Abstract
Producing antibodies usually takes more than three months. In the present study, we introduce a faster way of producing polyclonal antibodies based on preparation of the recombinant oligopeptide as antigen followed by immunization of rats. Using this method, we produced antisera against two mouse proteins: ERGIC-53 and c-Kit. An expression vector ligated with a pair of complementary synthetic oligodeoxyribonucleotides encoding the protein was introduced into bacteria, and the recombinant oligopeptide fused with the carrier protein glutathione-S-transferase was purified. Wistar rats were immunized by injecting the emulsified antigen subcutaneously into the hind footpads, followed by a booster injection after 2 weeks. One week after the booster, the sera were collected and examined for the antibody titer by immunohistochemistry. Antisera with 1600-fold titer at the maximum were obtained for both antigens and confirmed for their specificity by Western blotting. Anti-ERGIC-53 antisera recognized acinar cells in the sublingual gland, and anti-c-Kit antisera recognized spermatogenic and Leydig cells in the testis. These antisera were applicable to fluorescent double immunostaining with mouse monoclonal or rabbit polyclonal antibodies. Consequently, this method enabled us to produce specific rat polyclonal antisera available for immunohistochemistry in less than one month at a relatively low cost.
Keywords: rat polyclonal antibodies, recombinant oligopeptides, Wistar rat, immunohistochemistry
I. Introduction
Antibodies are indispensable tools to analyze protein functions in the broad area of life science. They are particularly useful for immunohistochemistry, a technique that visualizes the specific tissue- and cellular localization of proteins and other antigens [2, 8]. In many instances, we can purchase monoclonal and polyclonal antibodies from commercial sources, or obtain them from other researchers. However, in such cases where we are to study novel or rare proteins or when commercial antibodies are of unsatisfactory quality, we need to produce the antibodies for ourselves or order them as custom products.
In general, producing antibodies, whether they are polyclonal or monoclonal, is costly and time-consuming [3]. When producing polyclonal antibodies in rabbits, many researchers, unless they own the antigen proteins purified from living sources, employ either recombinant proteins or synthetic oligopeptides as antigens. Recombinant proteins are usually produced in bacteria by introducing the expression vector ligated with the entire coding sequence of antigen proteins. However, the full-length proteins produced by bacteria are often difficult to isolate because of their insolubility. Synthetic oligopeptides are composed of 10 to 25 amino acid residues representing portions of the antigen proteins that possess high specificity and antigenicity. However, it usually requires several weeks of time and considerable cost to obtain oligopeptides as custom products. In both cases, two or more rabbits are immunized by repeatedly injecting the antigens subcutaneously at two-week intervals over a period of a few months. Consequently, the total time required for antigen preparation and immunization exceeds three months. On the other hand, when producing monoclonal antibodies in mice, the whole procedure consisting of immunization of inbred (Balb/c) mice by injecting the antigens intraperitoneally, fusion of splenic B lymphocytes with myeloma cells, and cloning of the hybridoma cell strain in culture takes at least six months.
Recently, Sado et al. introduced a novel way of producing monoclonal antibodies in rats called the “rat lymph node method” [5, 9, 10]. This method is characterized by injection of antigens into the skin of footpads followed by collection of B cells from medial iliac lymph nodes in an inbred (WKY) rat strain. This method takes a much shorter time than the ordinary method of mouse monoclonal antibody production, because it employs only a single injection of antigen followed by collection of lymph nodes two weeks later. Although the same authors suggested that the antisera collected from the rats at the time of sacrifice would be available as polyclonal antibodies, the antisera often have too low antibody titers for use in immunohistochemistry, probably because of the short immunization period. The booster immunization was avoided in this method for the purpose of increasing the frequency of positive hybridomas after the fusion of B cells with myeloma cells.
These considerations led us to explore an improved method of obtaining polyclonal antibodies in a much shorter time. This method includes production of the recombinant oligopeptide in bacteria and modification of the rat lymph node method for producing polyclonal antibodies. In the present study, we used this new method to raise antisera against two distinct membranous proteins, ERGIC-53 and c-Kit. ERGIC-53 is a transmembrane animal L-type lectin known to be involved in the transport of certain glycoproteins from the rough endoplasmic reticulum (ER) to Golgi apparatus in the early secretory pathway. A subcellular membrane structure named ER-Golgi intermediate compartment (ERGIC) has been postulated based on the localization of ERGIC-53 [14]. C-Kit is a transmembrane receptor tyrosine kinase and postulated to play roles in the early developmental phenomena including hematopoiesis, gametogenesis and melanogenesis. In the testis, c-Kit is considered as a marker of the differentiating spermatogonia and Leydig cells [17]. Several new findings using these polyclonal antisera in mouse sublingual gland and testis will be presented.
II. Materials and Methods
Animals
Ten-week-old female Wistar rats for immunization and ten-week-old male Slc:ddY mice for immunohistochemistry, both in closed colonies, were purchased from Nippon SLC, Inc. (Hamamatsu, Japan). They were maintained under 12 hr light/12 hr dark laboratory conditions with free access to the standard food and water. All procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals in Kanazawa University.
Preparation of antigens
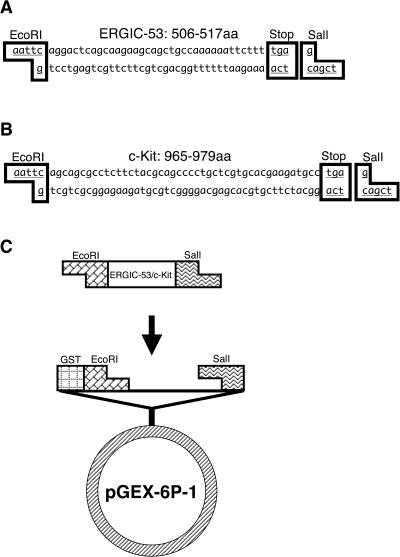
Pairs of complementary synthetic oligodeoxyribonucleotides for ERGIC-53 (GenBank accession number, AK011495), and c-Kit (GenBank accession number, NM_ 021099 [1]) were purchased from Japan BioService (Asaka, Japan) as custom products. The sense and antisense strands were designed to form a double stranded DNA that contains a cDNA portion coding 12 or 15 amino acids of ERGIC-53 or c-Kit, respectively, a stop codon, and the EcoRI and SalI restriction enzyme sites at the 5' and 3' ends, respectively (Fig. 1). They were annealed by incubating in TE buffer with 150 mM sodium chloride for 10 min at 65°C and then allowed to cool down to room temperature (RT). The resultant double stranded DNA was ligated with the bacterial expression vector pGEX-6p-1 (Amersham Pharmacia Biotech, Uppsala, Sweden) at the EcoRI/SalI restriction enzyme sites within the multicloning site located downstream of the promoter and glutathione-S-transferase (GST)-coding regions. The constructed vector was then introduced into the bacteria BL21 (Novagen, Madison, WI) and a transformed colony was picked up and cultured in ampicillin-containing LB medium up to the optical density 0.5 (600 nm) at 37°C. Expression of the recombinant oligopeptide fused with the carrier protein GST was induced by adding 1 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) to the media and further incubating the bacteria for 2 hr at 37°C. The bacteria were then homogenized in B-PER bacterial protein extraction reagent (Pierce, Rockford, IL). Following centrifugation of the homogenate, the supernatant containing the soluble recombinant GST-fused oligopeptide (subsequently called the recombinant protein) for ERGIC-53 or c-Kit was incubated with Glutathione Sepharose 4B (Amersham Pharmacia Biotech) overnight at 4°C. The complex of the recombinant protein and Glutathione Sepharose 4B was washed once with PBS containing 0.1% Triton-X by centrifugation, 4 times with PBS and then eluted 4 times with 20 mM glutathione in 0.1 M Tris-HCl (pH 8.0) containing 0.1 M sodium chloride. The concentration and molecular weight of the recombinant protein in the eluent was estimated by SDS-polyacrylamide gel electrophoresis (PAGE). An aliquot of the eluent was electrophoresed in an acrylamide gel, stained with Coomassie brilliant blue for 1 hr, and then washed in the destaining solution containing 5% acetic acid and 7.5% methanol overnight.
Fig. 1.
Antigen preparation method. (A, B) Pairs of complementary synthetic oligodeoxyribonucleotides for ERGIC-53 (A) or c-Kit (B) were annealed to form a double-stranded DNA that contains a cDNA portion encoding ERGIC-53 or c-Kit, a stop codon, and the EcoRI and SalI restriction enzyme sites. (C) The double-stranded DNA was ligated with the bacterial expression vector pGEX-6p-1 (Amersham Pharmacia Biotech, Uppsala, Sweden) at the EcoRI/SalI restriction enzyme sites located downstream of the promoter and Glutathione-S-transferase (GST)-coding regions.
Immunization of rats and production of polyclonal antibodies
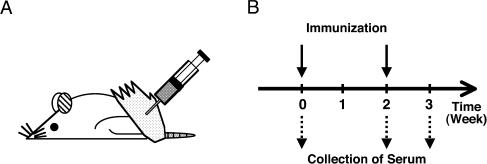
Female Wistar rats were anesthetized with sodium pentobarbital (75 mg/kg body weight) by intraperitoneal injection, and a 100 µl peripheral blood sample was collected from the tail vein for preparation of preimmune serum. Three rats were immunized for each antigen. Each rat was injected subcutaneously in the hind footpads with 200 µl emulsion containing 200 µg recombinant protein as antigen in physiological saline and Freund’s complete adjuvant (Difco, Detroit, MI) mixed at 1:1 (Fig. 2). Two weeks later, a 100 µl blood sample was collected for measurement of the serum antibody titers, followed by a booster injection of the animals with the same amount of antigen emulsified with Freund’s incomplete adjuvant. One week after the booster, the animals were sacrificed by deep anesthesia, the whole blood was collected through the pulmonary vein, and the serum was separated by leaving the blood for 30 min at 37°C, followed by centrifugation. After determination of the antibody titers, aliquots of the antisera for practical use were absorbed with GST at a concentration of 100 µg/ml for 1 hr at RT to block the antibodies reactive with GST and stored at 4°C with 0.05% sodium azide. The remaining antisera were stored at −80°C. All subsequent experiments were performed with the GST-absorbed antisera.
Fig. 2.
Immunization method. (A) The antigen emulsified with the adjuvant was injected subcutaneously into the hind footpads of rats. (B) The initial immunization was performed at 0 week followed by the booster after 2 weeks. Sera were collected at 0, 2 and 3 weeks after the initial immunization.
Western blot analysis
Aliquots of cell lysates from mouse sublingual gland and testis containing 30 µg proteins were subjected to SDS-PAGE followed by transfer to PVDF membranes (Bio-Rad Laboratories, Hercules, CA). The blots were incubated with rat anti-mouse ERGIC-53 antisera at 1:2000 dilution or anti-mouse c-Kit antisera at 1:3000 dilution at 4°C overnight. After washing, the blots were further incubated with horseradish peroxidase-labeled anti-rat IgG antibody (DAKO, Glostrup, Denmark) at 1:5000 dilution for 1 hr at RT. The immunoreaction was detected with X-ray film (Kodak, New Haven, CT, USA) after treatment of the blots with a chemiluminescent reagent ECL-plus (Amersham Pharmacia Biotech, Uppsala, Sweden).
Tissue preparation and antibody titration with immunohistochemistry
Male Slc:ddY mice were anesthetized with sodium pentobarbital, killed by bleeding from the right atrium, and perfused transcardially with cold physiological saline followed by 4% cold paraformaldehyde (Nacalai Tesque, Kyoto, Japan) in 0.1 M phosphate buffer (pH 7.4) for fixation. The sublingual gland and testis were dissected out, further immersed in the same fixative overnight at 4°C and then rinsed in PBS containing 30% sucrose overnight at 4°C for cryoprotection. They were then frozen and cut into 8-µm sections using a cryostat (Leica Microsystems, Wetzlar, Germany).
For fluorescent immunostaining, the sections were treated with 5% normal goat serum in PBS for 1 hr to prevent non-specific antibody binding. Subsequently, the sections of sublingual gland or testis were incubated overnight at 4°C with the samples of rat anti-mouse ERGIC-53 or anti-mouse c-Kit antisera diluted 25 to 3200 times with PBS. For control, the preimmune serum was used with the same dilution. After washing the sections with PBS, the immunoreaction was visualized by incubating the sections with either anti-rat IgG antibodies or anti-rat IgM antibodies conjugated with Alexa Fluor 594 (Molecular Probes, Eugene, OR) at 1:400 dilution for 1 hr at RT. After washing, the sections were mounted in glycerol and subjected to examination with a fluorescent microscope (Olympus BX50/BX-FLA) using green emission. The number of the maximum dilution of antisera to provide positive immunoreaction in the submandibular gland (for ERGIC-53) or testis (for c-Kit), as compared with the negative reaction with the same dilution of preimmune serum, was defined as the antibody titer of the antisera. Both the titers for IgG and IgM antibodies were obtained by using Alexa Fluor 594-labeled anti-IgG and anti-IgM secondary antibodies, respectively.
Fluorescent double-immunostaining
The sections of sublingual gland were incubated with the mixture of rat polyclonal anti mouse ERGIC-53 antisera (1:400 dilution) and mouse anti-BiP/GRP78 monoclonal antibody (BD Biosciences; San Jose, CA; 2 µg/ml) or mouse anti-GM130 monoclonal antibody (BD Biosciences; San Jose, CA; 5 µg/ml) overnight at 4°C. Similarly, the sections of testis were incubated with the mixture of rat anti-mouse c-Kit antisera (1:400 dilution) and rabbit anti-mouse SgIGSF polyclonal antibodies ([15, 16]; 1 µg/ml) overnight at 4°C. After washing with PBS, the sites of immunoreaction were visualized by incubating the sections successively with the mixture of Alexa Fluor 594-labelled anti-rat IgG (1:400 dilution) and Alexa Fluor 488-labelled anti-mouse IgG (1:400 dilution) or Alexa Fluor 488-labelled anti-rabbit IgG (1:400 dilution) antibodies for 1 hr at RT. After washing, the sections were counterstained in the nuclei with 10 ng/ml of Hoechst 33258 (Sigma-Aldrich, St. Louis, MO) for 5 min, mounted in glycerol and subjected to examination with a fluorescent microscope (Olympus BX50/BX-FLA, Tokyo, Japan) using green emission for Alexa Fluor 594, blue emission for Alexa Fluor 488 and UV emission for Hoechst 33258. To confirm specificity of the immunoreaction, the primary antisera were replaced with the preimmune serum or preabsorbed with the antigen used for the immunization (50 µg recombinant protein in 1 ml of diluted antisera) for 1 hr at RT prior to use.
III. Results
Production of recombinant oligopeptides as antigens
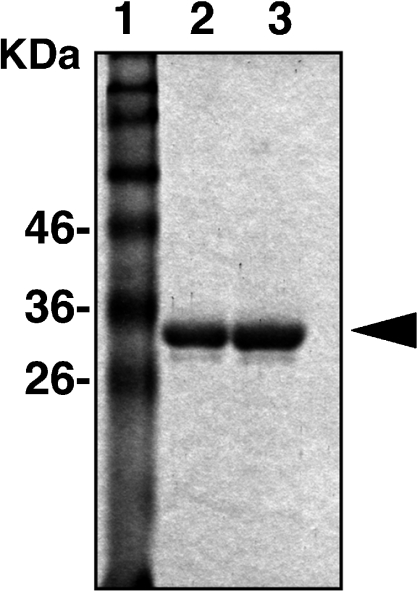
The recombinant oligopeptides for ERGIC-53 and c-Kit, which were purified by absorbing the bacterial homogenates with Glutathione-Sepharose 4B, were subjected to analysis with SDS-PAGE (Fig. 3). Single protein bands about 30 kDa in size, representing the fused proteins of GST (25 kDa) and oligopeptides, were detected without contamination with other bacterial proteins.
Fig. 3.
SDS-PAGE analysis of the recombinant GST-fused oligopeptides for ERGIC-53 (lane 2) and c-Kit (lane 3). Aliquots of the bacterial products purified with Glutathione Sepharose 4B were electrophoresed and stained with Coomassie brilliant blue. The standard markers were also electrophoresed (lane 1) with the molecular weights indicated.
Production of the antisera in rats
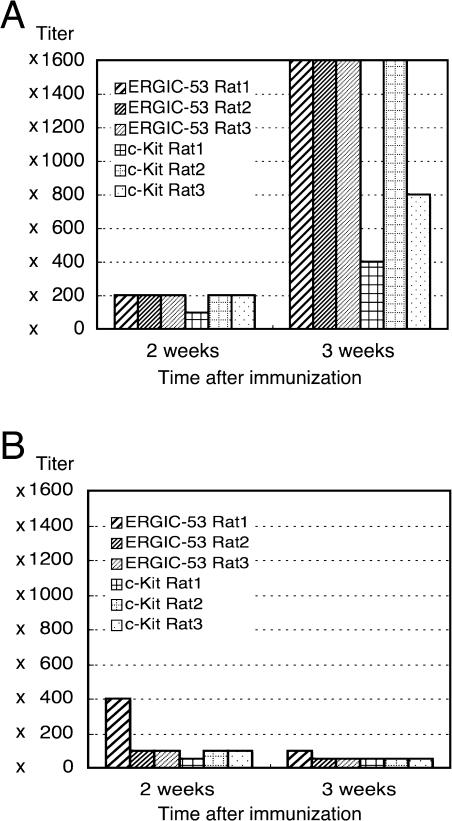
The titers of the IgG and IgM antibodies against ERGIC-53 and c-Kit were measured in the sera of 3 rats for each antigen at two time points, i.e., two weeks after the initial immunization and one week after the single booster immunization (Fig. 4A, B). For both antigens, the titers of the IgG antibodies were no more than 200-fold the first time but showed remarkable rises the second time, up to as high as 1600-fold, suggesting the effectiveness of the single booster immunization. There was some heterogeneity in the antibody titers among the rats immunized with c-Kit. Rats with a lower titer the first time tended to show a comparatively lower titer the second time. In contrast, those with titers of IgM antibodies that were 50–400-fold the first time tended to remain as low as 50–100-fold the second time, suggesting that the majority of antibodies produced after the booster belonged to IgG.
Fig. 4.
Histograms showing antibody titers in the antisera for ERGIC-53 and c-Kit collected at 2 and 3 weeks after the initial immunization. The antibody titer was defined as number of the maximum dilution of antisera that gives the positive immunostaining. (A) Titers of the IgG-class antibodies. (B) Titers of the IgM-class antibodies in the same antisera as in A.
Specificity of the antisera
On Western blot analysis, the mouse sublingual gland showed a single immunopositive band of about 58 kDa in size, which is consistent with known 517 amino acid residues for mouse ERGIC-53 (GenBank accession number, NP_081676). Also, the testis showed a broad immunopositive band of about 140–160 kDa in size representing mouse c-Kit [1] (Fig. 5). There was no extra immunopositive band including the one for GST about 25 kDa in size. These results indicated that the present rat antisera specifically recognized mouse ERGIC-53 or c-Kit.
Fig. 5.

Western blot analysis showing ERGIC-53 in mouse sublingual gland (A) and c-Kit in testis (B). The homogenate sample from each organ was electrophoresed, blotted and immunostained with anti-ERGIC-53 antisera or anti-c-Kit antisera. The positions of the molecular weight markers are indicated.
Immunohistochemical characterization of the antisera
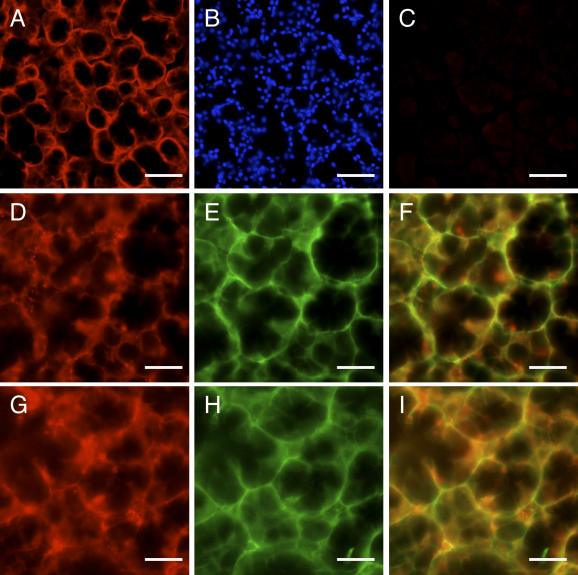
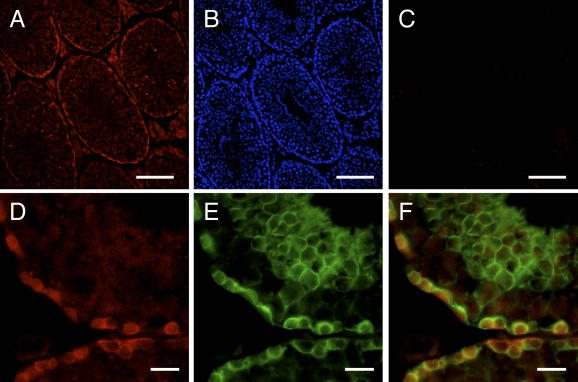
In the mouse sublingual gland, rat anti-ERGIC-53 antisera primarily immunostained acinar cells, where the immunoreactivity appeared to be localized to thin cytoplasmic areas outside the secretory granules (Fig. 6A, B). Absorption of the primary antibodies with the antigen recombinant protein eliminated the immunostaining completely, confirming the specificity of the antisera (Fig. 6C). To further determine the subcellular localization of ERGIC-53, double immunostaining was performed using rat anti-ERGIC-53 antisera combined with the mouse monoclonal antibody against BiP/GRP78, an ER marker [6], or GM130, a cis-Golgi marker [7]. The immunoreactivity for ERGIC-53 overlapped partially, but not completely, with that for both BiP/GRP78 and GM130 (Fig. 6D–I). In the mouse testis, rat anti-c-Kit antisera immunostained the plasma membrane of Leydig cells and a subpopulation of seminiferous epithelial cells (Fig. 7A, B). Again, absorption of the primary antibodies eliminated the immunostaining completely (Fig. 7C). Double immunostaining was performed using anti-c-Kit antisera combined with the rabbit polyclonal antibodies against SgIGSF, an adhesion molecule known to be expressed in spermatogenic cells [15] (Fig. 7A–F). SgIGSF was localized to the membrane of both elongating spermatids and the earlier-phase spermatogenic cells located on the basement membrane. The latter cell population overlapped with c-Kit-positive spermatogenic cell population almost completely.
Fig. 6.
Light micrographs showing the fluorescent immunohistochemical analysis of mouse sublingual gland for ERGIC-53. (A) A section was immunostained with anti-ERGIC-53 antisera at 400-fold dilution. The immunoreactivity (red) is present in the cytoplasmic regions of acinar cells outside the secretory granules. (B) The same section was counterstained with Hoechst 33258 (blue) in the nucleus. (C) The neighboring section was immunostained with the antigen-absorbed anti-ERGIC-53 antisera. No immunoreaction is recognized. (D–F) Double immunostaining of a section with anti-ERGIC-53 antisera and mouse monoclonal anti-BiP/GRP78 antibodies. The immunoreactivities for ERGIC-53 (red, D) and BiP/GRP78 (green, E) in the acinar cells slightly overlap (yellow) when the picture is merged (F). (G–I) Double immunostaining of a section with anti-ERGIC-53 antisera and mouse monoclonal anti-GM130 antibodies. The immunoreactivities for ERGIC-53 (red, G) and GM130 (green, H) in the acinar cells moderately overlap (yellow) when the picture is merged (I). Bars=100 µm (A–C) and 25 µm (D–I).
Fig. 7.
Light micrographs showing the fluorescent immunohistochemical analysis of mouse testis for c-Kit. (A) A section was immunostained with anti-c-Kit antisera at 400-fold dilution. The immunoreactivity (red) is present in the basal regions of seminiferous tubules and in Leydig cells. (B) The same section was counterstained with Hoechst 33258 (blue) in the nucleus. (C) The neighboring section was immunostained with the antigen-absorbed anti-c-Kit antisera. No immunoreaction is recognized. (D–F) Double immunostaining of a section with anti-c-Kit antisera and rabbit polyclonal anti-SgIGSF antibodies. The immunoreactivities for c-Kit (red, D) and SgIGSF (green, E) in the early spermatogenic cells located at the basal regions of seminiferous epithelium mostly overlap (yellow) when the picture is merged (F). The SgIGSF-positive elongating spermatids located at the apical regions of seminiferous epithelium are negative for c-Kit. Bars=100 µm (A–C) and 25 µm (D–F).
IV. Discussion
The present method of producing antibodies available for immunohistochemistry as well as Western blotting is novel in two respects: the preparation of the recombinant oligopeptide as antigen and the immunization of rats to raise polyclonal antisera. To prepare the antigen, we used synthetic complementary oligodeoxyribonucleotides designed to produce a double-stranded DNA flanked by the restriction enzyme sites, which was then ligated with the bacterial expression vector to produce recombinant oligopeptides. Similar use of oligodeoxyribonucleotides was introduced by Sasaki et al. [12] to produce labeled ribonucleotide probes for in situ hybridization. The recombinant oligopeptide adopted in the present method possesses advantages of both the recombinant full-length protein and the synthetic oligopeptide. The recombinant oligopeptide, like the recombinant full-length protein, can yield a large amount of antigen fused with the carrier protein GST as bacterial product. Furthermore, the former is more easily solubilized than the latter due to its lower molecular weight. On the other hand, the recombinant oligopeptide, like the synthetic oligopeptide, can be designed according to the selective sequence with high specificity and antigenecity. Although the former cannot be obtained as a custom product like the latter, it is less expensive and requires a shorter time to produce.
We employed the rat as an immune animal, which is much less expensive and requires much smaller amount of antigens (less than 1 mg per animal) than the rabbit. To obtain polyclonal rat antibodies, we employed the “rat lymph node method”, which was originally developed for monoclonal antibody production [9], with several modifications. Firstly, we used Slc:Wistar, a rat strain in a closed-colony, instead of WKY, an inbred rat strain used in the original method. The former is less expensive than the latter, whereas we confirmed in a preliminary experiment that there is no difference in serum antibody titers between the two strains. Secondly, we administered emulsified antigens into the hind footpad through the subcutaneous route, instead of the intracutaneous route adopted in the original method. This is because the original method was intended to restrict the immune reaction to medial iliac lymph nodes to obtain monoclonal antibody-producing lymphocytes in high frequencies [9], whereas the present method was intended simply to raise polyclonal antisera. Finally, we performed a booster immunization that was avoided in the original method. A single booster proved to be sufficient to produce more than 1000-fold titers. Repeating the booster twice or more did not necessarily yield higher antibody titers (data not shown). Titration of the antisera in the present method is based on immunohistochemical staining and thus reliable when diluting the antisera for practical use. The total period of 3 weeks required for immunization is much shorter than that in rabbits, which is usually 3 months or more. Despite the general concept that the class of immunoglobulins appearing earlier is IgM, we demonstrated that most of the rat antibodies raised after the booster is IgG. The total volume of antisera obtained from a rat is about 5 ml, which is much smaller than that from a rabbit but is sufficient for use in immunohistochemical studies conducted in a single laboratory. Also, although it is difficult to purify rat antibodies into the IgG fraction because of the lower binding affinity of rat IgG to protein A or G, they are sufficient for the use as antisera for immunohistochemical purposes, as long as the antibody titers are high enough.
By use of the present new method, we produced rat antisera against ERGIC-53 and c-Kit and successfully applied them to double-immunostaining with mouse monoclonal and rabbit polyclonal antibodies, respectively. Since the majority of antibodies used for immunohistochemistry are mouse monoclonal and rabbit polyclonal antibodies, it is a great advantage of rat polyclonal antisera to be able to be combined with both types of antibodies for double-immunostaining. The present anti-ERGIC-53 antisera immunostained the sublingual acinar cell, a mucus glycoprotein-producing cell population. ERGIC-53 is a type I membrane protein belonging to a family of animal L-type lectins [13]. ERGIC-53 with its luminal domain binds glycoprotein products in the ER and with its cytosolic domain binds COPII and COPI, the vesicle-coating proteins involved in ER-Golgi antero- and retrograde transports, respectively [4]. Based on functional and molecular biological studies conducted in cultured cells, ERGIC-53 is believed to be responsible for vesicular transport of certain glycoproteins from the ER to Golgi apparatus. Although the portion of internal membrane harboring ERGIC-53 was defined as the ERGIC, the ultrastructural basis for ERGIC is not clear. The present study demonstrated partial overlapping of ERGIC-53 with both ER and cis-Golgi markers, confirming that ERGIC represents the membranous compartment intermediate of ER and cis-Golgi. We recently discovered SLAMP, a new member of the animal L-type lectin family, in rat and mouse sublingual glands [11]. The intracellular localization and function of SLAMP by comparison with those of ERGIC-53 is under investigation using the present anti-ERGIC-53 antisera.
On the other hand, the present anti-c-Kit antisera immunostained the spermatogenic cells located adjacent to the basement membrane of seminiferous tubules, as well as Leydig cells. C-Kit is a transmembrane receptor of the cytokine Stem Cell Factor and is responsible for the survival and/or proliferation of the differentiating type A spermatogonia [17]. It is generally accepted that c-Kit is a marker of the spermatogonia populations other than the undifferentiated type A spermatogonia, the spermatogenic stem cell population. In the present study, double-immunostaining demonstrated that most of the c-Kit-immunopositive spermatogenic cells are simultaneously positive for SgIGSF. In our previous paper, we reported, based on the enzyme-labeled antibody method, that the spermatogenic cells in the step of intermediate (type B) spermatogonia or later are immunopositive for SgIGSF [15]. However, the present study, using the more sensitive fluorescent antibody method and double-immunostaining with c-Kit, suggested that the expression of SgIGSF begins as early as in the step of differentiating type A spermatogonia. This is also consistent with our previous finding that the seminiferous epithelium from WWv mutant mice, which are lacking in the c-Kit gene and hence occur in the spermatogenic cell lineage later than the undifferentiated spermatogonia, are devoid of SgIGSF immunoreactivity [15].
In conclusion, the present method will enable us to produce antibodies available for immunohistochemistry in shorter time and at lower cost.
V. References
- 1.Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 2.Coons A. H., Leduc E. H., Connolly J. M. Studies on antibody production, I. A method for the histochemical demonstration of specific antibody and its application of the hyperimmune rabbit. J. Exp. Med. 1955;102:49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harlow E., Lane D. Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 1988. [Google Scholar]
- 4.Hauri H. P., Kappeler F., Andersson H., Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- 5.Kishiro Y., Kagawa M., Naito I., Sado Y. A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct. Funct. 1995;20:151–156. doi: 10.1247/csf.20.151. [DOI] [PubMed] [Google Scholar]
- 6.Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakane P. K., Pierce G. G. Enzyme-labeled antibodies: Preparation and application for localization of antigens. J. Histochem. Cytochem. 1966;14:929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- 9.Sado Y., Kagawa M., Kishiro Y., Sugihara K., Naito I., Seyer J. M., Sugimoto M., Oohashi T., Ninomiya Y. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem. Cell Biol. 1995;104:267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- 10.Sado Y., Okigaki T. A novel method for production of monoclonal antibodies. Evaluation and expectation of the rat lymph node method in cell and molecular biology. Cell Biol. Int. 1996;20:7–14. doi: 10.1006/cbir.1996.0003. [DOI] [PubMed] [Google Scholar]
- 11.Sakulsak N., Wakayama T., Hipkaeo W., Yamamoto M., Iseki S. Cloning and characterization of a novel animal lectin expressed in the rat sublingual gland. J. Histochem. Cytochem. 2005;53:1335–1343. doi: 10.1369/jhc.5A6618.2005. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki J., Yamamoto H., Nomura T., Matsuura J., Seno M., Sato F. E., Inoue M. Multiple-labeling of oligonucleotide probes for in situ hybridization. Acta Histochem. Cytochem. 1998;31:275–279. [Google Scholar]
- 13.Schindler R., Itin C., Zerial M., Lottspeich F., Hauri H. P. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur. J. Cell Biol. 1993;61:1–9. [PubMed] [Google Scholar]
- 14.Schweizer A., Fransen J. A., Bachi T., Ginsel L., Hauri H. P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakayama T., Koami H., Ariga H., Kobayashi D., Sai Y., Tsuji A., Yamamoto M., Iseki S. Expression and functional characterization of the adhesion molecule spermatogenic immunoglobulin superfamily in the mouse testis. Biol. Reprod. 2003;68:1755–1763. doi: 10.1095/biolreprod.102.012344. [DOI] [PubMed] [Google Scholar]
- 16.Wakayama T., Koami H., Yamamoto M., Iseki S. Expression of the adhesion molecule spermatogenic immunoglobulin superfamily (SgIGSF) in mouse tissues. Acta Histochem. Cytochem. 2004;37:365–371. [Google Scholar]
- 17.Yoshinaga K., Nishikawa S., Ogawa M., Hayashi S., Kunisada T., Fujimoto T., Nishikawa S. Role of c-Kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-Kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]