature threshold for HSP induction | |
| Pyyrolidine dithiocarbamate | Antioxidant; inhibitor of NFκB | |
| Diethyldithiocarbamate | ||
| Bimoclomol | Hydroxylamine derivative, nontoxic | |
| Serine protease inhibitors | Concomitant inhibition of NF-_B | |
| Curcumin | Major constituent of tumeric; anti-inflammatory | |
| Glutamine | Clinically applicable amino acid | |
| Geranylgeranylacetone | Antiulcerative agent |
The structure, mode of regulation, and function of stress proteins are highly conserved among different species, with well-described bacterial homologs of mammalian stress proteins. These proteins range in molecular weight from 7 to 110 kDa and are found in virtually every cellular compartment, including the nucleus, cytoplasm, and mitochondria (Table 2). By convention, the stress proteins are classified according to their molecular weight, e.g., Hsp25, Hsp32, Hsp47, Hsp60, Hsp70, Hsp90, and Hsp110. Although the expression of stress proteins was initially noted in cells following acute stress (e.g. Hsp72), several members of this family of proteins are constitutively expressed and play important roles in cellular homeostasis. For example, Hsp 90 is constitutively expressed at high levels and is one of the most abundant intracellular proteins. There are also an increasing number of proteins that have well defined cellular functions not directly related to cellular stress, but have been demonstrated to be expressed in response to heat shock and other cellular stresses, e.g., ubiquitin (16, 17); Hsp32, or heme oxygenase (18); inhibitor of κB, IκBα (19–23); endothelial nitric oxide synthase, eNOS (24); and mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP-1) (25–27). While new functions continue to be discovered, the stress proteins are generally thought to maintain cellular homeostasis by acting as molecular chaperones, facilitating the proper folding and assembly of nascent polypeptides, as well as assisting in the refolding and stabilization of damaged peptides (16).
Table 2.
| NAME | SIZE (KDA) | LOCALIZATION | BACTERIAL HOMOLOG | SOME KNOWN AND POSSIBLE FUNCTIONS |
|---|---|---|---|---|
| Ubiquitin | 8 | Cytosol/nucleus | — | Nonlysosomal degradation pathways |
| HSP 27 | 27 | Cytosol/nucleus | Regulator of actin cytoskeleton; molecular chaperone; cytoprotection | |
| Heme | 32 | Bound to ER, | Degradation of heme to bilirubin; | |
| oxygenase | extends to cytoplasm | — | resistance to oxidant stress | |
| HSP 47 | 47 | ER | — | Collagen chaperone |
| HSP 60 | 60 | Mitochondria | Gro EL | Molecular chaperone |
| HSP 70 | 72 | Cytosol/nucleus | Dna K | Highly stress inducible; involved in cytoprotection against diverse agents |
| 73 | Cytosol/nucleus | — | Constitutively expressed chaperone | |
| HSP 90 | 90 | Cytosol/nucleus | htpG | Regulation of steroid hormone activity |
| HSP 110 | 110 | Nucleolus/cytosol | Clp family | Protects nucleoli from stress |
Regulation of Stress Protein Gene Expression
Cells respond to stressful stimuli (e.g., heat shock) by increasing stress protein gene expression at a level that is proportional to the severity of the stress (28). Again, heat stress is the stimulus that has been most thoroughly studied, and it appears that a temperature threshold of 4°C to 8°C above the normal growing temperature is required for induction of cellular stress protein expression (5). However, the temperature threshold at which stress protein genes are expressed may be cell-type specific (29–31). Heat stress affects the tertiary and quarternary structure of intracellular proteins, leading to the partial denaturation and unfolding of these proteins and their subsequent precipitation. The intracellular accumulation of denatured or improperly folded proteins is believed to be the universal signal resulting in the stress-induced gene expression of stress proteins (5, 28, 32, 33). Further experimental support for this purported mechanism has been shown by a series of elegant experiments in which microinjection of denatured proteins into cells results in the up-regulation of stress protein expression (5, 28, 34–37).
A family of transcription factors, known as heat shock factors (HSFs) controls the regulation of stress protein gene expression. Since the initial discovery of the HSF gene in Saccharomyces cerevisiae (38, 39), several different HSFs have been found in both vertebrate and nonvertebrate organisms (40, 41). The human HSFs that have been identified to date include HSF1, −2, and −4, all of which exhibit a similar structure with a highly conserved amino-terminal helix-turn-helix DNA binding domain and a carboxy-terminal transactivation domain (HSF-3 appears to be avian-specific and is not found in humans) (42–46). HSF1 appears to be the most important stress-inducible HSF, and biochemical and genetic studies have clearly demonstrated the critical role of HSF1 in stress-inducible stress protein expression, resistance to stress-induced apoptosis, and extra-embryonic development (41, 47–51). HSF is present under normal, resting conditions as a latent monomer that lacks both DNA binding and transcriptional activity. Following heat shock (or exposure to any of the myriad of stimuli that have been shown to induce the stress response), HSF1 undergoes homotrimerization and rapidly translocates into the nucleus (52, 53).
HSF1 also appears to possess intrinsic stress-sensing activity (51, 54–57). For example, homotrimerization of HSF1 appears to be directly activated in vitro and in vivo in a redox-dependent, reversible manner, via a process requiring two cysteine residues within the DNA-binding domain (51). These two cysteine residues appear to be crucial for nuclear translocation, DNA binding, and activation of stress protein target genes as well, perhaps through the formation of disulfide bonds which maintain HSF1 in a homotrimeric conformation (51, 58). While the exact mechanisms remain to be elucidated, a number of studies support a clear association between the cellular redox state and activation of the stress response (51, 57, 59, 60). For example, HSF1 activation is inhibited under conditions of hypoxia or in the presence of reducing agents such as dithiotreitol (DTT) (61–63).
Stress proteins appear to act as repressor proteins that maintain HSF1 in its non-active, monomeric conformation. Studies suggest that HSF1 monomers form a complex with multiple chaperones and co-chaperones, including Hsp90, Hsp50, and Hsp70 (64). Once a cell is exposed to stress, these chaperones and co-chaperones bind to denatured and damaged proteins, thereby “releasing” the HSF1 monomers to subsequently undergo homotrimerization (28, 40, 64–68). While homotrimerization is sufficient for DNA binding and nuclear translocation, additional steps are required for the complete activation of HSF1. The magnitude and duration of transcriptional activity appears to be regulated by several processes, including inducible phosphorylation of specific serine residues by an as yet undefined pathway (28, 38, 40, 64, 69–72). A number of candidate protein kinases have been shown to phosphorylate HSF1 in vitro, including Erk1/2, glycogen synthase kinase (GSK), JNK, and protein kinase C, among others (64). Once inside the nucleus, HSF1 binds to a heat shock element (HSE), which is defined by a tandem repeat of the pentamer nGAAn (“n” denoting a less conserved sequence) arranged in an alternating orientation either “head to head” (e.g., 5’-nGAAnnTTCn-3’) or “tail to tail” (e.g., 5’-nTTCnnGAAn-3’) (73), resulting in the upregulation of stress protein gene expression.
Hsp27
Hsp27 is an important member of what are commonly referred to as the small stress proteins (small heat shock proteins, sHsps). Hsp27, like many of the other stress proteins discussed below, is highly inducible in the lung following exposure to a variety of stressors, thereby enhancing cellular resistance to heat shock, oxidative stress, and inflammatory mediators such as tumor necrosis factor (TNF)- α in vitro and in vivo (74–79). Hsp27 has been shown in severl in vitro models to inhibit apoptosis, especially following oxidative stress (79). This anti-apoptotic effect appears to occur through mechanisms involving protection of anti-oxidant enzymes (e.g. glucose-6-phosphate dehydrogenase, glutathione reductase, and glutathione transferase – all of which critically affect the intracellular level of reduced glutathione, an important redox modulator in the cell), inhibition of lipid peroxidation, and stabilization of the cytoskeleton. In addition, Hsp27 may chaperone oxidized proteins through the ubiquitin-independent 20S proteasome degradation pathway (79).
Hsp27 is highly induced in peripheral blood monocytes from patients with systemic inflammatory response syndrome (SIRS) (80), and at least one study suggested that increased Hsp27 expression improved mortality in a rat model of endotoxic shock (81). Hsp27 is a substrate for phosphorylation by mitogen-activated protein kinase (MAPK)-activated protein kinase-2 (MAPKAPK-2) (82) and is believed to modulate the cytoskeletal arrangement of actin filaments in pulmonary endothelial cells, in a manner which is at least partially dependent upon its phosphorylation state (83, 84). Importantly, in contrast to the aforementioned studies, Hsp27 phosphorylation temporally correlates with LPS-induced lung injury and increased capillary permeability pulmonary edema in vivo (84).
A growing body of evidence (see below) suggests that extracellular stress proteins, including Hsp27, may be a “danger signal” for the innate immune system. In most cases, extracellular stress proteins are pro-inflammatory in nature. Of interest then is a recent study that showed that extracellular Hsp27 potently induced the anti-inflammatory cytokine, interleukin (IL)-10 in human monocytes in a dose- and time-dependent manner. This induction was independent of any autocrine effects of Hsp27-induced TNF-α (85).
Hsp 32 (Heme oxygenase)
Heme oxygenase (HO) is responsible for catalyzing what is one of the most well-known and common colorimetric reactions in humans - when a common bruise transforms through the spectrum of hues ranging from purple to green to yellow (86, 87). HO is the first and rate limiting step in the degradation of heme (purple hue) to biliverdin (green hue), and finally to bilirubin (yellow hue). Three known isoforms of HO exist: HO-1, -2, and -3. In the context of cytoprotection, HO-1 appears to be the most relevant isoform. HO-1 is identical to Hsp32 and is highly inducible by a variety of cellular stressors and stimuli, including heme, nitric oxide, cytokines, heavy metals, hyperoxia, hypoxia, endotoxin, heavy metals, and heat shock (18, 88–90). HO-1 activity is present in virtually all organs and is thought to primarily account for the cytoprotective properties of HO.
Since the discovery of HO in 1968, several observations have provided notable indications that HO-1 may serve an important cytoprotective role. These include the ability of HO-1 to be highly induced in response to potentially cytotoxic stimuli, its relative high level of conservation throughout evolution, and its wide tissue distribution. The prediction that HO-1 would confer broad cytoprotection has been confirmed by a variety of in vitro and in vivo studies (88, 91). For example, in vitro overexpression of HO-1 conferred protection against oxygen toxicity (hyperoxia) in hamster fibroblasts (92), rat fetal lung cells (93), and human respiratory epithelial cells (94). In a similar manner, overexpression of HO-1 in coronary endothelial cells conferred protection against heme and hemoglobin toxicity (95). In cultured murine fibroblasts, regulated overexpression of HO-1 conferred protection against tumor necrosis factor (TNF)-α -mediated apoptosis (96). Finally, in a human respiratory epithelial cell line representative of lung epithelial cells found in patients with cystic fibrosis, overexpression of HO-1 conferred protection against Pseudomonas-mediated cellular injury and apoptosis (97).
Experiments in animal models, involving either pharmacologic induction of HO-1 or genetic overexpression of HO-1, have confirmed that these in vitro observations apply to the in vivo context. For example, induction of HO-1 by intravenous hemoglobin protected rats against the lethal effects of endotoxemia. Protection in this model correlated with attenuation of endotoxin-mediated hypotension, renal dysfunction, hepatic dysfunction, and inflammation (98, 99). The direct role of HO-1 in conferring protection was further confirmed by co-administration of a competitive inhibitor of HO, tin protoporphyrin, which led to a substantial reduction of the protective effects induced by intravenous hemoglobin administration. Lung epithelial overexpression of HO-1, via an adenovirus vector, conferred protection in rats exposed to hyperoxia (100).
Gene knockout studies have provided further evidence regarding the cytoprotective properties of HO-1 and have been instrumental in further elucidating the biological roles of HO-1. HO-1 null mutant mice generally do not survive to term, and animals that do survive die during the first year of age (101). These animals display severe growth retardation, anemia, iron deposition in the kidneys and liver, and evidence of chronic inflammation in a variety of organs. In other investigations involving HO-1 null mutant mice the animals have been demonstrated to be more susceptible to: 1) renovascular-related hypertension, renal failure, and cardiac hypertrophy (102), 2) endotoxin-mediated lethality (103), and 3) right ventricular dilation and infarction secondary to chronic hypoxia (104). Collectively, the in vitro, in vivo, and gene deletion studies outlined above provide compelling evidence regarding the broad cytoprotective role of HO-1 in clinically relevant forms of cellular and tissue injury. What remains relatively elusive, however, is the mechanism(s) by which HO-1 confers this broad level of protection.
The byproducts of HO enzymatic activity include carbon monoxide (CO), bilirubin, and ferritin, and each of these byproducts have been postulated to play a role in cytoprotection (18, 88, 89). For example, ferritin is known to protect against oxidant stress and bilirubin can function as a potent antioxidant. While it us likely that the three byproducts synergize in some way to confer cytoprotection, the most recent work in the field implicates CO-related cell signaling as the key component of HO-1-mediated cytoprotection (18, 105, 106).
CO shares a variety of properties with another gaseous molecule having ubiquitous biological effects, nitric oxide. These include, neurotransmission, regulation of vascular tone, and activation of soluble guanylate cyclase (106). Other important connections between the CO pathway and the nitric oxide pathway include co-induction of inducible nitric oxide synthase and HO-1 by common stimuli (e.g. reactive oxygen species and cytokines), nitric oxide-dependent induction of HO-1 expression, and CO-dependent modulation of NO production (106–109). The reported biological effects of CO include potent anti-inflammatory effects (via the MAP kinase pathway), anti-apoptotic effects, and antioxidant effects (110–117). In the context of these biological effects, the cytoprotective properties of CO have been demonstrated in a variety of experiments involving direct administration of CO. For example, exogenous administration of CO protected cultured fibroblasts from tumor necrosis factor-α-mediated apoptosis (96). In vivo administration of low concentrations of inhaled CO protected rats from hyperoxia-mediated acute lung injury (114) and administration of exogenous CO to cardiac tissue protected the tissue from ischemia-reperfusion injury following transplantation (118). These in vivo studies are particularly intriguing because the amount of CO administered is within the range administered to humans undergoing lung diffusion scans (105). The rapidly evolving data strongly suggest that HO-1-derived CO is the key mechanism by which HO-1 confers cytoprotection, and further work in this area holds tremendous potential for therapeutic strategies involving HO-1 and/or CO. Interestingly, Kim and colleagues (119) recently demonstrated that Hsp70 can mediate the cytoprotective effects of CO both in vitro and in vivo.
Hsp60
The Hsp60 family of stress proteins are expressed both constitutively and under conditions of stress and can be classified into two groups – mitochondrial Hsp60 (mHsp60) and cytosolic Hsp60 (cHsp60) (120, 121). The mHsp60 forms heptamers and tetradecamers in association with Hsp10 and ATP and is an important part of the mitochondrial chaperone system, while the cHsp60 forms heteroligomeric ring structures to stabilize important cytoskeletal proteins such as actin and tubulin (122, 123). Like many of the other stress proteins, Hsp60 gene expression is highly inducible in response to cell stress. While less is known regarding the specific role of Hsp60 in acute lung injury, Hsp60 has been detected in the bronchial epithelium of patients with asthma (124), nonhuman primates following ozone exposure (77), and human pulmonary microvascular endothelial cells following both heat shock and heavy metal exposure (125). Furthermore, in vivo studies suggest that Hsp60 expression has been shown to ameliorate the progression to pulmonary fibrosis induced by paraquat intoxication (126). Collectively, these studies therefore suggest that Hsp60 confers stress tolerance in the lung. In keeping with the paradigm of extracellular stress proteins as “danger signals”, a recent clinical study suggested that serum levels of Hsp60 within 30 minutes of traumatic injury predicted the development of acute lung injury in adults (127).
Hsp70
Hsp70 expression is well described in both whole lungs and in specific lung cells from a variety of species and in response to a variety of stressors (128). More importantly, in vitro data, as well as data from various animal models of acute lung injury, demonstrate that stress proteins have an important cytoprotective role during lung inflammation and injury (128). For example, induction of the stress response by either thermal stress or sodium arsenite attenuates LPS-mediated apoptosis in cultured sheep pulmonary artery endothelial cells (8), perhaps via an anti-oxidant mechanism, as induction of the stress response was temporally associated with a reduction in LPS-mediated superoxide anion production. Hsp70 appears to be directly involved in this cytoprotective response, as overexpression of Hsp70 via stable transfection inhibited LPS-mediated apoptosis compared to wild-type cells and cells transfected with a control plasmid (8). Similarly, induction of Hsp70 by thermal stress protected cultured murine lung epithelial cells and bovine pulmonary artery endothelial cells from oxidant-mediated injury (11, 129). Overexpression of Hsp70 via stable transfection also protects lung epithelial cells from the deleterious effects of hyperoxia (130) and peroxynitrite (9, 131). Perhaps more compelling, direct delivery of the mature Hsp70 protein using a Tat-Hsp70 protein protects HSF1 null fibroblasts, which lack the intrinsic ability to mount a stress response and are therefore susceptible to hyperoxia-mediated cell death (132), against the deleterious effects of hyperoxia (133). Collectively, these data demonstrate that induction of the stress response protects lung cells against agents that are commonly implicated in the pathophysiology of acute lung injury.
Villar and colleagues were the first to demonstrate that induction of stress response using whole body hyperthermia (41 to 42°C 18 h prior to intratracheal administration of phospholipase A2) protected rats against acute lung injury (14). In addition, whole body hyperthermia increased both Hsp70 mRNA and protein as measured by Northern and Western blot analysis, respectively. These findings were further corroborated by these same investigators in a rat model of acute lung injury induced by cecal ligation and puncture (CLP) (134). Again, induction of Hsp70 mRNA and protein expression in the lung was temporally associated with reduced lung injury and improved survival. Finally, induction of lung Hsp70 expression via administration of sodium arsenite, in the absence of an increase in body temperature, protected rats against CLP-induced lung injury and mortality (135). Ribeiro and colleagues later demonstrated that prior induction of the stress response via whole body hyperthermia decreased lung inflammation and improved lung function in an ex vivo model of ventilator-induced lung injury (VILI) (136). Weiss and colleagues (137) demonstrated that CLP-induced polymicrobial sepsis impairs lung Hsp70 expression in rats and surmised that this intrinsic failure of the normal stress response contributes to acute lung injury in sepsis. Importantly, Hsp70 induction is decreased in the peripheral blood monocytes of patients with acute respiratory distress syndrome (ARDS), further suggesting that failure of the normal stress response contributes to sepsis-induced lung injury (138). Restoration of Hsp70 expression, at least in the pulmonary epithelium, via adenoviral delivery of mature Hsp70 ameliorates lung injury following CLP in rats (139).
Stress proteins and Cytoprotection
The mechanisms by which the stress response confers such broad cytoprotection are not fully understood, but Hsp70 certainly plays a central role. Hsp70 is the most highly induced stress protein in cells and tissues undergoing the stress response (3), and is known to be induced in patients with a variety of critical illnesses or injuries (140–143). Microinjection of anti-Hsp70 antibody into cells impairs their ability to achieve thermotolerance (144), and increased expression of Hsp70 by gene transfer/transfection has been demonstrated to confer protection against in vitro toxicity secondary to lethal hyperthermia (145), endotoxin (8), nitric oxide (9), hyperoxia (130), and in vivo ischemia-reperfusion injury (146–148). In addition, in vitro delivery of mature Hsp70 into the intracellular compartment has been shown to protect fibroblasts against lethal thermal injury and hyperoxia (133), and neuronal cells against nitrosative stress and excitotoxicity (149). Mice deficient in HSF1 have a drastically reduced ability to express Hsp70 (47, 48). Cell lines derived from these animals can not achieve thermotolerance and are highly susceptible to oxidant stress compared to cells from wild-type mice (47, 48, 132). When challenged with systemic endotoxin, HSF1-deficient mice have increased mortality compared to wild-type mice (48). Collectively, these data demonstrate that Hsp70 is central to the cytoprotective properties of the stress response. The mechanisms by which Hsp70 and other stress proteins confer protection are not fully understood, but most likely relate to the ability of stress proteins to serve as molecular chaperones by binding, re-folding, transporting, and stabilizing damaged intracellular proteins.
Another potential mechanism by which the stress response may confer cytoprotection is by modulating inflammatory responses. The stress response has been demonstrated to inhibit the expression of a number of genes related to inflammation, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, inducible nitric oxide synthase, IL-8, RANTES, C3, macrophage chemotactic protein-1, and intracellular adhesion molecule-1 (22, 129, 131, 150–157). In addition, it has been postulated that the inhibitory effects of the stress response are relatively selective for inflammation-associated genes (21). The mechanisms by which the stress response inhibits proinflammatory gene expression involve inhibition of NF-κB. Several in vitro and in vivo studies have demonstrated that induction of the stress response inhibits activation of NF-κB, a pluripotent transcription factor that regulates the expression of many genes associated with inflammation (21, 158, 159). The latest work in the area has identified IκB kinase (IKK) as the most upstream target through which the stress response modulates NF-κB activity. IKK is the rate limiting step in the activation of NF-κB in that it phosphorylates the endogenous NF-κB inhibitor, IκBα. Phosphorylation of IκBα leads to its rapid degradation by a proteasome/ubiquitin-dependent mechanism, thus releasing NF-κB to enter the nucleus. Induction of the stress response inhibits activation of IKK, in part by an intracellular phosphatase-dependent mechanism (159–161). Inhibition of IKK subsequently inhibits phosphorylation and degradation of IκBα (161), thus keeping NF-κB in an inactive state.
Recent work suggests that the modulating effects of the stress response on inflammation-related signal transduction also involves the de novo expression of proteins not traditionally considered to be classified as stress proteins. For example, it is now well established that the endogenous NF-κB inhibitory protein, IκBα, is expressed in response to heat shock both in vitro and in vivo (19, 23, 157). More recent work has demonstrated that the dual specific phosphatase, MKP-1, is also expressed in response to heat shock (26, 27). MKP-1 is a potent counter regulator of many proinflammatory signal transduction pathways. Thus, stress response-associated induction of IκBα and MKP-1 gene expression serves as another mechanism by which the stress response can inhibit NF-κB activity and inflammation-associated signal transduction.
Recent studies suggest that HSF1 acts directly as a transciptional repressor of several proinflammatory proteins, including IL-1β, c-fos, urokinase, and TNF-α (162, 163). For example, HSF1 binds the 5’-untranslated region of the murine TNF-α gene to repress transcription (162). In a similar manner, HSF1 represses transcription of the gene for IL-1β through physical interaction with the CCAAT/enhancer binding protein (163).
Stress proteins and Innate Immunity
Until relatively recently, stress proteins were considered to be exclusively intracellular proteins, but as mentioned briefly above, a growing body of literature suggests that stress proteins may also exist and function outside of the cell. For example, several studies suggest that cellular stress results in the increased surface expression and release of stress proteins (164–177). The release of stress proteins into the extracellular environment was first reported in the late 1980s, when Hsp70 release was demonstrated in cultured rat embryo cells following exposure to increased temperature (178). Importantly, Hsp70 release did not appear to be mediated via classic secretory pathways, as it was not inhibited by either colchicine or monensin. Cell lysis was not involved either, as Hsp70 release was not detected following exposure to non-ionic detergents. Hsp70 synthesized in the presence of a lysine amino acid analogue (aminoethyl cysteine) was not released from these cells, suggesting that the altered protein structure prevented interaction with the specific release mechanism (178).
While some studies suggest that Hsp70 is released in a non-specific manner from dying, necrotic cells (167, 179), we and others (169, 171, 178, 180–183) have shown that viable cells release Hsp70 in a specific and inhibitable manner. Monensin and brefeldin A are inhibitors of the classic endoplasmic reticulum (ER)/Golgi protein transport and secretory pathways. We have shown that Hsp70 release from THP-1 cells is not inhibited by either monensin or brefeldin A (Wheeler and Wong, unpublished data). Others have shown that Hsp70 release from peripheral blood monocytes (PBMC) is inhibited by brefeldin A, but not monensin (182, 183). In this study, Hsp70 release was also inhibited by methylamine and methyl-β-cyclodextrin, both of which inhibit protein secretion via lysosomal pathways (183). Soluble proteins are usually secreted via a classic, ER/Golgi dependent secretory pathway. The Hsp70 gene does not encode for the classic N-terminal peptide leader sequence targeting it for secretion via this pathway (184), which is consistent with the data presented here. Recent studies suggest that Hsp70 is actively released via an exosome-dependent, non-classical protein secretory pathway (177, 185).
Important work in this area suggests that the release of stress proteins from stressed cells may serve to signal an impending danger signal to neighboring cells, the so-called danger hypothesis (176, 186). For example, extracellular Hsp70 elicits NF-κB-dependent, pro-inflammatory gene expression via Toll-like receptor (TLR)4 and TLR2 (187, 188). Therefore, release of Hsp70, whether from dying, necrotic cells or viable, but damaged cells, can activate the host innate immune response.
Several properties would suggest Hsp70 to be a biologically plausible and likely candidate to serve as a host danger signal. Collectively stress proteins are the most abundant intracellular proteins, representing up to 10% of the total protein content in the cell (189). Hsp70 in particular is markedly induced in response to a diverse range of cellular insults, including increased temperature, oxidative stress, glucose deprivation, chemical exposure, I/R injury, ultraviolet radiation, and infectious agents such as LPS. Therefore, Hsp70 or additional inducible stress proteins, by virtue of their relative abundance during times of stress, are reliable markers of cell stress (i.e., danger).
As previously noted, stress proteins are ancient, highly conserved molecules that have been identified in virtually every organism examined to date. In comparison, LPS, an important exogenous danger signal, appeared relatively late on the evolutionary time-scale and is much less ubiquitous, being unique to only gram-negative bacteria. It is perhaps no mere coincidence that Hsp70-mediated and LPS-mediated activation of the host innate immune response occurs via similar mechanisms (i.e. TLR-4-mediated signal transduction), and it is plausible that the programmed response to the exogenous danger signal, LPS, is modeled on the more primitive programmed response to the endogenous danger signal (189).
Stress proteins, particularly Hsp70, are also highly immunogenic and have the capacity to mediate the induction of peptide-specific immunity. For example, as molecular chaperones, stress proteins bind to many peptides derived from the cells from which they are isolated. Stress protein-peptide complexes elicit potent T cell responses against the chaperoned peptide as well as the cell type from which the chaperoned peptide is derived, including tumors and viruses, and vaccination with stress protein-tumor peptide complexes as an immunotherapy for cancer is an active area of investigation (189–194). Similarly, stress protein-pathogen-derived peptide complexes have the capacity to elicit a pathogen-specific immune response (193). Finally, stress proteins themselves, especially members of the Hsp60 and Hsp70 families, have the capacity to activate the host innate immune response, resulting in dendritic cell activation and maturation, activation of complement, and release of proinflammatory cytokines (179, 187, 188, 195–203). Collectively, these observations would suggest the stress proteins to be likely candidates for endogenous danger signals - they are abundant, inducible, highly conserved, and appear to activate both the innate and adaptive immune response.
Extracellular Hsp70 (increased serum levels) is evident in a variety of clinical scenarios associated with physiologic stress. For example, increased extracellular Hsp70 levels are noted following strenuous exercise (180, 181, 204–208), following cardio-pulmonary bypass in both adults (209, 210) and children (Wheeler, unpublished data), in elderly adults (211, 212), and in children with acute lung injury (Wheeler, unpublished data). In addition, increased extracellular Hsp70 concentrations correlate with worse outcome in a variety of inflammatory disease processes, including liver disease (213), coronary artery disease (214–217), traumatic brain injury secondary to child abuse (218), pre-eclampsia (219), sickle cell disease vaso-occlusive crisis (220), septic shock (221), and traumatic brain injury (222). Conversely, increased extracellular Hsp70 levels correlated with improved outcome following multiple trauma in adults (141).
Given the signaling properties recently ascribed to Hsp70, these data generate a number of functional questions. First and foremost, is extracellular Hsp70 merely a marker of cellular stress, or could the release of Hsp70 potentiate an already active host immune response, thereby leading to poor outcome? Second, could it be possible that extracellular Hsp70 serves an as yet undefined cytoprotective function at lower levels as a normal response to infection or stress, and once a certain critical threshold is attained potentiate the dysregulated inflammatory response that subsequently results in significant auto-injury to the host? The results correlating increased Hsp70 levels to improved outcome in adults with trauma support this latter concept (141). Future investigation in this area will likely address these questions.
Conclusion
In this review, we have attempted to describe the stress response, one of the most powerful and ubiquitous endogenous mechanisms that exists to counter-act the multitude of cytotoxic stimuli that can adversely affect the human host. While stress protein expression is not unique to the lung compartment, the well described biochemical properties and regulation of stress proteins suggest that they can play an important role in the context of acute lung injury. Much of the lung-specific literature indicates that this role is primarily cytoprotective. However, the emerging data involving extracellular Hsp72 indirectly suggest that within a certain milieu, extracellular Hsp72 could contribute to the pathophysiology of acute lung injury. Ongoing work will further elucidate these issues and it remains to be determined if stress protein expression can be manipulated as a therapeutic strategy. In this regard, Weischmeyer and colleagues have generated interesting data indicating that the amino acid, glutamine, may be a feasible approach to inducing Hsp72 in the clinical setting as a strategy to protect against acute lung injury (223–226).
Figure 1. Potential roles of heat shock proteins (HSP) in acute lung injury.
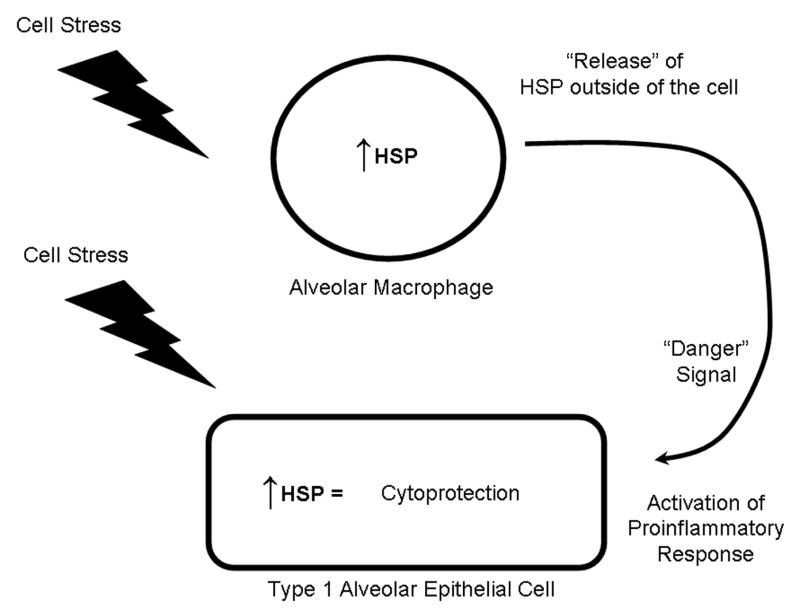
The expression of heat shock proteins in both alveolar macrophages and airway and alveolar epithelial cells is upregulated in response to a myriad of cell stressors, including LPS, free radicals, thermal stress, and hypoxia. Increased expression of heat shock proteins results in a cytoprotective response in these cell types. Release of heat shock proteins (by an as yet unidentified mechanism) may serve as a “danger signal” to activate the inflammatory response in surrounding cells.
Footnotes
Supported by the Children’s Hospital Research Foundation and the National Institutes of Health, R01-GM61723 (HRW), R01-GM064619 (HRW), K08GM077432-01 (DSW)
References
- 1.Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 2.Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92(5):2177–86. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler DS, Wong HR. Immunobiology of Organ Transplantation. Kluwer Academic/Plenum Publishers; New York: 2004. The heat shock response and transplantation immunology. [Google Scholar]
- 5.De Maio A. The heat shock response. New Horiz. 1995;3:198–207. [PubMed] [Google Scholar]
- 6.Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature. 1975;256(5517):500–2. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- 7.Hasday JD, I, Singh S. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. 2000;5(5):471–80. doi: 10.1379/1466-1268(2000)005<0471:fathsr>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HR, Mannix RJ, Rusnak JM, Boota A, Zar H, Watkins SC, Lazo JS, Pitt BR. The heat-shock response attenuates lipopolysaccharide-mediated apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1996;15(6):745–51. doi: 10.1165/ajrcmb.15.6.8969269. [DOI] [PubMed] [Google Scholar]
- 9.Wong HR, Ryan M, Menendez IY, Denenberg A, Wispe JR. Heat shock protein induction protects human respiratory epithelium against nitric oxide-mediated cytotoxicity. Shock. 1997;8(3):213–8. doi: 10.1097/00024382-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C, Wong HR, Salzman AL. Pre-exposure to heat shock inhibits peroxynitrite-induced activation of poly(ADP) ribosyltransferase and protects against peroxynitrite cytotoxicity in J774 macrophages. Eur J Pharmacol. 1996;315(2):221–6. doi: 10.1016/s0014-2999(96)00628-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang YR, Xiao XZ, Huang SN, Luo FJ, You JL, Luo H, Luo ZY. Heat shock pretreatment prevents hydrogen peroxide injury of pulmonary endothelial cells and macrophages in culture. Shock. 1996;6(2):134–41. doi: 10.1097/00024382-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ryan AJ, Flanagan SW, Moseley PL, Gisolfi CV. Acute heat stress protects rats against endotoxin shock. J Appl Physiol. 1992;73(4):1517–22. doi: 10.1152/jappl.1992.73.4.1517. [DOI] [PubMed] [Google Scholar]
- 13.Hauser GJ, Dayao EK, Wasserloos K, Pitt BR, Wong HR. HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am J Physiol. 1996;271(6 Pt 2):H2529–35. doi: 10.1152/ajpheart.1996.271.6.H2529. [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Edelson JD, Post M, Mullen JB, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis. 1993;147(1):177–81. doi: 10.1164/ajrccm/147.1.177. [DOI] [PubMed] [Google Scholar]
- 15.Hiratsuka M, Yano M, Mora BN, Nagahiro I, Cooper JD, Patterson GA. Heat shock pretreatment protects pulmonary isografts from subsequent ischemia-reperfusion injury. J Heart Lung Transplant. 1998;17(12):1238–46. [PubMed] [Google Scholar]
- 16.Bond U, Schlesinger MJ. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985;5:949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller-Taubenberger A, Hagmann J, Noegel A, Gerisch G. Ubiquitin gene expression in Dictyostelium is induced by heat and cold shock, cadmium, and inhibitors of protein synthesis. J Cell Sci. 1988;90:51–58. doi: 10.1242/jcs.90.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235(1–2):249–63. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong HR, Ryan MA, Menendez IY, Wispe JR. Heat shock activates the I-kappaBalpha promoter and increases I-kappaBalpha mRNA expression. Cell Stress Chaperones. 1999;4(1):1–7. doi: 10.1006/csac.1998.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMeester SL, Buchman TG, Qui Y, Jacob AK, Dunnigan K, Hotchkiss RS, Karl I, Cobb JP. Heat shock induces IkappaB-alpha and prevents stress-induced endothelial cell apoptosis. Arch Surg. 1997;132:1283–1287. doi: 10.1001/archsurg.1997.01430360029005. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra V, Wong HR. Interactions between the heat shock response and the nuclear factor-kappa B signaling pathway. Crit Care Med. 2002;30(1 Suppl):S89–95. [PubMed] [Google Scholar]
- 22.Thomas SC, Ryan MA, Shanley TP, Wong HR. Induction of the stress response with prostaglandin A1 increases I-kappaBalpha gene expression. Faseb J. 1998;12(13):1371–8. doi: 10.1096/fasebj.12.13.1371. [DOI] [PubMed] [Google Scholar]
- 23.Wong HR, Ryan M, Wispe JR. Stress response decreases NF-kappaB nuclear translocation and increases I-kappaBalpha expression in A549 cells. J Clin Invest. 1997;99(10):2423–8. doi: 10.1172/JCI119425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285:H333–H340. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- 25.Gorostizaga A, Brion L, Maloberti P, Maciel FC, Podesta EJ, Paz C. Heat shock triggers MAPK activation and MKP-1 induction in Leydig testicular cells. Biochem Biophys Res Commun. 2005;327:23–28. doi: 10.1016/j.bbrc.2004.11.129. [DOI] [PubMed] [Google Scholar]
- 26.Sanlorenzo L, Zhao B, Spight D, Denenberg AG, Page K, Wong HR, Shanley TP. Heat shock inhibition of lipopolysaccharide-mediated tumor necrosis factor expression is associated with nuclear induction of MKP-1 and inhibition of mitogen-activated protein kinase activation. Crit Care Med. 2004;32(11):2284–92. doi: 10.1097/01.ccm.0000145580.96994.c9. [DOI] [PubMed] [Google Scholar]
- 27.Wong HR, Dunsmore KE, Page K, Shanley TP. Heat shock-mediated regulation of MKP-1. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00138.2005. [DOI] [PubMed] [Google Scholar]
- 28.Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukary Gene Expr. 1994;4:375–401. [PubMed] [Google Scholar]
- 29.Gothard LQ, Ruffner ME, Woodward JG, Park-Sarge OK, Sarge KD. Lowered temperature set point for activation of the cellular stress response in T-lymphocytes. J Biol Chem. 2003;278:9322–9326. doi: 10.1074/jbc.M209412200. [DOI] [PubMed] [Google Scholar]
- 30.Ostberg JR, Kaplan KC, Repasky EA. Induction of stress proteins in a panel of mouse tissue by fever-range whole body hyperthermia. Int J Hyperthermia. 2002;18:552–562. doi: 10.1080/02656730210166168. [DOI] [PubMed] [Google Scholar]
- 31.Sarge KD. Male germ cell-specific alteration in temperature set point of the cellular stress response. J Biol Chem. 1995;270:18745–18748. doi: 10.1074/jbc.270.32.18745. [DOI] [PubMed] [Google Scholar]
- 32.Kelly PM, Schlessinger MJ. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978;15:1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- 33.De Maio A. Heat shock proteins: Facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Baler R, Welch WJ, Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: Hsp70 as a potential autoregulatory factor. J Cell Biol. 1992;117:1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anathan J, Goldberg AL, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger activation of heat shock genes. Science. 1986;232:252–254. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 36.Trotter EW, Kao CM, Berenfeld L, Botstein D, Petsko GA, Gray JV. Misfolded proteins are competent to mediate a subset of the responses to heat shock in Saccharomyces cerevisiae. J Biol Chem. 2002;277:44817–44825. doi: 10.1074/jbc.M204686200. [DOI] [PubMed] [Google Scholar]
- 37.Hightower LE. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980;102:407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- 38.Sorger PK, Pelham HR. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:841–853. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- 39.Wiederrecht G, Oehler R, Derkits S, Oismuller C, Fugger R, Roth E. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54:841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- 40.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 41.Christians ES, Yan LJ, Benjamin IJ. Heat shock factor 1 and heat shock proteins: Critical partners in protection against acute cell injury. Crit Care Med. 2002;30:S43–S50. [PubMed] [Google Scholar]
- 42.Rabindran SK, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci U S A. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuetz TJ, Gallo GJ, Sheldon L, Tempst P, Kingston RE. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc Natl Acad Sci U S A. 1991;88:6911–6915. doi: 10.1073/pnas.88.16.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green M, Schuetz TJ, Sullivan EK, Kingston RE. A heat-shock responsive domain of human HSF1 that regulates transcription activation domain function. Mol Cell Biol. 1995;15:3354–3362. doi: 10.1128/mcb.15.6.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sistonen L, Sarge KD, Morimoto RI. Human heat shock factors 1 and 2 are differentially activated and can synergistically induce hsp70 gene transcription. Mol Cell Biol. 1994;14:2087–2099. doi: 10.1128/mcb.14.3.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273(13):7523–8. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 48.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. Embo J. 1999;18(21):5943–52. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christians E, Davis AA, Thomas SD, Benjamin IJ. Maternal effect of HSF1 on reproductive success. Nature. 2000;407:693–694. doi: 10.1038/35037669. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem. 2002;86:376–393. doi: 10.1002/jcb.10232. [DOI] [PubMed] [Google Scholar]
- 51.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor 1. Mol Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodson ML, Sarge KD. Heat-inducible DNA binding of purified heat shock transcription factor 1. J Biol Chem. 1995;270:2447–2450. doi: 10.1074/jbc.270.6.2447. [DOI] [PubMed] [Google Scholar]
- 55.Morimoto RI. Regulation of the heat shock transcriptional response: Cross-talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 56.Zhong M, Kim SJ, Wu C. Sensitivity of Drosophila heat shock transcription factor to low pH. J Biol Chem. 1999;274:3135–3140. doi: 10.1074/jbc.274.5.3135. [DOI] [PubMed] [Google Scholar]
- 57.Ahn SG, Liu PCC, Klyachko K, Morimoto RI, Thiele DJ. The loop domain of heat shock transcription factor 1 dictates DNA-binding specificity and responses to heat stress. Genes Dev. 2001;15:2134–2145. doi: 10.1101/gad.894801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manalo DJ, Lin Z, Liu AYC. Redox-dependent regulation of the conformation and function of human heat shock factor 1. Biochemistry. 2002;41:2580–2588. doi: 10.1021/bi0159682. [DOI] [PubMed] [Google Scholar]
- 59.Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem. 2000;275:35377–35383. doi: 10.1074/jbc.M005287200. [DOI] [PubMed] [Google Scholar]
- 60.Paroo Z, Meredith MJ, Locke M, Haist JV, Karmazyn M, Noble EG. Redox signaling of cardiac HSF1 DNA binding. Am J Cell Physiol. 2002;283:C404–C411. doi: 10.1152/ajpcell.00051.2002. [DOI] [PubMed] [Google Scholar]
- 61.Huang LE, Zhang H, Bae SW, Liu AYC. Thiol reducing agents inhibit the heat shock response. J Biol Chem. 1994;269:301718–30725. [PubMed] [Google Scholar]
- 62.Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1996;93:5116–5121. doi: 10.1073/pnas.93.10.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquier-Sarlin MR, Polla B. Dual regulation of heat shock transcription factor (HSF) activation and DNA-binding activity by H2O2: Role of thioredoxin. J Biol Chem. 1996;318:187–193. doi: 10.1042/bj3180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satyal S, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev. 1998;12:1962–1974. doi: 10.1101/gad.12.13.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baler R, Zhou J, Voellmy R. Evidence for a role of Hsp70 in the regulation of the heat shock response in mammalian cells. Cell Stress Chaperones. 1996;1:33–39. doi: 10.1379/1466-1268(1996)001<0033:efaroh>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding XZ, Tsokos GC, Kiang JG. Overexpression of HSP-70 inhibits the phosphorylation of HSF1 by activating protein phosphatase and inhibiting protein kinase C activity. FASEB J. 1998;12:451–459. doi: 10.1096/fasebj.12.6.451. [DOI] [PubMed] [Google Scholar]
- 69.Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor I DNA binding precedes stress-induced serine phosphorylation: Evidence for a multistep pathway of regulation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 70.Xia W, Voellmy R. Hyperphosphorylation of heat shock transcription factor 1 is correlated with transcriptional competence and slow dissociation of active factor trimers. J Biol Chem. 1997;272:4094–4102. doi: 10.1074/jbc.272.7.4094. [DOI] [PubMed] [Google Scholar]
- 71.Morimoto RI, Kline M, Bimston DN, Cotto JJ. The heat shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- 72.Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: Cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999;64:105–118. [PubMed] [Google Scholar]
- 73.Amin J, Ananthan J, Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988;8:3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blake MJ, Gershon D, Fargnoli J, Holbrook NJ. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990;265:15275–15279. [PubMed] [Google Scholar]
- 75.Hastie AT, Everts KB, Zangrilli J, Shaver JR, Pollice MB, Fish JE, Peters SB. HSP27 elevated in mild allergic inflammation protects airway epithelium from H2SO4 effects. Am J Physiol. 1997;273:L401–L409. doi: 10.1152/ajplung.1997.273.2.L401. [DOI] [PubMed] [Google Scholar]
- 76.Williams KJ, Cruikshank MK, Plopper CG. Pulmonary heat shock protein expression after exposure to a metabolically activated Clara cell toxicant: relationship to protein adduct formation. Toxicol Appl Pharmacol. 2003;192(2):107–18. doi: 10.1016/s0041-008x(03)00302-8. [DOI] [PubMed] [Google Scholar]
- 77.Wu R, Zhao YH, Plopper CG, Chang MM, Chmiel K, Cross JJ, Weir A, Last JA, Tarkington B. Differential expression of stress proteins in nonhuman primate lung and conducting airway after ozone exposure. Am J Physiol. 1999;277:L511–L522. doi: 10.1152/ajplung.1999.277.3.L511. [DOI] [PubMed] [Google Scholar]
- 78.Meredino AM, Paul C, Vignola AM, Costa MA, Melis M, Chiappara G, Izzo V, Bousquet J, Arrigo AP. Heat shock protein-27 protects human bronchial epithelial cells against oxidative stress-mediated apoptosis: Possible implication in asthma. Cell Stress Chaperones. 2002;7:269–280. doi: 10.1379/1466-1268(2002)007<0269:hspphb>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arrigo AP. Hsp27: A novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 80.Hashiguchi N, Ogura H, Tanaka H, Koh T, Nakamori Y, Noborio M, Shiozaki T, Nishino M, Kuwagata Y, Shimazu T, Sugimoto H. Enhanced expression of heat shock proteins in activated polymorphonuclear leukocytes in patients with sepsis. J Trauma. 2001;51(6):1104–9. doi: 10.1097/00005373-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 81.Wischmeyer P, Wolfson R, Musch M, Chang E, Kahana M. Glutamine induces heat shock protein and prevents mortality from endotoxemia in the rat. Shock. 2000;13:28. (abstract). [Google Scholar]
- 82.Knauf U, Jakob U, Engel K, Buchner J, Gaestel M. Stress- and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1994;13:54–60. doi: 10.1002/j.1460-2075.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 MAP-kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 84.Hirano S, Rees RS, Yancy SL, Welsh MJ, Remick DG, Yamada T, Hata J, Gilmont RR. Endothelial barrier dysfunction caused by LPS correlates with phosphorylation of HSP27 in vivo. Cell Biol Toxicol. 2004;20:1–14. doi: 10.1023/b:cbto.0000021019.50889.aa. [DOI] [PubMed] [Google Scholar]
- 85.De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exagerrated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- 86.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244(23):6388–94. [PubMed] [Google Scholar]
- 88.Morse D, Choi AM. Heme oxygenase-1: the "emerging molecule" has arrived. Am J Respir Cell Mol Biol. 2002;27(1):8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 89.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1029–37. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 90.Taketani S, Kohono H, Yoshinaga T, Tokunaga R. The human 32kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989;245:173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- 91.Morse D, Choi AMK. Heme oxygenase-1: From bench to bedside. Am J Respir Crit Care Med. 2005;172:660–670. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 92.Dennery PA, Sridhar KJ, Lee CS, Wong HE, Shokoohi V, Rodgers PA, Spitz DR. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997;272(23):14937–42. doi: 10.1074/jbc.272.23.14937. [DOI] [PubMed] [Google Scholar]
- 93.Suttner DM, Sridhar K, Lee CS, Tomura T, Hansen TN, Dennery PA. Protective effects of transient HO-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am J Physiol. 1999;276(3 Pt 1):L443–51. doi: 10.1152/ajplung.1999.276.3.L443. [DOI] [PubMed] [Google Scholar]
- 94.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996;93(19):10393–8. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci U S A. 1995;92(15):6798–802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000;278(2):L312–9. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 97.Zhou H, Lu F, Latham C, Zander DS, Visner GA. Heme oxygenase-1 expression in human lungs with cystic fibrosis and cytoprotective effects against Pseudomonas aeruginosa in vitro. Am J Respir Crit Care Med. 2004;170(6):633–40. doi: 10.1164/rccm.200311-1607OC. [DOI] [PubMed] [Google Scholar]
- 98.Otterbein L, Sylvester SL, Choi AM. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol. 1995;13(5):595–601. doi: 10.1165/ajrcmb.13.5.7576696. [DOI] [PubMed] [Google Scholar]
- 99.Otterbein L, Chin BY, Otterbein SL, Lowe VC, Fessler HE, Choi AM. Mechanism of hemoglobin-induced protection against endotoxemia in rats: a ferritin-independent pathway. Am J Physiol. 1997;272(2 Pt 1):L268–75. doi: 10.1152/ajplung.1997.272.2.L268. [DOI] [PubMed] [Google Scholar]
- 100.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103(7):1047–54. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94(20):10919–24. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, DiFonzo N, Rennke HG, Layne MD, Yet SF, Lee ME, Perrella MA. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ Res. 2001;88(10):1088–94. doi: 10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- 103.Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102(24):3015–22. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- 104.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103(8):R23–9. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi AM, Otterbein LE. Emerging role of carbon monoxide in physiologic and pathophysiologic states. Antioxid Redox Signal. 2002;4(2):227–8. doi: 10.1089/152308602753666271. [DOI] [PubMed] [Google Scholar]
- 106.Morse D, Sethi J, Choi AM. Carbon monoxide-dependent signaling. Crit Care Med. 2001;30(1 Supp):S12–S17. [PubMed] [Google Scholar]
- 107.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, Bach FH, Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198(11):1707–16. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mayer RD, Wang X, Maines MD. Nitric oxide inhibitor N omega -nitro-l-arginine methyl ester potentiates induction of heme oxygenase-1 in kidney ischemia/reperfusion model: a novel mechanism for regulation of the oxygenase. J Pharmacol Exp Ther. 2003;306(1):43–50. doi: 10.1124/jpet.102.048686. [DOI] [PubMed] [Google Scholar]
- 109.Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG, Jr, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L346–53. doi: 10.1152/ajplung.00385.2001. [DOI] [PubMed] [Google Scholar]
- 110.Sethi JM, Otterbein LE, Choi AM. Differential modulation by exogenous carbon monoxide of TNF-alpha stimulated mitogen-activated protein kinases in rat pulmonary artery endothelial cells. Antioxid Redox Signal. 2002;4(2):241–8. doi: 10.1089/152308602753666299. [DOI] [PubMed] [Google Scholar]
- 111.Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L209–16. doi: 10.1152/ajplung.2001.281.1.L209. [DOI] [PubMed] [Google Scholar]
- 112.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166(6):4185–94. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 113.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 114.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276(4 Pt 1):L688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 115.Ke B, Buelow R, Shen XD, Melinek J, Amersi F, Gao F, Ritter T, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum Gene Ther. 2002;13(10):1189–99. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 116.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2002;55(2):396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 117.Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4(2):321–9. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- 118.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. Faseb J. 2004;18(6):771–2. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 119.Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AMK. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: Involvement of p38beta MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 120.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 121.Levy-Rimler G, Bell RE, Ben-Tal N, Azem A. Type I chaperonins: Not all are created equal. FEBS Lett. 2002;529:1–5. doi: 10.1016/s0014-5793(02)03178-2. [DOI] [PubMed] [Google Scholar]
- 122.Richardson A, Landry SJ, Georgopoulos C. The ins and outs of a molecular chaperone machine. Trends Biochem Sci. 1998;23:138–143. doi: 10.1016/s0968-0004(98)01193-1. [DOI] [PubMed] [Google Scholar]
- 123.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 124.Fajac I, Roisman GL, Lacronique J, Polla BS, Dusser DJ. Bronchial gamma delta T-lymphocytes and expression of heat shock proteins in mild asthma. Eur Respir J. 1997;10:633–638. [PubMed] [Google Scholar]
- 125.Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 126.Kaetsu A, Fukushima T, Inoue S, Lim H, Moriyama M. Role of heat shock protein 60 (HSP60) on paraquat intoxication. J Appl Toxicol. 2001;21:425–430. doi: 10.1002/jat.774. [DOI] [PubMed] [Google Scholar]
- 127.Pespeni M, Mackersie RC, Lee H, Morabito D, Hodnett M, Howard M, Pittet JF. Serum levels of Hsp60 correlate with the development of acute lung injury after trauma. J Surg Res. 2005;126:41–47. doi: 10.1016/j.jss.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 128.Wong HR, Wispe JR. The stress response and the lung. Am J Physiol Lung Cell Mol Physiol. 1997;17:L1–L9. doi: 10.1152/ajplung.1997.273.1.L1. [DOI] [PubMed] [Google Scholar]
- 129.Wong HR, Ryan M, Gebb S, Wispe JR. Selective and transient in vitro effects of heat shock on alveolar type II cell gene expression. Am J Physiol. 1997;272(1 Pt 1):L132–8. doi: 10.1152/ajplung.1997.272.1.L132. [DOI] [PubMed] [Google Scholar]
- 130.Wong HR, I, Menendez Y, Ryan MA, Denenberg AG, Wispe JR. Increased expression of heat shock protein-70 protects A549 cells against hyperoxia. Am J Physiol. 1998;275(4 Pt 1):L836–41. doi: 10.1152/ajplung.1998.275.4.L836. [DOI] [PubMed] [Google Scholar]
- 131.Wong HR, Finder JD, Wasserloos K, Pitt BR. Expression of iNOS in cultured rat pulmonary artery smooth muscle cells is inhibited by the heat shock response. Am J Physiol. 1995;269(6 Pt 1):L843–8. doi: 10.1152/ajplung.1995.269.6.L843. [DOI] [PubMed] [Google Scholar]
- 132.Malhotra V, Kooy NW, Denenberg AG, Dunsmore KE, Wong HR. Ablation of the heat shock factor-1 increases susceptibility to hyperoxia-mediated cellular injury. Exp Lung Res. 2002;28(8):609–22. doi: 10.1080/01902140260426724. [DOI] [PubMed] [Google Scholar]
- 133.Wheeler DS, Dunsmore KE, Wong HR. Intracellular delivery of HSP70 using HIV-1 Tat protein transduction domain. Biochem Biophys Res Commun. 2003;301(1):54–9. doi: 10.1016/s0006-291x(02)02986-8. [DOI] [PubMed] [Google Scholar]
- 134.Villar J, Ribeiro SP, Mullen BM, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–921. [PubMed] [Google Scholar]
- 135.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22:922–929. doi: 10.1097/00003246-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Ribeiro SP, Rhee K, Tremblay L, Veldhuizen R, Lewis JF, Slutsky AS. Heat stress attenuates ventilator-induced lung dysfunction in an ex vivo rat lung model. Am J Respir Crit Care Med. 1999;163:1451–1456. doi: 10.1164/ajrccm.163.6.9908076. [DOI] [PubMed] [Google Scholar]
- 137.Weiss YG, Bouwman A, Gehan B, Schears G, Raj N, Deutschman CS. Cecal ligation and double puncture impairs heat shock protein 70 (Hsp-70) expression in the lungs of rats. Shock. 2000;13:19–23. doi: 10.1097/00024382-200013010-00004. [DOI] [PubMed] [Google Scholar]
- 138.Durand P, Bachelet M, Brunet F, Richard MJ, Dhainaut JF, Dall'ava J, Polla BS. Inducibility of the 70 kD heat shock protein in peripheral blood monocytes is decreased in human acute respiratory distress syndrome and recovers over time. Am J Respir Crit Care Med. 2000;161:286–292. doi: 10.1164/ajrccm.161.1.9812150. [DOI] [PubMed] [Google Scholar]
- 139.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kindas-Mugge I, Hammerle AH, Frohlich I, Oismuller C, Micksche M, Trautinger F. Granulocytes of critically ill patients spontaneously express the 72 kD heat shock protein. Circ Shock. 1993;39(4):247–52. [PubMed] [Google Scholar]
- 141.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52(4):611–7. doi: 10.1097/00005373-200204000-00001. discussion 617. [DOI] [PubMed] [Google Scholar]
- 142.Lai Y, Kochanek PM, Adelson PD, Janesko K, Ruppel RA, Clark RS. Induction of the stress response after inflicted and non-inflicted traumatic brain injury in infants and children. J Neurotrauma. 2004;21(3):229–37. doi: 10.1089/089771504322972022. [DOI] [PubMed] [Google Scholar]
- 143.Wheeler DS, Fisher LE, Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6(3):308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 144.Riabowol KT, Mizzen LA, Welch WJ. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988;242(4877):433–6. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- 145.Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991;88(5):1681–5. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hiratsuka M, Mora BN, Yano M, Mohanakumar T, Patterson GA. Gene transfer of heat shock protein 70 protects lung grafts from ischemia-reperfusion injury. Ann Thorac Surg. 1999;67(5):1421–7. doi: 10.1016/s0003-4975(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 147.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95(4):1446–56. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95(4):1854–60. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lai Y, Du L, Dunsmore KE, Jenkins LW, Wong HR, Clark RS. Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J Neurochem. 2005;94(2):360–6. doi: 10.1111/j.1471-4159.2005.03212.x. [DOI] [PubMed] [Google Scholar]
- 150.Schmidt JA, Abdulla E. Down-regulation of IL-1 beta biosynthesis by inducers of the heat-shock response. J Immunol. 1988;141(6):2027–34. [PubMed] [Google Scholar]
- 151.Snyder YM, Guthrie L, Evans GF, Zuckerman SH. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukoc Biol. 1992;51(2):181–7. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- 152.Ayad O, Stark JM, Fiedler MM, Menendez IY, Ryan MA, Wong HR. The heat shock response inhibits RANTES gene expression in cultured human lung epithelium. J Immunol. 1998;161(5):2594–9. [PubMed] [Google Scholar]
- 153.Moon R, Pritts TA, Parikh AA, Fischer JE, Salzman AL, Ryan M, Wong HR, Hasselgren PO. Stress response decreases the interleukin-1beta-induced production of complement component C3 in human intestinal epithelial cells. Clin Sci (Lond) 1999;97(3):331–7. [PubMed] [Google Scholar]
- 154.Malhotra V, Eaves-Pyles T, Odoms K, Quaid G, Shanley TP, Wong HR. Heat shock inhibits activation of NF-kappaB in the absence of heat shock factor-1. Biochem Biophys Res Commun. 2002;291(3):453–7. doi: 10.1006/bbrc.2002.6470. [DOI] [PubMed] [Google Scholar]
- 155.Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, Morris C, Stark J, Shanley TP. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17(2):91–7. doi: 10.1097/00024382-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 156.Wong HR, Ryan M, Wispe JR. The heat shock response inhibits inducible nitric oxide synthase gene expression by blocking I kappa-B degradation and NF-kappa B nuclear translocation. Biochem Biophys Res Commun. 1997;231(2):257–63. doi: 10.1006/bbrc.1997.6076. [DOI] [PubMed] [Google Scholar]
- 157.Pritts TA, Wang Q, Sun X, Fischer DR, Hungness ES, Fischer JE, Wong HR, Hasselgren PO. The stress response decreases NF-kappaB activation in liver of endotoxemic mice. Shock. 2002;18(1):33–7. doi: 10.1097/00024382-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 158.Curry HA, Clemens RA, Shah S, Bradbury CM, Botero A, Goswami P, Gius D. Heat shock inhibits radiation-induced activation of NF-kappaB via inhibition of I-kappaB kinase. J Biol Chem. 1999;274(33):23061–7. doi: 10.1074/jbc.274.33.23061. [DOI] [PubMed] [Google Scholar]
- 159.Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164(10):5416–23. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- 160.Grossman BJ, Shanley TP, Odoms K, Dunsmore KE, Denenberg AG, Wong HR. Temporal and mechanistic effects of heat shock on LPS-mediated degradation of IkappaBalpha in macrophages. Inflammation. 2002;26(3):129–37. doi: 10.1023/a:1015552515183. [DOI] [PubMed] [Google Scholar]
- 161.Shanley TP, Ryan MA, Eaves-Pyles T, Wong HR. Heat shock inhibits phosphorylation of I-kappaBalpha. Shock. 2000;14(4):447–50. doi: 10.1097/00024382-200014040-00005. [DOI] [PubMed] [Google Scholar]
- 162.Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5'-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem. 2002;277:4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- 163.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002;277:11802–11810. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- 164.Gao YL, Brosnan CF, Raine CS. Experimental autoimmune encephalomyelitis. Qualitative and semiquantitative differences in heat shock protein 60 expression in the central nervous system. J Immunol. 1995;154:3548–3556. [PubMed] [Google Scholar]
- 165.Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;5:315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- 166.Belles C, Kuhl A, Nosheny R, Carding SR. Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect Immun. 1999;67:4191–4200. doi: 10.1128/iai.67.8.4191-4200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 168.Hantschel M, Pfister K, Jordan A, et al. HSP 70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5:438–442. doi: 10.1379/1466-1268(2000)005<0438:hpmeop>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914(1–2):66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 170.Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood. 2001;97:3505–3512. doi: 10.1182/blood.v97.11.3505. [DOI] [PubMed] [Google Scholar]
- 171.Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 172.Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171(11):5736–42. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 173.Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- 174.Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumour growth and metastasis. Tumour Biol. 2004;25:243–251. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: Relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 176.Lang A, Benke F, Eitner D, Engel S, Ehrlich M, Breloer E, Hamilton-Williams S, Specht A, Hoerauf J, Floege A, von Bonin, Kurts C. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: In vivo evidence for an immunologic danger signal. J Am Soc Nephrol. 2005;16:383–391. doi: 10.1681/ASN.2004040276. [DOI] [PubMed] [Google Scholar]
- 177.Bausero M, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 179.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappaB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 180.Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting skeletal muscle. J Physiol. 2002;538:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ernani FP, Teale JM. Release of stress proteins from Mesocestoides corti is a brefeldin A-inhibitable process: evidence for active export of stress proteins. Infect Immun. 1993;61(6):2596–601. doi: 10.1128/iai.61.6.2596-2601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hunter-Lavin C, Davies EL, Bacelar MMFVG, Marshall MJ, Andrew SM, Williams JHH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 184.Nickel W. The mystery of nonclassical protein secretion: A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 185.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 186.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 187.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 188.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 189.Anderson KM, Srivastava PK. Heat, heat shock, heat shock proteins and death: A central link in innate and adaptive immune responses. Immunol Lett. 2000;74:35–39. doi: 10.1016/s0165-2478(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 190.Robert J. Evolution of heat shock protein and immunity. Dev Comp Immunol. 2003;27(6–7):449–64. doi: 10.1016/s0145-305x(02)00160-x. [DOI] [PubMed] [Google Scholar]
- 191.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 192.Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: Primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 193.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 194.Pockley AG. Heat shock proteins in health and disease: Therapeutic targets or therapeutic agents? Expert Rev Mol Med. 2001;2001:1–21. doi: 10.1017/S1462399401003556. [DOI] [PubMed] [Google Scholar]
- 195.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/Interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 197.Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, Galle PR, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169(11):6141–8. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 198.Panjwani NN, Popova L, Srivastava PK. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol. 2002;168:2997–3003. doi: 10.4049/jimmunol.168.6.2997. [DOI] [PubMed] [Google Scholar]
- 199.Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7(1):17–22. doi: 10.1379/1466-1268(2002)007<0017:hspiap>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 201.Radsak MP, Hilf N, Singh-Jasuja H, Braedel S, Brossart P, Rammensee HG, Schild H. The heat shock protein Gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood. 2003;101:2810–2815. doi: 10.1182/blood-2002-07-2261. [DOI] [PubMed] [Google Scholar]
- 202.Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionall significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tsan MF, Gao B. Endogenous ligans of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 204.Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6(4):386–93. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schneider EM, Niess AM, Lorenz I, Northoff H, Fehrenbach E. Inducible Hsp70 expression analysis after heat and physical exercise: Transcriptional, protein expression, and subcellular localization. Ann N Y Acad Sci. 2002;973:8–12. doi: 10.1111/j.1749-6632.2002.tb04598.x. [DOI] [PubMed] [Google Scholar]
- 206.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Campisi J, Fleshner M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol. 2003;94:43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- 208.Banfi G, Dolci A, Verna R, Corsi MM. Exercise raises serum heat-shock protein 70 (Hsp70) levels. Clin Chem Lab Med. 2004;42(12):1445–6. doi: 10.1515/CCLM.2004.268. [DOI] [PubMed] [Google Scholar]
- 209.Sharma M, Ganguly NK, Chaturvedi G, Thingnam SKS, Majumdar S, Suri RK. A possible role of HSP70 in mediating cardioprotection in patients undergoing CABG. Mol Cell Biochem. 2003;247:31–36. doi: 10.1023/a:1024148825262. [DOI] [PubMed] [Google Scholar]
- 210.Dybdahl B, Wahba A, Haaverstad R, Kirkeby-Garstad I, Kierulf P, Espevik T, Sundan A. On-pump versus off-pump coronary artery bypass grafting: more heat-shock protein 70 is released after on-pump surgery. Eur J Cardiothorac Surg. 2004;25(6):985–92. doi: 10.1016/j.ejcts.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 211.Njemini R, Demanet C, Mets T. Inflammatory status as an important determinant of heat shock protein 70 serum concentrations during aging. Biogerontology. 2003;5:31–38. doi: 10.1023/b:bgen.0000017684.15626.29. [DOI] [PubMed] [Google Scholar]
- 212.Terry DF, McCormick M, Andersen S, Pennington J, Schoenhofen E, Palaima E, Bausero M, Ogawa K, Perls TT, Asea A. Cardiovascular disease delay in centenarian offspring: role of heat shock proteins. Ann N Y Acad Sci. 2004;1019:502–5. doi: 10.1196/annals.l297.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kimura F, Itoh H, Ambiru S, Shimizu H, Togawa A, Yoshidome H, Ohtsuka M, Shimamura F, Kato A, Nukui Y, Miyazaki M. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg. 2004;187(6):777–84. doi: 10.1016/j.amjsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 214.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20(9):1815–20. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 215.Pockley AG, Georgiades A, Thulin T, de Faire U, Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 2003;42(3):235–8. doi: 10.1161/01.HYP.0000086522.13672.23. [DOI] [PubMed] [Google Scholar]
- 216.Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ishaemia and the inflammatory response: Release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Genth-Zotz S, Bolger AP, Kalra PR, von Haehling S, Doehner W, Coats AJS, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: Relation to disease severity and survival. Int J Cardiol. 2003;96:397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 218.Lai Y, Kochanek PM, Adelson PD, Janesko K, Ruppel RA, Clark RSB. Induction of the stress response after inflicted and non-inflicted traumatic brain injury in infants and children. J Neurotrauma. 2004;21:229–237. doi: 10.1089/089771504322972022. [DOI] [PubMed] [Google Scholar]
- 219.Fukushima A, Kawahara H, Isurugi C, Syoji T, Oyama R, Sugiyama T, Horiuchi M. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res. 2005;31:72–77. doi: 10.1111/j.1447-0756.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 220.Adewoye AH, Klings ES, Farber HW, Palaima E, Bausero MA, McMahon L, Odhiambo A, Surinder S, Yoder M, Steinberg MH, Asea A. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am J Hematol. 2005;78:240–242. doi: 10.1002/ajh.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wheeler DS, Fisher J, Catravas LEJD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6:308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 222.Da Rocha AB, Zanoni C, De Freitas GR, Andre C, Himelfare S, Schneider RF, Grivicich I, Borges L, Schwartsmann G, Kaufmann M, Regner A. Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma. 2005;22:966–977. doi: 10.1089/neu.2005.22.966. [DOI] [PubMed] [Google Scholar]
- 223.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med. 2005;31(8):1079–86. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 224.Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005;33(6):1206–13. doi: 10.1097/01.ccm.0000166357.10996.8a. [DOI] [PubMed] [Google Scholar]
- 225.Singleton KD, Serkova N, Banerjee A, Meng X, Gamboni-Robertson F, Wischmeyer PE. Glutamine attenuates endotoxin-induced lung metabolic dysfunction: potential role of enhanced heat shock protein 70. Nutrition. 2005;21(2):214–23. doi: 10.1016/j.nut.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 226.Wischmeyer P. John M. Kinney International Award for General Nutrition. Glutamine, heat shock protein, and inflammation--opportunity from the midst of difficulty. Nutrition. 2004;20(6):583–5. doi: 10.1016/j.nut.2004.03.006. [DOI] [PubMed] [Google Scholar]


