Abstract
The neuropeptide substance P (SP) modulates bone metabolism. This study examined the temporal appearance of the neuropeptides SP and brain-derived nerve growth factor (BDNF) and their receptors (neurokinin-1 receptor (NK1-R) and Trk B, respectively) in the rat trigeminal ganglion to investigate the role of neuropeptides in healing after tooth extraction. Rats were anesthetized and their upper right first molars were extracted; the rats were sacrificed 3 hours and 1–21 days after extraction. Their trigeminal ganglion and maxilla were removed, and cryosections were prepared and immunostained using specific antibodies against SP, BDNF, NK1-R, and Trk B. In the tooth sockets after extraction, new bone and a few SP-immunoreactive nerve fibers were first seen at day 7, and bone completely filled the sockets at day 21. In the trigeminal ganglion, the proportions of NK1-R-, BDNF-, and Trk B-immunoreactive neurons changed similarly, i.e., they initially decreased, increased rapidly to maximum levels by day 3, and then decreased gradually to control levels until 21 days. These findings suggest that the appearance of neuropeptides in the trigeminal ganglion, the reinnervation of SP-immunoreactive nerve fibers, and bone repair in the tooth socket during healing after extraction were correlated.
Keywords: trigeminal ganglion, tooth extraction, substance P, brain-derived nerve growth factor (BDNF)
I. Introduction
Extraction of vital teeth damages the nerve fibers innervating the teeth. The pulpal nerve fibers are cut at the apical foramen and the periodontal nerve fibers are crushed. During socket healing after extraction, these traumatized nerve fibers regenerate into and through the soft tissues of the socket. Hansen examined neural reorganization after tooth extraction [9]: in the healing socket, initial degeneration was followed 2 days later by progressive axon regeneration; within the first postoperative week, the alveolus was filled with connective tissue with many long thin axons; cancellous bone then filled the tooth sockets; and the axons were concentrated into central fascicles, directed toward the limbus. Although the regeneration of nerve fibers in extracted root sockets has been examined, the roles of these regenerated nerve fibers have not been evaluated in terms of bone repair.
Peripheral nerve injury (such as an extraction) induces profound structural, biochemical, and physiological changes in primary sensory neurons [2, 5, 29, 30]. The production of neuropeptides in the neurons may either decrease or increase following peripheral axotomy [3, 7, 16, 21]. For example, Itotagawa et al. demonstrated that NPY-immunoreactive cells appeared in the trigeminal ganglion 14 days after extraction [11]. Trauma to dental tissues involving nerve injury is often encountered in clinical practice, and some of these lesions may allow complete reinnervation of denervated tissues.
Substance P (SP) is an important member of the tachykinin family of neuropeptides, which are neurotransmitters or neuromodulators [13, 19]. Recent advances in the analysis of SP receptors, particularly neurokinin-1 receptors (NK1-R), which have high affinity for SP, have demonstrated that they are distributed not only in the cells of the neuronal or immune systems but also in peripheral cells. Therefore, the effect of SP and its cellular receptors is not limited to the nervous system but is more extensive than previously appreciated. SP-immunoreactive axons have been localized in bone, and SP receptors are widely distributed in osteoclasts and osteoblasts [8]. Chung and George indicated that SP has an osteogenic stimulating effect in vitro [4], while Mori et al. reported SP activates osteoclastic bone resorption activity [17]. The distribution of SP-immunoreactive axons and SP receptors suggests that SP modulates bone metabolism directly via SP receptors.
Brain-derived neurotrophic factor (BDNF) promotes developing or regenerating sensory neurons and is transported to the sensory ganglia retrogradely, where it promotes neuron survival [6, 10]. Recently, it has been suggested that BDNF plays an important neuromodulatory role in the dorsal horn of the spinal cord in inflammation [24]. Using an inflammatory model, it was also reported that SP and BDNF increased in the dorsal root ganglions (DRGs) [18, 24] and that SP and BDNF receptors (tyrosine kinase receptor (Trk B)) increased in the dorsal horn [1, 24].
Therefore, this study examined the role of SP and BDNF in healing after tooth extraction. We investigated the temporal appearance of SP and BDNF, and their receptors (NK1-R and Trk B, respectively) in the rat trigeminal ganglion. To confirm the damaged neurons we observed the appearance of activating transcription factor 3 (ATF3) after tooth extraction [25]. Furthermore, we analyzed the appearance of SP, growth associated protein-43 (GAP-43: a marker for regenerating nerve fibers), and protein gene product 9.5 (PGP-9.5: a marker for mature nerve fibers) [22, 23, 27, 28] in nerve fibers in the healing socket after extraction.
II. Materials and Methods
Animals
The experimental protocol in rats was reviewed and approved by the Animal Care Committee at Kyushu Dental College, Fukuoka, Japan. Seventy male Sprague-Dawley rats weighing 200–250 g were used. The animals were acclimatized for at least 1 week before the experiment started.
Surgical procedures
The rats were anesthetized with an intramuscular injection of ketamine (Daiichi Pharmaceutical, Tokyo, Japan; 70 mg/kg) and xylazine (Bayer, Tokyo, Japan; 13 mg/kg). After sweeping up around the tooth, the right maxillary first molars were extracted. After extraction, rats were fed with powdered food. Untreated animals were used as the control group.
Tissue preparations
The experimental periods were set at 3 hr, 1, 3, 7, 14 and 21 days after tooth extraction. Under deep anesthesia with diethyl ether, the operated rats (7 animals for each time period), unoperated controls (4 animals for each time period) and controls (on 0 day, 4 animals) were perfused transcardially with 4% paraformaldehyde in 0.2 M phosphate buffer containing 0.2% picric acid. The right trigeminal ganglion in 28 control and 42 experimental animals were then removed and post-fixed in the same fixative overnight. The maxillae were also removed and decalcified with 10% EDTA in 0.1 M phosphate buffer for 3 weeks.
Immunohistochemistry of trigeminal ganglion
The trigeminal ganglion was frozen rapidly and cut into 6-µm-thick sections with a cryostat (Leica Instruments, Wetzlar, Germany).
Double labeling for SP, NK1-R, BDNF, TrkB, or ATF3 and Protein-gene product 9.5 (PGP-9.5), a marker for neurons, was performed as follows. The sections were pre-incubated in 0.1 M phosphate-buffered saline (PBS, pH 7.4) with 1% normal goat serum (ICN Pharmaceuticals, Aurora, OH, USA) for 30 min at room temperature. Then, the sections were incubated with rabbit polyclonal antibodies against SP (1:1000; Affiniti Research Products, Devon, UK), rabbit polyclonal antibodies against NK1-R (1:1000; Calbiochem–Novabiochem, San Diego, CA, USA), sheep polyclonal antibodies against BDNF (1:100; Chemicon International, Temecula, CA, USA), rabbit polyclonal antibodies against TrkB (1:2000; Chemicon International) or rabbit polyclonal antibodies against ATF3 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hr at 37°C. After rinsing with 0.1 M PBS, the sections were incubated with goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC) (1:100; Molecular Probes, Eugene, OR, USA) or goat anti-sheep IgG conjugated with FITC (1:100; Chemicon International) for 1.5 hr at 37°C. After rinsing with 0.1 M PBS, the sections were incubated with guinea pig polyclonal antibodies against PGP-9.5 (1:500; Neuromics Antibodies, Northfield, MN, USA) for 2 hr at 37°C, washed in PBS, and incubated with goat anti-guinea pig IgG conjugated to tetramethylrhodamine-5-isothiocyanate (TRITC) (1:100; Molecular Probes) for 1.5 hr at 37°C. Finally, the sections were washed in 0.1 M PBS and placed under a coverslip.
Double labeling for SP and NK1-R was performed as follows. The sections were pre-incubated in 0.1 M PBS with 1% normal goat serum for 30 min at room temperature. Then, the sections were incubated with rabbit polyclonal antibodies against SP for 2 hr at 37°C. After rinsing with 0.1 M PBS, the sections were incubated with goat anti-rabbit IgG conjugated with FITC. After rinsing with 0.1 M PBS, the sections were also incubated in 0.1 M PBS with 1% normal goat serum for 30 min at room temperature for blocking the unlabeled antibodies. Sections were incubated with rabbit polyclonal antibodies against NK1-R for 2 hr at 37°C, washed in PBS, and incubated with goat anti-rabbit IgG conjugated to TRITC (1:100; Molecular Probes) for 1.5 hr at 37°C. Finally, the sections were washed in 0.1 M PBS and placed under a coverslip.
The samples were examined under a fluorescence microscope (Olympus Optical, Tokyo, Japan) equipped with a CoolSNAP CCD camera (RS Photometrics, Tucson, AZ, USA) or a confocal laser scanning microscope (Radiance 2100, Bio-Rad, Herts, UK).
Cell counts
In each rat, three to five sections (at least 30-µm intervals) of the trigeminal ganglion were selected randomly and observed under a fluorescence microscope. The total number of immunopositive neurons in the maxillary and mandibular nerve regions was counted on each section.
Immunohistochemistry of the maxilla
After decalcification, the maxilla was frozen and cut into 20-µm-thick sections using a cryostat (Leica Instruments).
For immunostaining for SP, NK1-R, PGP-9.5, or human growth-associated phosphoprotein 43 (GAP-43), a marker for new nerve fibers, the sections were pre-incubated for 5 min at room temperature in 0.1 M PBS with 0.3% H2O2, rinsed with PBS, and pre-incubated for 30 min at room temperature in 0.1 M PBS with 1% normal goat serum. The sections were incubated with rabbit polyclonal antibodies against SP (1:1000; Affiniti Research Products), rabbit polyclonal antibodies against NK1-R (1:1000; Calbiochem-Novabiochem), rabbit polyclonal antibodies against PGP-9.5 (1:500; Neuromics Antibodies), or mouse monoclonal antibodies against GAP-43 (1:40; Novocastra Laboratories, Benton Lane, UK) for 2 hr at 37°C. After rinsing with PBS, the sections were incubated with goat anti-rabbit IgG (1:1000) and then with avidin–biotin–peroxidase complex using a VECTASTAIN Elite ABC KIT (Vector Laboratories, Burlingame, CA, USA), or goat anti-mouse IgG conjugated with peroxidase (1:70; Amersham Biosciences, Buckinghamshire, UK). The peroxidase activity was developed using 0.02% 3,3'-diaminobenzidine (Dojindo, Kumamoto, Japan) and 0.02% 30% hydrogen peroxidase solution. Then, the sections were observed under an optical microscope (Olympus Optical).
Statistical analysis
One-way ANOVA was used to analyze the proportions of neurons labeled with NK1-R, BDNF, or Trk B, followed by individual post hoc comparisons (Scheffè).
III. Results
SP-, NK1-R-, BDNF-, TrkB-, ATF3-immunoreactive neurons in trigeminal ganglion after extraction
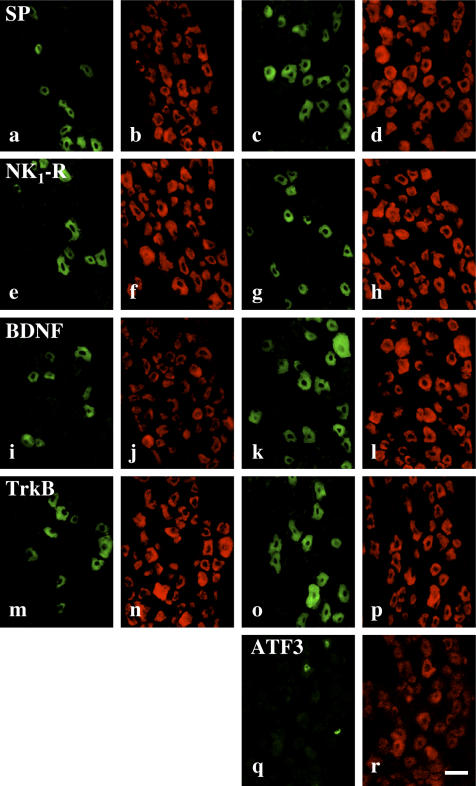
SP-, NK1-R-, BDNF-, and TrkB-immunoreactive neurons in the trigeminal ganglion were observed as medium or small neurons, which ranged from 520 to 953 µm2 (Fig. 1a–p). Double staining for PGP-9.5 and SP, NK1-R, BDNF, and TrkB confirmed that some PGP-9.5-immunoreactive neurons in the maxillary nerve region were immunopositive for SP, NK1-R, BDNF, and TrkB. After tooth extraction, some PGP-9.5 immunopositive neurons appeared to be immunopositive for ATF3 (Fig. 1q, r).
Fig. 1.
SP-, NK1-R-, BDNF-, TrkB-, and ATF3-immunoreactive neurons in the trigeminal ganglion. (a, c) SP-, (e, g) NK1-R-, (i, k) BDNF-, (m, o) TrkB- and (q) ATF3-immunoreactive neurons in the maxillary nerve regions in the non-operated rat trigeminal ganglion (a, e, i, m) and at 3 days after extracted rat trigeminal ganglion (c, g, k, o, q). (b, d, f, h, j, l, n, p, r) Immunohistochemical images for PGP-9.5 in the same region as a, c, e, g, i, k, m, o, q. Bar=50 µm.
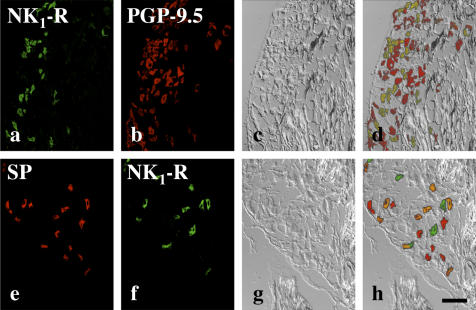
After tooth extraction, degenerative changes of neurons were not observed in double staining for NK1-R and PGP-9.5 and differential interference contrast images of the maxillary nerve region (Fig. 2a–d).
Fig. 2.
SP- and NK1-R immunoreactive neurons in the rat trigeminal ganglion after 3 days following extraction. (a) NK1-R- and (b) PGP-9.5-immunoreactive neurons in the maxillary nerve region. (e) SP- and (f) NK1-R-immunoreactive neurons in the maxillary nerve region. (c, g) Differential interference contrast image in the same region as a, b and e, f respectively. (d, h) are merged image of (a, b, c) and (e, f, g). The cells indicated by orange color represent immunopositive for both SP and NK1-R. Bar=100 µm.
Double staining for SP and NK1-R confirmed that some neurons were immunopositive for both NK1-R and SP (Fig. 2e–h).
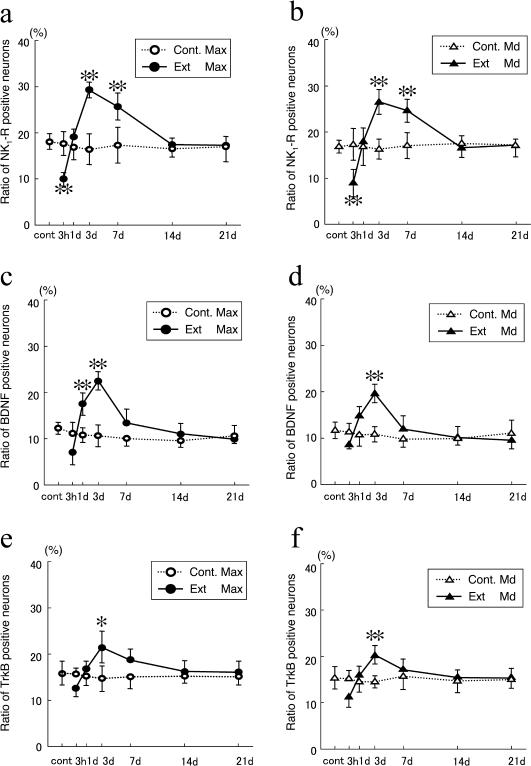
In the control, the proportion of PGP-9.5-immunoreactive neurons containing NK1-R (NK1-R/PGP-9.5 neurons) in the maxillary and mandibular nerve regions was 18.1 and 16.8%, respectively. Three hours after extraction, the respective proportion of NK1-R/PGP-9.5 neurons in the maxillary and mandibular nerve region decreased to 9.9 and 9.0%. Then, the ratio increased 1 day after extraction, and reached the maximum respective values of 29.3 and 26.5% after 3 days in the maxillary and mandibular nerve regions. At days 7, 14, and 21 post-extraction, the proportion of NK1-R/PGP-9.5 neurons in both the maxillary and mandibular nerve regions had decreased to control levels (Fig. 3a, b).
Fig. 3.
The ratio of NK1-R- (a), BDNF- (b), and TrkB-immunoreactive neurons per PGP-9.5-immunoreactive neuron in the maxillary (a, c, e) and mandibular (b, d, f) nerve regions between 3 hr and 21 days after extraction. Cont., control; Ext, extracted groups; Max, maxillary nerve region; Md, mandibular nerve region. Data are expressed as the mean±SD. Control group n=4. Experimental group n=7. ** p<0.01, * p<0.05.
Like NK1-R-immunoreactive neurons, after extracting the upper first molar, the number of BDNF-immunoreactive neurons decreased initially and then increased to a maximum after 3 days. In contrast, on day 1, the proportion of BDNF/PGP-9.5 neurons was already greater than that in the control. These changes in the ratio of BDNF-immunoreactive neurons were observed for both the maxillary and mandibular nerve regions. The proportion of BDNF/PGP-9.5 was 12.1 and 11.7% in the control, 6.9 and 8.6% after 3 hours, 17.4 and 14.9% on day 1, and 22.4 and 19.7% on day 3 post-extraction, respectively. At 7, 14, and 21 days, the proportions of BDNF/PGP-9.5 neurons in both the maxillary and mandibular nerve regions had decreased to control levels in a similar manner (Fig. 3c, d).
The changes in the proportion of TrkB/PGP-9.5 neurons followed a similar tendency to that for BDNF/PGP-9.5 neurons after extraction. The proportion decreased initially, and then increased until 3 days after extraction. These changes in the number of Trk B-immunoreactive neurons were observed in both the maxillary and mandibular nerve regions. The proportion of Trk B/PGP-9.5 was 15.8 and 15.2% in the control, 12.5 and 11.2% after 3 hours, 16.6 and 15.7% on day 1, and 21.4 and 20.3% on day 3 post-extraction, respectively. At 7, 14, and 21 days, the proportions of Trk B/PGP-9.5 neurons in both the maxillary and mandibular nerve regions had decreased to the control levels (Fig. 3e, f).
Bone formation in tooth sockets
To examine the expression of SP-immunoreactive nerve fibers, we performed immunostaining using SP antibodies. Furthermore, to confirm the expression of new and old nerve fibers, we conducted immunostaining using GAP-43 and PGP-9.5.
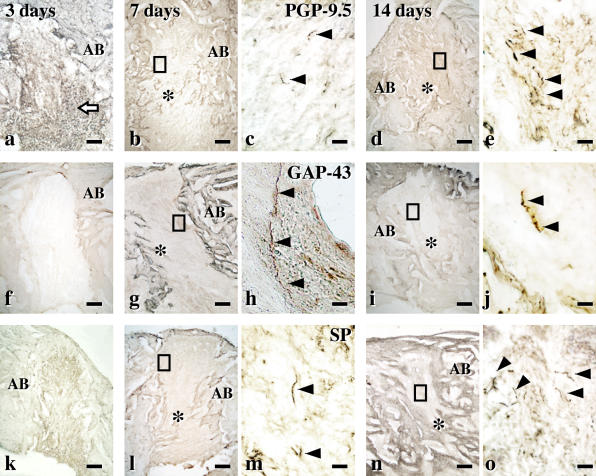
In the tooth sockets at 3 days after extraction, inflammatory cells were present (Fig. 4a, f, k) and there were no PGP-9.5-immunoreactive nerve fibers (Fig. 4a). Seven days after extraction, new bone had appeared (Fig. 4b, g, l) with GAP-43-immunoreactive nerves (new nerve fibers) (Fig. 4g, h) and a few SP-immunoreactive nerve fibers (Fig. 4l, m). The number of PGP-9.5- and SP-immunoreactive nerve fibers had increased by day 14 (Fig. 4d, e, n, o). Bone completely filled the sockets on day 21 (data not shown).
Fig. 4.
Reinnervation during the healing of extracted sockets. Immunohistochemical images for PGP-9.5 (a–e), GAP-43 (f–g) and SP (k–o) at the tooth extracted socket. (a, f, k) Three days after extraction, the extracted root sockets are filled with inflammatory cells (arrow) and (a) no PGP-9.5-immunoreactive nerve fibers are seen. (b, c, g, h, l, m) Seven days after extraction. (b, g, l) The root socket is filled with fibrous tissues. (h) High-power images of the square area in (g). GAP-43-immunoreactive nerve fibers (arrowheads) appeared in the extracted socket. (m) Some SP-immunoreactive nerve fibers (arrowheads) is seen. (d, e, i, j, n, o) Fourteen days after extraction. (d, i, n) The extracted sockets are partly filled with new bone. (o) SP-immunoreactive nerve fibers (arrowheads) was often observed in the extracted root sockets. AB, alveolar bones; *, new bone. Bars=200 µm (a, b, d, f, g, i, k, l, n) and 20 µm (c, e, h, j, m, o).
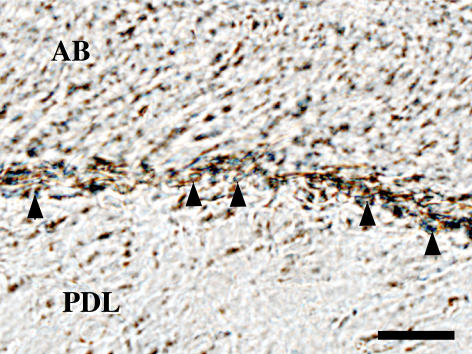
Immunostaining for NK1-R confirmed that the presence of NK1-R-immunoreactive osteoblasts on bone surface (Fig. 5).
Fig. 5.
NK1-R immunoreactive osteoblasts (arrowheads) on the bone surface at the root socket. AB, alveolar bones; PDL, periodontal ligaments. Bar=20 µm.
IV. Discussion
This study demonstrated that extraction of an upper molar induced an increase in ratio of NK1-R-immunoreactive neurons in both the maxillary and mandibular nerve regions of the trigeminal ganglion, and this synchronized change in the maxillary and mandibular nerve regions was also observed for the appearance of BDNF and TrkB. Marked degeneration of neurons was not observed, whereas there were injured ATF3 positive neurons after extraction. These results indicate that the number of NK1-R-, BDNF-, TrkB-immunoreactive neurons were increased in both the maxillary and mandibular nerve regions of the trigeminal ganglion at 3 days after extraction of upper molar. Shortly after the peak expression of NK1-R-, BDNF-, and TrkB-immunoreactive neurons in the trigeminal ganglion, SP-, and GAP-43-immunoreactive new nerve fibers were observed in the root cavity. The bone healing was complete when the number of SP-immunoreactive neurons returned to the control level. These findings strongly suggest that neuropeptides in the trigeminal ganglion are associated with bone healing after extraction.
In this study, we found temporal changes in the trigeminal ganglion neuropeptides in both the maxillary and mandibular nerve regions, which is similar to our earlier observation for SP-immunoreactive neurons in the trigeminal ganglion following tooth extraction [12]. Previously, we observed that after maxillary first molar extraction, the ratio of PGP-9.5-immunoreactive neurons containing SP (SP/PGP-9.5 neurons) in the maxillary and mandibular nerve regions decreased initially and then increased to a maximum 3 days after extraction. The proportion of SP/PGP-9.5 neurons in both the maxillary and mandibular nerve regions decreased to control levels. In this study, we observed similar changes in the NK1-R/PGP-9.5, BDNF/PGP-9.5, and TrkB/PGP-9.5 ratios of neurons in the trigeminal ganglion after tooth extraction, which suggests that peripheral nerve injury not only induces the up-regulation of neuropeptides, but also that of their receptors. Additionally there were both SP- and NK1-R-immunoreactive neurons in maxillary nerve region of trigeminal ganglion. These findings suggest that peripheral nerve injury up-regulates neuropeptide expression and causes autocrine-like reactions in the trigeminal ganglion.
The NK1-R/PGP-9.5, BDGF/PGP-9.5, and TrkB/PGP-9.5 ratios of neurons increased not only in the maxillary nerve region, but also in the mandibular nerve region of the trigeminal ganglion. As primary afferent neurons in the mammalian ganglion are anatomically isolated from one another and not interconnected synaptically, our findings suggest that the same interactions exist between the maxillary and mandibular nerve regions in the trigeminal ganglion. The most plausible explanation for the synchronized occurrence of neuropeptides and their receptors in both the maxillary and mandibular nerve regions after extraction of the upper molar is cross-excitation between injured and neighboring neurons. Cross-excitation occurs via non-synaptic neurotransmitters, such as ATP, which binds the P2X3 receptor, an ATP-gated cation channel receptor associated with primary nociceptive sensory neurons. In sensory ganglia, the differential regulation of P2X3 receptor expression in peripheral nerve injury in intact and injured neurons suggests the existence of cross-excitation [26]. Another study demonstrated that SP is synthesized by neurons after the interaction of ATP with P2X3 receptors [15]. Although we found no direct evidence of cross-excitation in the trigeminal ganglion, the synchronized appearance of neuropeptides in the maxillary and mandibular nerve regions of the trigeminal ganglion strongly suggests the existence of cross-excitation via a non-synaptic neurotransmission system.
In this study, 1 day after tooth extraction, the BDNF/PGP-9.5 ratio of neurons was already greater than in the control, whereas those of NK1-R/GAP-9.5 and TrkB/PGP-9.5 were still close to control levels. Another study has reported that BDNF- and TrkB-immunoreactive neurons increase following peripheral injury [31]. Since GAP-43-immunoreactive nerves appeared initially and then SP-immunoreactive nerve fibers increased in the healing root socket after extraction, after nerve injury, BDNF is produced earlier than other neuropeptides for the regeneration of nerve fibers.
The association between reinnervation in the root socket and alveolar bone repair is still unclear. Sandhu et al. [20] reported that sympathectomy greatly increases the number of osteoclasts in the root socket after extraction; i.e., neuropeptides from sympathetic neurons may inhibit osteoclastic activity. We observed the appearance of SP-immunoreactive neurons in the root socket a few days after the SP-production increase in the trigeminal ganglion and NK1-R-immunoreactive osteoblasts on bone surface of sockets. Mason and Holland [14] reported that the amount of innervation in the healing socket after extraction grew until 1 month after the extraction, and then decreased gradually. These findings suggest that after the nerve injury caused by extraction, the neuropeptides produced in the sensory ganglion are transported to the damaged site retrogradely, where the released neuropeptides stimulate bone remodeling during healing of the root socket.
In conclusion, the nerve injury caused by extracting the maxillary first molar induces increases in SP, BDNF, and their receptors in the trigeminal ganglion. The proportions of NK1-R-, BDNF-, and TrkB-immunoreactive neurons grew not only in the maxillary nerve region, but also in the mandibular nerve region of the trigeminal ganglion. The reinnervation in the extracted socket occurred before the new bone repaired the root socket. These findings demonstrate the relationships among the appearance of neuropeptides in the trigeminal ganglion, the reinnervation of SP-immunoreactive nerve fibers, and bone repair in the tooth socket during the healing process after extraction.
V. Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15591941) to Dr. T. Goto.
VI. References
- 1.Abbadie C., Brown J. L., Mantyh P. W., Basbaum A. I. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 2.Aldskogius H., Arvidsson J., Grant G. The reaction of primary sensory neurons to peripheral nerve injury with particular emphasis on transganglionic changes. Brain Res. 1985;357:27–46. doi: 10.1016/0165-0173(85)90006-2. [DOI] [PubMed] [Google Scholar]
- 3.Bisby M. A., Keen P. Regeneration of primary afferent neurons containing substance P-like immunoreactivity. Brain Res. 1986;365:85–95. doi: 10.1016/0006-8993(86)90725-0. [DOI] [PubMed] [Google Scholar]
- 4.Chung S., George W. B. Neurogenic substance P stimulates osteogenesis in vitro. Peptides. 1997;18:323–326. doi: 10.1016/s0196-9781(96)00280-x. [DOI] [PubMed] [Google Scholar]
- 5.Devor M., Govrin-Lippmann R., Frank I., Raber P. Proliferation of primary sensory neurons in adult rat dorsal root ganglion and the kinetics of retrograde cell loss after sciatic nerve section. Somatosens. Res. 1985;3:139–167. doi: 10.3109/07367228509144581. [DOI] [PubMed] [Google Scholar]
- 6.DiStefano P. S., Friedman B., Radziejewski C., Alexander C., Boland P., Schick C. M., Lindsay R. M., Wiegand S. J. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 7.Fried K., Brodin E., Theodorsson E. Substance P-, CGRP- and NPY-immunoreactive nerve fibers in rat sciatic nerve-end neuromas. Regul. Pept. 1989;25:11–24. doi: 10.1016/0167-0115(89)90244-9. [DOI] [PubMed] [Google Scholar]
- 8.Goto T., Yamaza T., Kido M. A., Tanaka T. Light- and electron-microscopic study of the distribution of axons containing substance P and the localization of neurokinin-1 receptor in bone. Cell Tissue Res. 1998;293:87–93. doi: 10.1007/s004410051100. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J. H. Neurohistological reactions following tooth extractions. Int. J. Oral Surg. 1980;9:411–426. doi: 10.1016/s0300-9785(80)80070-6. [DOI] [PubMed] [Google Scholar]
- 10.Hofer M. M., Barde Y. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988;331:261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- 11.Itotagawa T., Yamanaka H., Wakisaka S., Sasaki Y., Kato J., Kurisu K., Tsuchitani Y. Appearance of neuropeptide Y-like immunoreactive cells in the rat trigeminal ganglion following dental injuries. Arch. Oral Biol. 1993;38:725–728. doi: 10.1016/0003-9969(93)90013-c. [DOI] [PubMed] [Google Scholar]
- 12.Kometani K., Goto T., Konoo T., Kobayashi S., Yamaguchi K. Changes in neuron containing substance P and brain-derived nerve growth factor in the rat trigeminal ganglion during healing after extraction. J. Dent. Res. 2003;82(Spec Iss B):B-160. [Google Scholar]
- 13.Maggi C. A., Patacchini R., Rovero P., Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 14.Mason A. G., Holland G. R. The reinnervation of healing extraction sockets in the ferret. J. Dent. Res. 1993;72:1215–1221. doi: 10.1177/00220345930720080901. [DOI] [PubMed] [Google Scholar]
- 15.Matsuka Y., Neubert J. K., Maidment N. T., Spigelman I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Res. 2001;915:248–255. doi: 10.1016/s0006-8993(01)02888-8. [DOI] [PubMed] [Google Scholar]
- 16.McGregor G. P., Gibson S. J., Sabate I. M., Blank M. A., Christofides N. D., Wall P. D., Polak J. M., Bloom S. R. Effect of peripheral nerve section and nerve crush on spinal cord neuropeptides in the rat; increased VIP and PHI in the dorsal horn. Neuroscience. 1984;13:207–216. doi: 10.1016/0306-4522(84)90270-7. [DOI] [PubMed] [Google Scholar]
- 17.Mori T., Ogata T., Okumura H., Shibata T., Nakamura Y., Kataoka K. Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone resorption. Biochem. Biophys. Res. Commun. 1999;262:418–422. doi: 10.1006/bbrc.1999.1220. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi K., Ruda M. A. Gene regulation in an ascending nociceptive pathway: inflammation-induced increase in preprotachykinin mRNA in rat lamina I spinal projection neurons. J. Neurosci. 1992;12:2563–2572. doi: 10.1523/JNEUROSCI.12-07-02563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu H. S., Herskovits M. S., Singh I. J. Effect of surgical sympathectomy on bone remodeling at rat incisor and molar root sockets. Anat. Rec. 1987;219:32–38. doi: 10.1002/ar.1092190107. [DOI] [PubMed] [Google Scholar]
- 21.Shehab S. A., Atkinson M. E. Vasoactive intestinal polypeptide increases in areas of the dorsal horn of the spinal cord from which other neuropeptides are depleted following peripheral axotomy. Exp. Brain Res. 1986;62:422–430. doi: 10.1007/BF00238861. [DOI] [PubMed] [Google Scholar]
- 22.Stewart H. J., Cowen T., Curtis R., Wilkin G. P., Mirsky R., Jessen K. R. GAP-43 immunoreactivity is widespread in the autonomic neurons and sensory neurons of the rat. Neuroscience. 1992;47:673–684. doi: 10.1016/0306-4522(92)90175-2. [DOI] [PubMed] [Google Scholar]
- 23.Tetzlaff W., Zwiers H., Lederis K., Cassar L., Bisby M. A. Axonal transport and localization of B-50/GAP-43-like immunoreactivity in regenerating sciatic and facial nerves of the rat. J. Neurosci. 1989;9:1303–1313. doi: 10.1523/JNEUROSCI.09-04-01303.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson S. W., Bennett D. L., Kerr B. J., Bradbury E. J., McMahon S. B. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc. Natl. Acad. Sci. U S A. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujino H., Kondo E., Fukuoka T., Dai Y., Tokunaga A., Miki K., Yonenobu K., Ochi T., Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol. Cell. Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 26.Tsuzuki K., Kondo E., Fukuoka T., Yi D., Tsujino H., Sakagami M., Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 27.Vento P., Soinila S. Quantitative comparison of growth-associated protein GAP-43, neuron-specific enolase, and protein gene product 9.5 as neuronal markers in mature human intestine. J. Histochem. Cytochem. 1999;47:1405–1416. doi: 10.1177/002215549904701107. [DOI] [PubMed] [Google Scholar]
- 28.Verhaagen J., Oestreicher A. B., Gispen W. H., Margolis F. L. The expression of the growth associated protein B50/GAP43 in the olfactory system of neonatal and adult rats. J. Neurosci. 1989;9:683–691. doi: 10.1523/JNEUROSCI.09-02-00683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villar M. J., Cortes R., Theodorsson E., Wiesenfeld-Hallin Z., Schalling M., Fahrenkrug J., Emson P. C., Hokfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- 30.Wells M. R., Vaidya U. Morphological alterations in dorsal root ganglion neurons after peripheral axon injury: association with changes in metabolism. Exp. Neurol. 1989;104:32–38. doi: 10.1016/0014-4886(89)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler E. F., Naftel J. P., Pan M., von Bartheld C. S., Byers M. R. Neurotrophin receptor expression is induced in a subpopulation of trigeminal neurons that label by retrograde transport of NGF or fluoro-gold following tooth injury. Brain Res. Mol. Brain Res. 1998;61:23–38. doi: 10.1016/s0169-328x(98)00179-x. [DOI] [PubMed] [Google Scholar]