Abstract
Elevated blood pressure (BP) variability has been linked to an increased risk of adverse cardiovascular events, but the biological factors which promote elevated BP variability are not entirely understood. We conducted a cross-sectional study to examine whether inflammatory factors might be associated with elevated BP variability during 24-hour ambulatory BP monitoring. Subjects were 140 healthy, normotensive adults. Inflammatory markers included C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α). Blood pressure variability was calculated as the within-subject standard deviation of BP values obtained during the daytime, nighttime, and 24-hour periods. In linear regression models that were adjusted for mean BP and other factors, CRP quartiles were positively associated with daytime systolic BP variability; for subjects in the lowest to highest CRP quartiles, the mean within-subject standard deviations of daytime systolic BP were 9.31, 9.62, 10.55, and 11.17, respectively (p for linear trend = .001). C-reactive protein showed similar positive associations with nighttime and 24-hour systolic BP variability. In contrast, TNF-α was not independently associated with systolic BP variability during any of the time periods. With respect to diastolic BP variability, we found significant positive associations between CRP and diastolic BP variability during all time periods, and between TNF-α and daytime diastolic BP variability. In conclusion, there are positive associations between markers of inflammation and BP variability in healthy, normotensive adults, suggesting that inflammation may be one of the factors that promotes increased BP variability.
Keywords: blood pressure, blood pressure monitoring, blood pressure variability, inflammation, C-reactive protein, tumor necrosis factor-alpha
Elevated blood pressure (BP) variability during 24-hour ambulatory BP monitoring is associated with an increased risk of target organ damage,1,2 early atherosclerosis,3 and cardiovascular events.3–5 The causes of increased BP variability, however, are not entirely understood. Inflammatory factors, by promoting vasoconstriction6–10 and facilitating acute blood pressure increases,11 might play a role in increased BP variability. Yet data on the association between inflammatory markers and BP variability in humans are sparse. The present study examined whether two markers of inflammation, C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α), were positively associated with BP variability during 24-hour ambulatory BP monitoring in healthy men and women.
The study design was cross-sectional. The study population was a sample of male and female subjects drawn from the Atlanta, Georgia metropolitan area. Subjects were eligible to participate in the study if they: (1) were 30 to 60 years of age; (2) had no history of hypertension and were not using antihypertensive medications; (3) had no history of cardiovascular disease (coronary disease, stroke, peripheral vascular disease) and were not using lipid lowering drugs; (4) were free of any other major systemic illnesses (e.g. a form of cancer); (5) were non-smokers; (6) were not pregnant. We recruited 161 subjects who met these criteria. Of these 161 subjects, 140 had complete data on all variables of interest and formed the study population for this report. All subjects provided written informed consent, and the study protocol was approved by the Emory University Institutional Review Board.
Data collection took place at the Emory General Clinical Research Center. Subjects arrived during morning hours in a fasting state (since 12 a.m. the previous night). Self-reported information on age, sex, and race (White, African American, Asian, Hispanic, or other) was obtained by questionnaire. Additionally, a research nurse directly measured each subject’s height and weight. From the height and weight data, body mass index (BMI) was calculated as weight (kg)/height (m2).
The research nurse then obtained fasting blood samples from each subject via veinipuncture. Samples were placed into EDTA tubes. The tubes underwent centrifugation and plasma was aliquoted and stored at −80° C. From the stored plasma samples, inflammatory markers were measured using commercially available enzyme-linked immunosorbant assay (ELISA) kits according to the manufacturers’ instructions. C-reactive protein was measured using an ELISA kit from ALPCO Diagnostics (309710-s) that had a lower detection limit of .12 ng/ml. Tumor necrosis factor-α was measured using an ELISA kit from R&D Systems (HSTA00C) that had a minimum detection limit of .12 pg/ml.
After blood samples had been obtained, the research nurse initiated a subject’s ambulatory BP monitoring session. Ambulatory BP monitoring was conducted with a Spacelabs 90217 monitor (Spacelabs Inc., Redmond Washington.). The nurse placed an appropriately sized blood pressure cuff on the subject’s non-dominant arm, and instructed the subject to go about his/her normal activities during the 24-hour ambulatory BP monitoring period, but to refrain from vigorous physical activity. The ambulatory monitor was programmed to record a subject’s blood pressure every 30 minutes during the 24-hour period, for a maximum of 48 measurements. All subjects in this study had complete data on at least 36 (75%) of the 48 possible measurements. Blood pressure measurements that occurred after 6 AM and before 10 PM were regarded as “daytime” measurements, while measurements that occurred between 10 PM and 6 AM were designated as “nighttime” measurements. A subject’s mean systolic BP and diastolic BP were calculated for each of these time periods. A subject’s systolic or diastolic BP variability was calculated as the standard deviation of the subject’s BP measurements during each time period. Other studies have also used the standard deviation to quantify a subject’s BP variability during ambulatory BP monitoring.5
Statistical analysis proceeded according to the following 3 steps. First, we calculated descriptive statistics for the study population. Continuous variables were described in terms of the mean (± the standard deviation) or, when extremely skewed, in terms of the median (interquartile range). Categorical variables were described in terms of frequencies and percents. Next, we ran a series of multivariable linear regression models which examined whether the inflammatory markers were related to BP variability after adjustment for other factors. For a given model, the independent variables of interest were CRP and TNF-α and the dependent variable was the standard deviation of systolic or diastolic ambulatory BP for a particular time period (daytime, nighttime, or 24-hours). Because they were highly skewed, C-reactive protein and TNF-α were divided into quartiles and entered into models as a series of indicator variables. This allowed us to calculate the mean (95% confidence interval) of a given BP variability measure according to each quartile of an inflammatory marker. In order to model linear trends, we also ran models in which C-reactive protein and TNF-α quartiles were entered as ordinal variables. Since the inflammatory markers were entered into the models simultaneously, the markers were adjusted for each other. Additionally, all models were adjusted for age, sex, race, BMI, and the corresponding mean blood pressure value (e.g. if the dependent variable for a given model was the standard deviation of daytime systolic BP, then the model adjusted for mean daytime systolic BP). Age, mean blood pressure and BMI were entered into models as continuous, linear variables, while race was entered as three categories (White, African American, Other). Statistical significance was calculated by F-tests and partial r-squared values for the inflammatory markers were obtained. Finally, we explored whether the inflammatory markers showed any statistical interactions with age, sex, or race.
Descriptive statistics for the study population are presented in Table 1. In Table 2, the regression models for inflammatory factors and systolic BP variability are presented. We found that C-reactive protein quartiles showed positive and statistically significant linear trends with the standard deviations of daytime, nighttime, and 24-hour systolic BP, independent of other factors. In contrast, TNF-α levels were not independently associated with the standard deviations of systolic BP. The partial r-squared values for the combined effects of the ordinal CRP and TNF-α variables were .07, .05, and .04, for the standard deviations of daytime, nighttime, and 24-hour systolic BP, respectively. Regression models for inflammatory factors and diastolic BP variability are presented in Table 3. C-reactive protein quartiles showed positive, statistically significant associations with the standard deviations of diastolic BP during all time periods, and TNF-α quartiles showed a significant positive trend with daytime diastolic BP variability. The partial r-squared values for the combined effects of the ordinal CRP and TNF-α variables were .09, .05, and .04 for the standard deviations of daytime, nighttime and 24-hour diastolic BP, respectively.
Table 1.
Descriptive statistics for study population (n = 140)
| Variable | Mean (± SD) or Median (Interquartile Range) | n (%) |
|---|---|---|
| Age, years | 43.4 (± 8.0) | |
| Sex, male | 46 (34.6) | |
| Race: | ||
| White | 84 (60.0) | |
| African American | 37 (26.4) | |
| Other | 19 (13.6) | |
| BMI, kg/m2 | 27.2 (± 5.4) | |
| CRP, mg/L | 1.2 (0.5, 2.9) | |
| TNF-α, pg/ml | 0.9 (0.7, 1.4) | |
| Ambulatory Systolic BP, mmHg: | ||
| Daytime | 117.4 (± 10.4) | |
| Nighttime | 105.4 (± 11.3) | |
| 24-hour | 113.5 (± 10.1) | |
| Daytime Within-Subject Standard Deviation | 10.2 (± 2.4) | |
| Nighttime Within-Subject Standard Deviation | 9.3 (± 3.1) | |
| 24-Hour Within-Subject Standard Deviation | 11.8 (± 2.6) | |
| Ambulatory Diastolic BP, mmHg: | ||
| Daytime | 75.1 (± 7.0) | |
| Nighttime | 63.9 (± 7.2) | |
| 24-hour | 71.5 (± 6.4) | |
| Daytime Within-Subject Standard Deviation | 8.6 (± 2.1) | |
| Nighttime Within-Subject Standard Deviation | 7.7 (± 2.6) | |
| 24-Hour Within-Subject Standard Deviation | 10.2 (± 2.1) | |
BMI = Body Mass Index; BP = blood pressure; CRP = C-reactive protein; TNF-α = tumor necrosis factor-alpha
Table 2.
Systolic blood pressure variability according to quartiles of C-reactive protein and tumor necrosis factor- alpha. Results of multivariable-adjusted linear regression models.
| Inflammatory Markers | Mean* (95% CI) Within-Subject Standard Deviation of Daytime Systolic Blood Pressure | Mean† (95% CI) Within-Subject Standard Deviation of Nighttime Systolic Blood Pressure | Mean‡ (95% CI) Within-Subject Standard Deviation of 24-Hour Systolic Blood Pressure |
|---|---|---|---|
| CRP Quartiles | |||
| (< 0.5 mg/L) | 9.3 (8.5, 10.2) | 7.9 (6.8, 9.0) | 11.2 (10.2, 12.2) |
| (0.5 mg/L to < 1.2 mg/L) | 9.6 (8.8, 10.4) | 9.2 (8.2, 10.2) | 11.3 (10.4, 12.2) |
| (1.2 mg/L to < 2.9 mg/L) | 10.6 (9.8, 11.4) | 9.7 (8.7, 10.7) | 12.1 (11.2, 12.9) |
| (≥ 2.9 mg/L) | 11.2 (10.4, 12.0) | 10.3 (9.3, 11.3) | 12.8 (11.9, 13.7) |
| P for linear trend = .001 | P for linear trend = .008 | P for linear trend = .01 | |
| TNF-α Quartiles | |||
| (< 0.7 pg/ml) | 10.2 (9.4, 11.0) | 9.5 (8.5, 10.4) | 12.1 (11.2, 12.9) |
| (0.7 pg/ml to < 0.9 pg/ml) | 9.7 (8.9, 10.5) | 9.7 (8.7, 10.7) | 11.8 (11.0, 12.7) |
| (0.9 pg/ml to < 1.4 pg/ml) | 10.5 (9.7, 11.3) | 8.0 (7.0, 9.1) | 11.6 (10.7, 12.5) |
| (≥ 1.4 pg/ml) | 10.3 (9.5, 11.0) | 9.9 (8.9, 10.8) | 11.8 (11.0, 12.7) |
| P for linear trend = .66 | P for linear trend = .99 | P for linear trend = .48 | |
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean daytime systolic BP
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean nighttime systolic BP
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean 24-hour systolic BP
BMI = body mass index; BP = blood pressure; CRP = C-reactive protein; TNF-α = tumor necrosis factor-alpha
Table 3.
Diastolic blood pressure variability according to quartiles of C-reactive protein and tumor necrosis factor- alpha. Results of multivariable-adjusted linear regression models.
| Inflammatory Markers | Mean* (95% CI) Within-Subject Standard Deviation of Daytime Diastolic Blood Pressure: | Mean† (95% CI) Within-Subject Standard Deviation of Nighttime Diastolic Blood Pressure: | Mean‡ (95% CI) Within-Subject Standard Deviation of 24-hour Diastolic Blood Pressure: |
|---|---|---|---|
| CRP Quartiles | |||
| (< 0.5 mg/L) | 8.0 (7.3, 8.8) | 6.7 (5.8, 7.6) | 9.9 (9.1, 10.7) |
| (0.5 mg/L to < 1.2 mg/L) | 7.9 (7.2, 8.6) | 7.8 (7.0, 8.7) | 9.7 (8.9, 10.4) |
| (1.2 mg/L to < 2.9 mg/L) | 9.0 (8.3, 9.7) | 7.8 (7.0, 8.7) | 10.4 (9.7, 11.2) |
| (≥ 2.9 mg/L) | 9.4 (8.7, 10.1) | 8.6 (7.8, 9.5) | 11.0 (10.2, 11.7) |
| P for linear trend = .003 | P for linear trend = .01 | P for linear trend = .03 | |
| TNF-α Quartiles | |||
| (< 0.7 pg/ml) | 8.1 (7.4, 8.8) | 7.8 (7.0, 8.7) | 9.9 (9.1, 10.6) |
| (0.7 pg/ml to < 0.9 pg/ml) | 8.4 (7.7, 9.1) | 7.7 (6.9, 8.6) | 10.4 (9.7, 11.1) |
| (0.9 pg/ml to < 1.4 pg/ml) | 8.8 (8.1, 9.6) | 6.9 (6.0, 7.7) | 10.1 (9.3, 10.8) |
| (≥ 1.4 pg/ml) | 9.1 (8.4, 9.7) | 8.4 (7.6, 9.2) | 10.7 (10.0, 11.4) |
| P for linear trend = .04 | P for linear trend = .52 | P for linear trend = .28 | |
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean daytime diastolic BP
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean nighttime diastolic BP
Means adjusted for age, sex, race, BMI, quartiles of the other inflammatory marker, and mean 24-hour diastolic BP
BMI = body mass index; BP = blood pressure; CRP = C-reactive protein; TNF-α = tumor necrosis factor-alpha
We then explored whether the inflammatory markers statistically interacted with age, sex, or race. There was evidence of an interaction between race (White vs. African-American) and TNF-α levels on daytime diastolic BP variability (p = .03 for interaction). We then ran separate models in Whites and African-Americans to examine the race-specific relationship between TNF-α and diastolic BP variability. Adjusted means from these models (Figure 1) indicated that TNF-α levels were positively related to daytime diastolic BP variability in African Americans but not Whites. Similar race-specific findings were observed for CRP and daytime diastolic BP variability, but the interaction between race and CRP did not quite reach statistical significance (p = .07). None of the other interactions tested reached statistical significance (data not shown).
Figure 1.
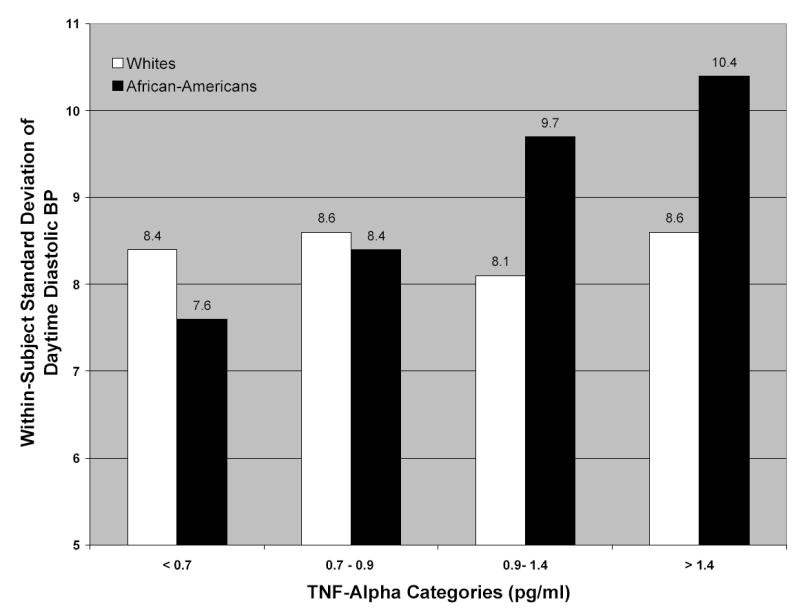
Within-subject standard deviation of daytime ambulatory diastolic BP, according to tumor necrosis factor-α categories and race (p for interaction between race and tumor necrosis factor-α = .03). Results are adjusted for age, sex, body mass index, and mean daytime diastolic BP. BP = blood pressure; TNF-alpha = tumor necrosis factor-alpha.
Our study found that CRP and, to a lesser extent, TNF-α, showed positive associations with ambulatory BP variability among normotensive middle-aged adults. These associations were observed after statistical adjustment for mean blood pressure, BMI, and other factors. The associations we observed were of fairly modest magnitude. However, our ability to observe associations of greater magnitude may have been limited by the fact that our healthy subjects had relatively normal inflammatory and BP variability values that were within fairly narrow ranges.
Our study had a number of limitations. First, our results were based on cross-sectional, observational data, so we were unable to prove causality. Second, we only measured inflammatory markers and BP variability at one point in time, and it is unclear whether these one-time measurements resulted in valid estimates of subjects’ true inflammation and BP variability levels. Third, our study was based on a relatively small sample, so it is unclear whether our results are generalizable to other populations. In spite of these limitations, we believe that our study provides new scientific information, because it appears to be the first study to report statistically significant positive associations between inflammatory factors and BP variability during ambulatory BP monitoring of healthy adults. The finding may be important clinically, because it may suggest that controlling inflammation could reduce BP variability and the adverse cardiovascular consequences which follow from it.
Acknowledgments
The authors would like to thank Steven D. Rhodes, R.N., for his valuable assistance with data collection.
Footnotes
Dr. Abramson is supported by grant 0330018N from the American Heart Association and grant K23 HL075295-01A1 from the National Institutes of Health
Dr. Vaccarino is supported by grants K24HL077506, R01 HL68630, and R01 AG026255 from the National Institutes of Health.
Cheryl Lewis, Grant T. Anderson, and Nancy C Murrah were supported by grant 0330018N from the American Heart Association
Additional study supported was provided by Emory University General Clinical Research Center grant M01-RR00039
References
- 1.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G. Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni) Hypertension. 2002;39:710–714. doi: 10.1161/hy0202.104376. [DOI] [PubMed] [Google Scholar]
- 3.Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation. 2000;102:1536–1541. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen G, Delcourt A, Toussaint G, Otsuka K, Watanabe Y, Siegelova J, Fiser B, Dusek J, Homolka P, Singh RB, Kumar A, Singh RK, Sanchez S, Gonzalez C, Holley D, Sundaram B, Zhao Z, Tomlinson B, Fok B, Zeman M, Dulkova K, Halberg F. Opportunity of detecting pre-hypertension: worldwide data on blood pressure overswinging. Biomed Pharmacother. 2005;59 (Suppl 1):S152–157. doi: 10.1016/s0753-3322(05)80023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 6.Baudry N, Rasetti C, Vicaut E. Differences between cytokine effects in the microcirculation of the rat. Am J Physiol. 1996;271:H1186–1192. doi: 10.1152/ajpheart.1996.271.3.H1186. [DOI] [PubMed] [Google Scholar]
- 7.Iversen PO, Nicolaysen A, Kvernebo K, Benestad HB, Nicolaysen G. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflugers Arch. 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- 8.Klemm P, Warner TD, Hohlfeld T, Corder R, Vane JR. Endothelin 1 mediates ex vivo coronary vasoconstriction caused by exogenous and endogenous cytokines. Proc Natl Acad Sci U S A. 1995;92:2691–2695. doi: 10.1073/pnas.92.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuki T, Duling BR. TNF-alpha modulates arteriolar reactivity secondary to a change in intimal permeability. Microcirculation. 2000;7:411–418. [PubMed] [Google Scholar]
- 10.White LR, Juul R, Skaanes KO, Aasly J. Cytokine enhancement of endothelin ET(B) receptor-mediated contraction in human temporal artery. Eur J Pharmacol. 2000;406:117–122. doi: 10.1016/s0014-2999(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee DL, Leite R, Fleming C, Pollock JS, Webb RC, Brands MW. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension. 2004;44:259–263. doi: 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]


