Abstract
This study is aimed at investigating the potentials of ex vivo expansion and pluri-differentiation of cryopreservation of adult human bone marrow mesenchymal stem cells (hMSCs) into chondrocytes, adipocytes and neurocytes. Cryopreserved hMSCs were resuscitated and cultured for 15 passages, and then induced into chondrocytes, adipocytes and neurocytes with corresponding induction medium. The induced cells were observed for morphological properties and detected for expressions of type II collagen, triglyceride or neuron-specific enolase and nestin. The result showed that the resuscitated cells could differentiate into chondrocytes after exposure to transforming growth factor β1 (TGF-β1), insulin-like growth factor I (IGF-I) and vitamin C (VC), and uniformly changed morphologically from a spindle-like fibroblastic appearance to a polygonal shape in three weeks. The induced cells were heterochromatic to safranin O and expressed cartilage matrix-procollagenal (II) mRNA. The resuscitated cells cultured in induction medium consisting of dexamethasone, 3-isobutyl-1-methylxanthine, indomethacin and IGF-I showed adipogenesis, and lipid vacuoles accumulation was detectable after 21 d. The resuscitated hMSCs were also induced into neurocytes and expressed nestin and neuron specific endolase (NSE) that were special surface markers associated with neural cells at different stage. This study suggested that the resuscitated hMSCs should be still a population of pluripotential cells and that it could be used for establishing an abundant hMSC reservoir for further experiment and treatment of various clinical diseases.
Keywords: Bone marrow mesenchymal stem cells, Cryopreservation, Expansion, Differentiation
INTRODUCTION
It has been demonstrated that adult human bone marrow mesenchymal stem cells (hMSCs) are a subset of multipotential precursor cells from the bone marrow. hMSCs express several surface epitopes with the majority of the cells being in the G0/G1 phase (Yamaguchi et al., 2001) . They are notable for their ability to self-proliferate and differentiate along multiple lineages including bone, cartilage, adipose and muscle cells (Pittenger et al., 1999; Jiang et al., 2002; Shi et al., 2002; Simonsen et al., 2002), with other researches showing that the cultured hMSCs also have the potential to differentiate into neural cells (Sanchez-Ramos et al., 2000; Woodbury et al., 2000; Xiang et al., 2001a; Blondheim et al., 2006). hMSCs can be easily isolated, cultured and expanded in vitro due to their adherent characteristics. hMSCs may be of use in the treatment of a diverse variety of clinical conditions (Collas and Hakelien, 2003; Korbling and Estrov, 2003).
The long-term cultivation of hMSCs may fail due to many factors, such as genotypic drift, senescence, transformation, phenotypic instability, contamination or incubator failure. The failure of cultivation will result in absence of hMSCs for experimental and clinical use. Therefore, it is necessary to cryopreserve hMSCs as cell seeds. Although increasing telomerase expression of cells may overcome the senescence of cells (Poh et al., 2005), the cryopreservation of hMSCs may be more practical to save much time and culture materials. Resuscitated MSCs could be subcultivated for many passages without noticeable loss of viability and capability of osteogenic differentiation (Bruder et al., 1997; Kotobuki et al., 2004; 2005). In the present study, to confirm the potentials of proliferation and pluri-differentiation of post-cryopreserved hMSCs, we resuscitated the hMSCs cryopreserved for 12 months and cultured them for 15 passages, and then analysed their growth, phenotypical and pluri-differentiation characteristics. Finally, the induction conditions of chondrocytic, adipocytic and neurocytic differentiations are discussed.
MATERIALS AND METHODS
Cell culture
hMSCs were isolated from 7 healthy adult human donors from the First People’s Hospital of Zhejiang, China. Each donor was obtained his informed consent. hMSCs were isolated and cultured according to a previously reported method with some modification (Digirolamo et al., 1999). Briefly, the aspirate was diluted 1:1 with phosphate buffer solution (PBS) to 6 ml and layered over 3 ml Ficoll (Ficoll-Paque, Pharmacia, Uppsala, Sweden). After centrifugation at 900×g for 25 min, the mononuclear cell layer was recovered from the gradient interface and washed with PBS. The cells were centrifuged at 900×g for 6 min and resuspended in complete medium [α-MEM, Gibco BRL, Grand Island, NY; 10% fetal bovine serum (FBS), Atlanta Biologicals, Norcross, GA; 100 U/ml penicillin; 100 μg/ml streptomycin; 2 mmol/L L-glutamine, Gibco BRL]. Cells are seeded in a 25-cm2 flask (Nunc, Naperville, IL) at a concentration of 1×106 cells/cm2, and then were incubated at 37 °C in 5% CO2. After 48 h, nonadherent cells were discarded, and adherent cells were washed twice with PBS. Fresh complete medium was added and replaced every 3 or 4 d. Cells grew to 70%~90% confluence after about 14 d, then harvested with 0.25% trypsin/(1 mmol·L−1) EDTA (Life Technologies, Gaithersburg, MD) and diluted 1:3 for passage.
Flow cytometry analysis
Harvested hMSCs at passage 3, 4, 8, 13 and 18 of pre-cryopreservation and at passage 1, 5, 10 and 15 of post-cryopreservation were trypsinized and stained with anti-CD34-FITC, CD45-FITC, CD44-FITC, CD90-PE, CD166-PE, CD29-PE (Becton Dickinson, San Jose, CA), SH2 and SH3 monoclonal antibodies (Osiris Therapeutics, Baltimore, MD) and were analyzed by FACScalibur flow cytometry (Becton Dickinson).
Cell cryopreservation and resuscitation
hMSCs at passage 3 of pre-cryopreservation were harvested and centrifuged at 400×g for 15 min. Cells were resuspended with 30% (v/v) sterile serum-containing α-MEM containing 10% (v/v) dimethylsulfoxide (DMSO, Sigma, St. Louis, MO) in a concentration of 1×106 cells/ml, and then loaded into a cryopreservating ampoule at 1 ml aliquots. Cells were incubated at 4 °C for 10 min, then cooled to −80 °C at a rate of 1 °C/min in a controlled-rate freezer (Labquip Asia Pte, Unity Centre, Singapore), and finally, frozen in a liquid nitrogen-fill storage vessel at −196 °C.
After freezing for 12 months, the frozen stocks of hMSCs were thawed in a constant-temperature heating bath at 37 °C by shaking lightly. After 1 or 2 min, cells were resuspended in complete medium and centrifuged at 400×g for 5 min. Cell number and viability were determined using supravital stains fluorescein diacetate and propidium iodide. hMSC viability at the time of thawing ranged from 84.6% to 100%. Then the cells were cultured at a concentration of 3×104 cells/ml in a 25-cm2 flask under 37 °C and 5% CO2. At 70%~90% confluence over about 4~5 d, cells were diluted 1:3 for passage as described above. For analysis of growth curves of hMSCs, hMSCs in passage 1, 5, 10 and 15 of post-cryopreservation were cultured in 14-well plates (Costar, Cambridge, MA) at density of 1×104 cells per well and the numbers of hMSCs from 3 wells were counted each 24 h.
Colonies of post-cryopreserved hMSCs after initial plating were stained with Wright-Giemsa. The cells were fixed with 100% methanol for at least 5 min and stained with Wright-Giemsa for 5 min. Then, Giemsa stain solution was removed and cells were rinsed thrice with distilled water and finally, cells were dried and rinsed with 95% methanol and 100% methanol successively.
Post-cryopreserved hMSCs at different passages were evaluated for expression of Oct4 by RT-PCR. Total RNA from MSCs was isolated with Trizol reagent. Five micrograms of total RNA from induced hMSCs were used for first strand cDNA synthesis with 200 U RevertAid™ M-MuLV reverse transcriptase (Fermentas, Hanover, MD) and 500 ng oligo(dT) primers. Twenty microlitres of reaction volume contained 4 μl of 5×reaction buffer and 2 μl of 10 mmol/L dNTP Mix. Reactions were incubated at 42 °C for 1 h. RT-PCR amplification was performed using specific primers for Oct4. RT-PCR was achieved after 35 cycles of 94 °C for 30 s, 62 °C for 30 s and 72 °C for 45 s. The following primers were used for RT-PCR analysis: 5′-ATC TGC TGA AGC AGA AGA GG-3′, 5′-GGT TCT CAT TGT TGT CGG CT-3′.
Chondrogenesis
Harvested hMSCs at passage 15 of post-cryopreservation were seeded into 25 cm2 flasks at a density of 3×104 cells/ml for chondrogenic differentiation. Cells were cultured to 90% confluence in a low-glucose DMEM medium with 10% FBS, and then were replaced with chondrogenic induction medium of high-glucose DMEM containing transforming growth factor β1 (TGF-β1) (10 ng/ml), IGF-I (10 ng/ml), vitamin C (VC) (50 μg/ml), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/L L-glutamine (Gibco BRL) and 10% FBS (Digirolamo et al., 1999; Yin et al., 2002). The induced cells were observed for morphological properties each day.
For histochemical analysis of induced cells, we performed the pellet culture of Johnstone et al.(1998) with a little modification. Briefly, 2×105 hMSCs harvested from passage 15 of post-cryopreservation were centrifuged in a 15-ml polypropylene tube, and the pellets were cultured with chondrogenic induction medium. For control, cells were cultured in a low-glucose DMEM medium with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/L L-glutamine and 10% FBS. The medium was replaced every 2 to 3 d for three weeks. Then, the pellets were embedded in paraffin, cut into 5 μm sections and stained with type 2 collagen specific antibody. Induced cells were also evaluated by RT-PCR. The first strand cDNA synthesis was performed as described above. The corresponding cDNA reaction was added to 48 ml of reaction mix with 5 μl of 10×PCR buffer, 1 U Taq polymerase, 25 mmol/L MgCl2, 2 mmol/L dNTP mix and 10 mmol/L of each primer. Transcripts of collagen type II gene were determined using the primers C-1 (forward): 5′-TTC AGC TAT GGA GAT GAC AAT C-3′ and C-2 (reverse): 5′-AGA GTC CTA GAG TGA CTG AG-3′ (475 bp fragment). The amplification condition was as follows: denaturation at 94 °C for 30 s, annealing at 55 °C for 60 s and extension at 72 °C for 30 s during 35 cycles. The samples were separated on a 2% agarose gel, stained with ethidium bromide and photographed under UV light.
Adipogenesis
hMSCs harvested from passage 15 of post-cryopreservation were seeded in a 30-mm dish (Costar) at the density of 2×104 cells per dish and cultured in α-MEM with 10% FBS. Cells with nearly 90% confluence were exposed to α-MEM supplemented with 10 ng/ml IGF-I, 100 μmol/L indomethacin, 1 μmol/L dexamethasone, 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma), and 10% FBS for 21 d. For control, cells were cultured in the regular medium above. Medium was changed every third or fourth day. After 3 weeks of induction, cells were washed with PBS, stained with oil red O, and observed under a light microscope (Xiang et al., 2001b). Briefly, after fixation in 10% formal calcium, induced hMSCs were stained in filtered oil red O for 2~3 h, then rinsed with 60% isopropyl alcohol.
Neurogenesis
The neuronal differentiation protocol was modified from a previously published method (Sanchez-Ramos et al., 2000). hMSCs at passage 3 of pre-crypreservation and at passage 15 of post-preservation were cultured in in a 30-mm dish (Costar) described above. At 70%~90% confluence, medium was replaced with pre-induction medium consisting of α-MEM/10% FBS/1 mmol/L β-mercaptoethanol (BME; Sino-American Biotechnology, Shanghai, China) and cultured for 24 h before neuronal induction. For neuronal differentiation, the pre-induction medium was removed, and then cells were washed with PBS and induced in a neuronal induction medium composed of α-MEM with 2% dimethylsulfoxide (DMSO), 200 μmol/L butylated hydroxyanisole (BHA), 1 μmol/L hydrocortisone, 10 ng/ml insulin-like growth factor (IGF-I) (Sigma), and 0.5 μmol/L all-trans retinoic acid (ATRA) (Sigma).
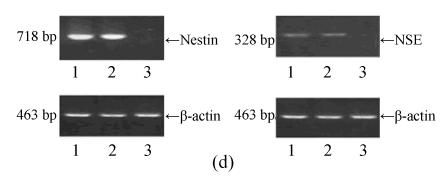
Induced cells were processed for morphological observation, immuno-cytochemistry and RT-PCR at 1 and 6 d of post-induction (Woodbury et al., 2000; Xiang et al., 2001a). For immunocytochemistry, induced cells were fixed in 4% paraformaldehyde (Sigma) for 15 min, rinsed with PBS, and blocked with 10% bovine serum albumin (BSA) (Atlanta Biologicals) for 30 min. Fixed cells were stained in 10% BSA with rb (rabbit polyclonal) nestin (1:400) or rb neuron specific endolase (NSE) (1:400) (Boster, Wuhan, China) at 4 °C overnight. Then, cells were incubated for 1 h with secondary antibodies (Abcom, Cambridge, MA). Diaminobenzidine (DAB, BioVision, Mountain View, CA) served as chromagen. Labelled cells were visualized using reflected light fluorescence microscope and color images were generated using Adobe Photoshop (Adobe Systems, Mountain View, CA). For RT-PCR, total RNA from the induced cells was isolated with Trizol reagent. The first strand cDNA synthesis was performed as above. The corresponding cDNA reaction was added to 48 ml reaction mix containing 5 μl of 10×PCR buffer, 1 U Taq polymerase, 25 mmol/L MgCl2, 2 mmol/L dNTP mix and 10 mmol/L of each primer for the corresponding amplication. As a marker of neuron differentiation, transcripts of nestin and NSE were determined using the primers as follows: for nestin, C-1 (forward) 5′-GGC AGC GTT GGA ACA GAG GTT GGA-3′ and C-2 (reverse) 5′-CTC TAA ACT GGA GTG GTC AGG GCT-3′ (718 bp fragment); for NSE, C-1 (forward) 5′-AAG GAC AAA TAC GGC AAG GA-3′ and C-2 (reverse) 5′-TGG ACC AGG CAG CCC AAT C-3′ (328 bp fragment); for β-actin, C-1 (forward) 5′-GGC GAC GAG GCC CAG A-3′ and C-2 (reverse) 5′-CGA TTT CCC GCT CGG C-3′ (463 bp fragment). The amplification conditions were as follows: pre-denaturation at 94 °C for 5 s, denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 10 s during 30 cycles (for nestin), 32 cycles (for NSE) and 26 cycles (for β-actin). The samples were separated on a 2% agarose gel, stained with ethidium bromide and photographed under UV light.
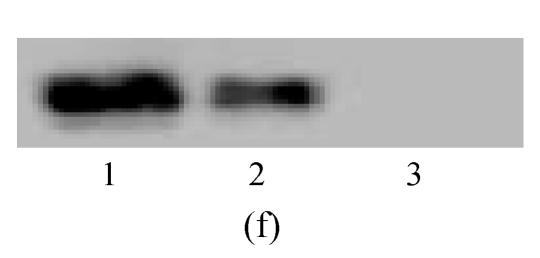
Western blot assay was performed to analyze neural markers at the level of protein. Cells were lysed with sodium dodecyl sulfate (SDS) 2%. Loading buffer (75 mmol/L Tris-HCl, pH 6.8, 20% glycerol, 5% 2-mercaptoethanol, 0.001 bromophenol blue) was subsequently added and samples placed in boiling water for 3 min. Fifteen to thirty micrograms of protein was run on 10% or 8% SDS polyacrylamide gel, depending on molecular weight. Separated proteins were electroblotted onto PVDF (polyvinylidene difluoride) membrane (Hybond-P, Amersham) and blocked with 5% nonfat dry milk overnight at 4 °C. Immunodetection was performed with antibodies against nestin (1:1000, monoclonal, Chemicon, Temecula, CA, USA) and NSE (1:5000, monoclonal, Cymbus Biotechnology). The membrane was washed and incubated with a horseradish-peroxidase-conju-gated antimouse IgG (1:1000, Chemicon). After washing, protein bands were detected with SuperSignal West Pico chemiluminescent substrate (Pierce).
Statistical analysis
Statistical significance between groups was determined for percentages of hMSCs expressing specific phenotypes using mean±SEM, and statistical comparisons were performed using the Student’s t test. A level of P<0.05 was accepted as significant.
RESULTS
Growth and phenotypes of post-cryopreserved hMSCs
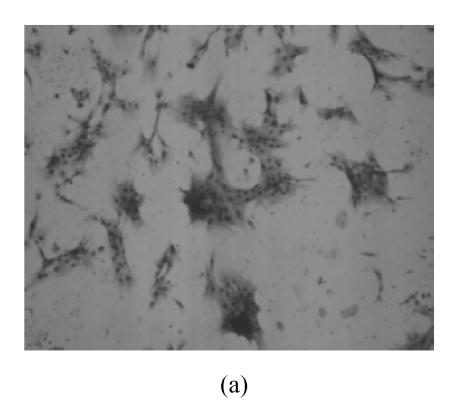
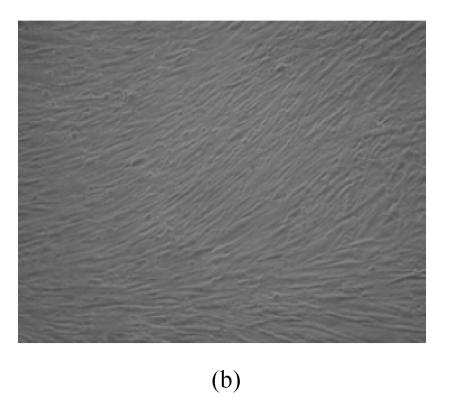
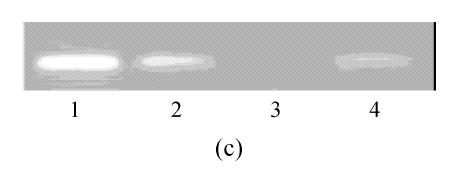
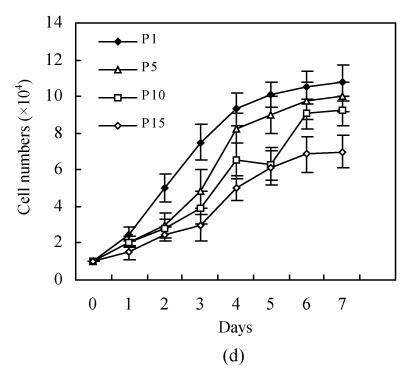
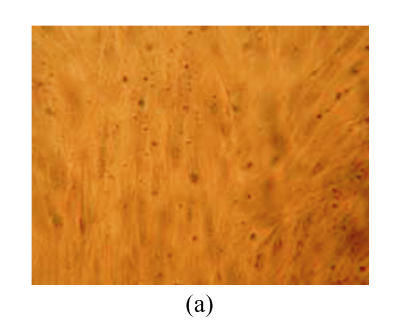
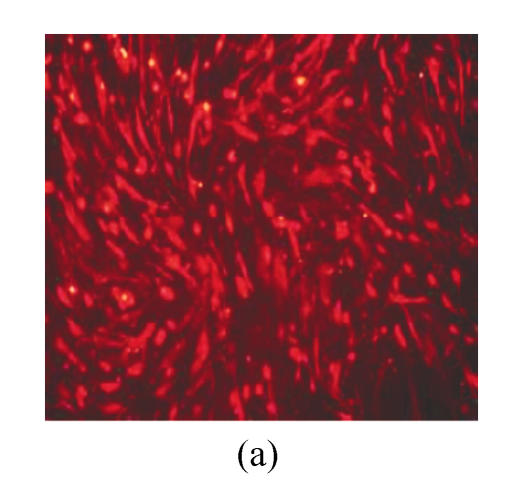
hMSCs from pre- and post-cryopreservation had similar colony-formation and cellular morphology. The resuscitated hMSCs grew first as isolated colonies after initial plating (Fig.1a) and then these adherent cells grew in typically fibroblastic or spindle shape. As cells approached confluence, they assumed a more spindle shape, and fibroblastic morphology (Fig.1b). The resuscitated hMSCs kept expression of Oct4 at different passages of post-cryopreservation (Fig.1c). Fig.1d showed the growth curves of passage 1, 5, 10 and 15 of post-cryopreservation.
Fig. 1.
Ex vivo growth morphology of resuscitated hMSCs. (a) Colonies of post-cryopreserved hMSCs stained with Wright-Giemsa (×100); (b) Confluence of post-cryopreserved hMSCs (×100); (c) RT-PCR for Oct4 (line 1 for passage 1 of resuscitated hMSCs, line 2 for passage 5 of resuscitated hMSCs, line 3 for control cells (fibroblast cells) and line 4 for passage 10 of resuscitated hMSCs); (d) The growth curves of hMSCs at passage 1 (P1), 5 (P5), 10 (P10) and 15 (P15) of post-cryopreservation
Fluorescent sorting of cells at passage 3, 4, 8, 13 and 18 of pre-cryopreservation and at passage 1, 5, 10 and 15 of post-cryopreservation showed that these cells were negative for CD34 and CD45 (leukocyte common antigen), cell surface markers associated with lymphohematopoietic cells, which demonstrated that there were not hematopoietic precursors in the cultures. In contrast, cells expressed CD44 (Pgp-1/ly-24), CD29 (integrin β1), CD90 (Thy-1), CD166 (activated leukocyte cell adhesion molecule, ALCAM), SH2 and SH3 (Src-homology domains) (Table 1), which are similar to the surface antigens of human mesenchymal stem cells. And percentages of cells expressing these surface markers increased with passage. A comparison of cryopreserved hMSCs with non-cryopreserved hMSCs at similar passages (Post-1 with Pre-4, Post-5 with Pre-8; Post-10 with Pre-13 and Post-15 with Pre-18) was performed to evaluate the effect of cryopreservation on the phenotype of the cell population. Table 1 shows that there was no significant difference at similar passages between cryopreserved and non-cryopreserved populations (P>0.05). It was concluded that cryopreservation did not affect the phenotype of hMSCs populations.
Table 1.
Percentage of bone marrow mesenchymal stem cells expressing specific phenotypes
| Passage | CD34** | CD45 | CD29 | CD44 | CD90 | CD166 | SH2 | SH3 |
| Pre-3* | 3.8±5.2a | 5.2±4.7a | 30.2±11.2a | 29.6±7.8a | 37.6±19.2a | 47.5±6.9a | 37.8±5.7a | 27.2±15.2a |
| Pre-4 | 3.8±4.3a | 5.1±3.3a | 24.8±9.2a | 27.4±11.4a | 35.6±10.5a | 48.6±16.2a | 35.8±8.9a | 41.2±14.2a |
| Pre-8 | 2.0±0.9 | 3.3±1.1b | 57.3±9.4a | 43.5±12.2a | 59.8±12.7b | 75.4±9.5b | 60.4±7.9a | 55.5±11.2a |
| Pre-13 | 1.2±0.5 | 0.9±0.3 | 75.2±11.6 | 81.4±15.7 | 70.3±14.4 | 81.5±16.6 | 83.7±18.2 | 80.9±12.4 |
| Pre-18 | 1.1±0.7 | 0.5±0.3 | 82.6±10.4 | 85.8±15.8 | 80.2±18.6 | 90.4±14.5 | 84.2±14.2 | 82.4±10.8 |
| Post-1 | 3.3±2.5a | 5.9±6.2a | 27.3±13.0a | 29.1±17.2a | 34.7±9.9a | 50.8±14.6a | 41.4±9.3a | 39.4±11.7a |
| Post-5 | 1.5±0.7 | 3.6±1.3a | 54.8±8.2a | 46.4±13.3a | 57.2±15.4b | 79.5±12.8b | 62.2±10.2a | 53.9±7.5a |
| Post-10 | 1.7±0.9 | 1.1±0.6 | 73.3±16.1 | 79.6±18.7 | 68.8±17.3 | 84.5±20.2 | 86.4±6.8 | 82.5±9.3 |
| Post-15 | 0.9±1.2 | 0.2±0.5 | 87.4±7.7 | 88.3±11.5 | 81.7±12.5 | 93.5±10.4 | 80.1±9.5 | 79.3±10.4 |
Pre-: Pre-cryopreservation; Post-: Post-cryopreservation
CD34: Hematopoietic stem cell marker; CD45: Leucocyte common antigen (LCA); CD29: Beta-1 integrin; CD44: Family of cell surface glycoproteins; CD90: Thy-1; CD166: Activated leukocyte cell adhesion molecule (ALCAM); SH2: An Src-homology 2 domain; SH3: An Src-homology 3 domain. CD34−, CD45−, CD29+, CD44+, CD90+, CD166+, SH2+ and SH3+ are immunophenotypic properties of hMSCs (Pittenger et al., 1999)
Comparison with Post-10 passage: P<0.01
Comparison with Post-10 passage: P<0.05
The “stemness” of resuscitated hMSCs was also demonstrated by analysis of Oct4 expression (Fig.1d). It was shown that Oct4 expression was maintained at different passages of post-cryopreservation. It suggests that resuscitated hMSCs were undifferentiated cells with high proliferative capacity.
Pluripotential differentiation of post-cryopreserved hMSCs
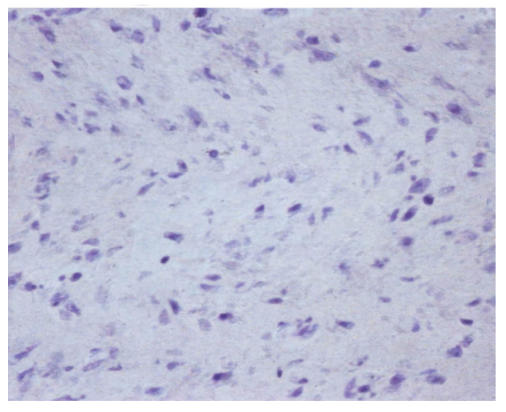
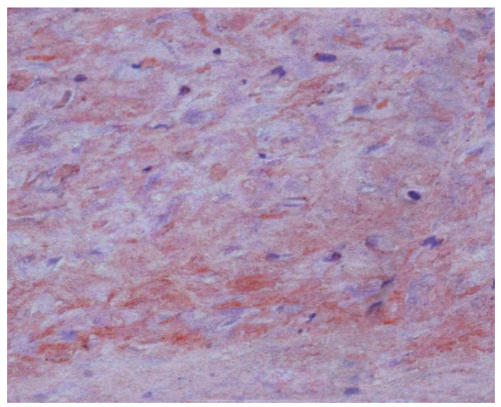
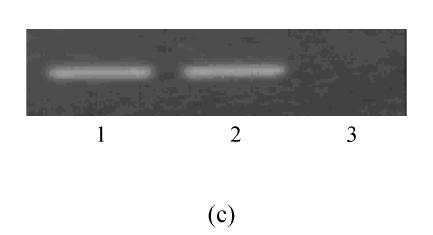
During the assay of three weeks, hMSCs cultured in chondrogenic induction medium underwent a dramatic change in cellular morphology that was accompanied by a significant increase in type II collagen production. In the first three days of induction, the morphology of cells did not evidently change, but the cell body began to retract from the fourth to fifth day of induction, and up to 21 d the decrease in size of differentiated cells resulted in bigger space between them. Meanwhile, the differentiated cells showed a strong safranin O staining in the center of cultured pellet that revealed the expression of aggrecan (Fig.2b) and the control cells were negative for safranin O staining (Fig.2a). The expression of type II collagen was uniformly probed by RT-PCR for passage 3 of pre-cryopreservation and passage 15 of post-cryopreservation (Fig.2c).
Fig. 2.
The differentiation of resuscitated hMSCs into chondrocytes. Induced cells from pellet cultures at passage 15 of post-cryopreservation in paraffin section immunohistochemically stained with type 2 collagen specific antibody after three weeks of culture in control media (a) and with chondrogenic induction medium (b). The expression of type II collagen of induced cells (c) (line 1: Induced cells from passage 15 of post-cryopreservation; line 2: Induced cells from passage 3 of pre-cryopreservation; line 3: Uninduced cells from passage 15 of post-cryopreservation)
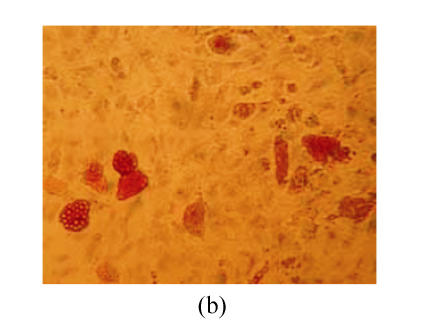
After culture with adipogenic induction medium, lipid vacuoles accumulation was detectable in cells induced for 3 d (not shown). For three weeks of induction, oil red O staining showed lipid vacuoles with orange red colour, which indicated the differentiation of MSCs into adipoblast (Fig.3b), but the control cells showed no detectable lipid vacuoles (Fig.3a).
Fig. 3.
The differentiation of resuscitated hMSCs into adipocytes oil red O staining of cells from passage 15 of post-cryopreservation not under induction (×250) (a) and under induction for 3 weeks (×250) (b)
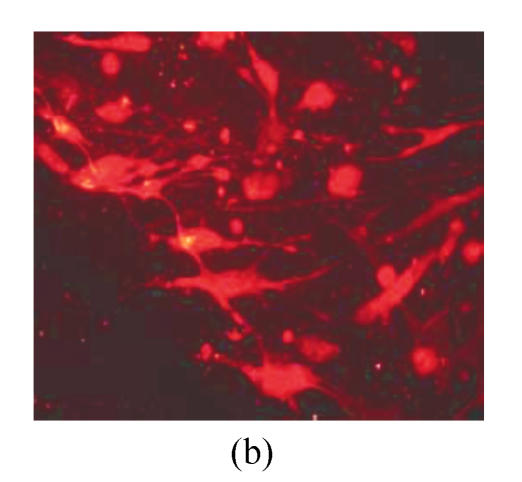
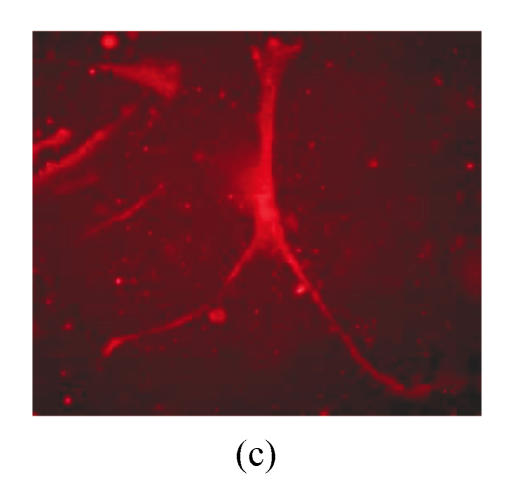
Under culture with neuronal induction medium, hMSCs turned bipolar, multipolar and tapered, and then intersected as network structure. After induction for 24 h, hMSCs with fibroblastic shape transformed into neural progenitor cells with refractile cell bodies and short branching processes. Immunocytofluorescence analysis of cells induced for 24 h revealed the expression of nestin in induced cells (Fig.4a). RT-PCR and Western blotting showed the expression of nestin (Figs.4d and 4f). For induction of 6 d, induced cells prolonged their branching processes that produced cone-like terminal structures. Immunocytofluorescence analysis showed that cells induced for 6 d expressed NSE cells (Figs.4b and 4c). RT-PCR and Western blotting showed the expression of NSE, which revealed that hMSCs had been induced into neurons (Figs.4e and 4g).
Fig. 4.
The differentiation of resuscitated hMSCs into neurocytes. (a) Nestin staining of cells from passage 15 of post-cryopreservation induced for 1 d (×100); (b) NSE staining of cells from passage 15 of post-cryopreservation induced for 6 d (×250); (c) NSE staining of cells from passage 15 of post-cryopreservation induced for 6 d (×400); (d) RT-PCR of nestin mRNA in cells induced for 1 d; (e) RT-PCR of NSE mRNA in cells induced for 6 d; (f) Western blotting of nestin in cells induced for 1 d; (g) Western blotting of NSE in cells induced for 6 d
(d)~(f) Line 1: induced cells from passage 15 of post-cryopreservation, line 2: induced cells from passage 3 of pre-cryopreservation, line 3: uninduced cells from passage 15 of post-cryopreservation; (g) Line 1: uninduced cells from passage 15 of post-cryopreservation, line 2: induced cells from passage 15 of post-cryopreservation, line 3: induced cells from passage 3 of pre-cryopreservation
DISCUSSION
Cryopreservation is an important method to keep cells as seeds. The use of cryopreserved hMSCs in gene and cell therapy also requires preservation of their differentiation and proliferative capacities. Therefore, it is necessary to determine whether the process of cryopreservation affected either the proliferative capacity of hMSCs, or their developmental potential. Bruder et al.(1997) found that resuscitated hMSCs could be subcultivated for many passages without noticeable loss of capability of osteogenic differentiation. Recently Kotobuki et al.(2005) revealed that hMSCs do not loose their osteogenic differentiation capacity after cryopreservation. Here, resuscitated hMSCs kept a high proliferative potential. hMSCs first grew as clones after limiting dilution and then expanded rapidly over 15 cumulative population doublings. We had propagated resuscitated hMSCs for more than 25 passages (Qiu et al., 2004). Resuscitated hMSCs have a homogenous morphology and cell-surface antigen profile with pre-cryopreserved cells. In our experiment, the undifferentiated hMSCs, including pre-cryopreserved, resuscitated and propagated cells for 15 passages, showed the typical features of spindle-shaped cell bodies and of confluence after a lag phase of 6~10 d. These cells showed a uniform cell surface expression profile (positive for CD29, CD44, CD90, CD166, SH2 and SH3, negative for CD34 and CD45, which demonstrated that these cells were not hematopoietic cells) similar to previous results (Pittenger et al., 1999). The percentage of positive immunophenotypes increased and the percentage of negative immunophenotypes decreased with passaging (Table 1) with the immunophenotypic profile of resuscitated hMSCs not change significantly after 10 passages in culture (P>0.05). It showed that the passage procedure was an hMSC selection procedure. It could be inferred that the passage of resuscitated hMSCs enhanced the homogeneity of the cells. In addition to characteristics described above, another defining feature that resuscitated hMSCs kept was that Oct4, a transcriptional binding factor present in undifferentiated cells with high proliferative capacity (Tondreau et al., 2005; Ren et al., 2006), kept expression at different passages of post-cryopreservation. Also, their multipotentiality or their ability to acquire multiple cellular phenotypes was shown when exposed to appropriate stimuli. Our present study demonstrated that the post-cryopreserved hMSCs from bone marrow were still pluripotential and that the resuscitated hMSCs indeed exhibitted two essential stem cells’ characteristics: extensive self-renewal capacity and multi-lineage potential. These resuscitated cells could be not only maintained in an undifferentiated state in vitro as well, but also directed into the plurilineage in vitro upon the addition of some appropriate bioactive factors. These observations suggest that the “memory” of proliferation and differentiation in hMSCs is not affected by the process of cryopreservation. To be sure, cells which were frozen and stored for as long as 27 years have been shown to behave the same way as the starting cells which were never cryopreserved (Hayflick, 1989). Our result may also be an indicator of results obtained with hMSCs stored for a long time though it was only 12 months in the present study.
Insulin is regarded as an essential hormone for various differentiations of hMSCs. Insulin binds to cell-surface receptors to stimulate the production of intra-cellular second messenger(s). Many of the responses to insulin involved alterations in phosphorylation of specific proteins. Insulin regulation of transcription of a number of genes has been described (Meisler and Howard, 1989). So insulin is very important for cell differentiation. It was suggested that insulin response may be duplicated with much lower concentrations of IGF-I (Wang et al., 2002). Some researches showed that IGF-I was able to induce chondrocytic differentiation (Worster et al., 2001; Zhang et al., 2004a; Indrawattana et al., 2004). In the present study, we used IGF-I to induce chondrocytic, adipocytic and neurocytic differentiation. The results showed that IGF-I could be used to replace insulin for multi-differentiations of hMSCs.
Furthermore, we tried to modify the combination of induction factors in differentiation of resuscitated hMSCs, such as chondrocytic and neurocytic differentiations. For chondrocytic differentiation in vitro of MSCs, it had been demonstrated that TGF-β was necessary for induction in vitro (Worster et al., 2001; Indrawattana et al., 2004; Lisignoli et al., 2005; Bai et al., 2004; Lee et al., 2004; Chen et al., 2003). In addition, other inducers were supplemented for chondrogenic induction, such as CDMP-1 (Bai et al., 2004), BMP (Indrawattana et al., 2004; Lee et al., 2004), GDF-5 (Lee et al., 2004), IGF-I (Worster et al., 2001; Zhang et al., 2004a; Indrawattana et al., 2004) or dexamethasone (Mackay et al., 1998). TGF-β induced-chondrogenesis was regulated through ERK1/2 signaling pathway. This pathway is necessary to promote type II collagen expression. Aggrecan was not regulated completely through ERK1/2 signaling pathway. It was reported that aggrecan gene expression was regulated by cross-talk between Smad, ERK1/2, and p38MAPK pathway (Watanabe et al., 2001). Therefore, in vitro induction of chondrogenesis from hMSCs needs a combination of various inducers though TGF-β is essential. In the present study, it may be a result of cross talk between different signaling pathways that cells induced with differentiation medium consisting of TGF-β, IGF-I and VC expressed aggrecan and type II collagen. For neurogenesis, various inducers, alone or in combination, are involved in neurocytic differentiation in vitro of MSCs. These include retinoic acid (Cho et al., 2005), DMSO and BHA (Chu et al., 2004; Lu et al., 2004), GM1 and bFGF (Dezawa et al., 2004; Zhang et al., 2004b; Munoz-Elias et al., 2003), and 2-mercapto-ethanol (2-ME) (Guo et al., 2001). It was suggested that cAMP signaling pathway should be involved in neurocytic differentiation of MSCs (Suon et al., 2004; Jori et al., 2005), but it could not rule out the synergistic effect of other signaling pathways on neurogenesis of hMSCs, in regard to neural differentiation of embryonic stem cells (Kleber et al., 2005; Bainter et al., 2001; Otero et al., 2004). Neuhuber et al.(2004) highlighted the possible deficiencies of many protocols for differentiating MSCs into neurocytes. They suggested that DMSO alone was responsible for the change in morphological characteristics of the cell and that neurogenesis had not occurred. The combination of inducers in the present study promoted expression of genes (nestin and NSE) associated with neurogenesis in different differentiation stages. The signaling pathways involved in these inducers requires further study.
In conclusion, it was verified that resuscitated hMSCs have the growth and differentiation characteristics similar to pre-cryopreserved cells even if they are propagated for many passages. This study supplied the supportive data that cryopreserved hMSCs could still undergo differentiation towards the chondrogenic, adipogenic or neurogenic lineage. Therefore, it supported the suggestion that cryopreserved hMSCs could indeed be used as prospective adult stem cells in tissue engineering, cell transplantation and gene therapy (Bruder et al., 1997; Kotobuki et al., 2005).
Footnotes
Project supported by the Science Foundation (No. 2003C23015) and the Natural Science Foundation (No. Y204139) of Zhejiang Province, China
References
- 1.Bai X, Xiao Z, Pan Y, Hu J, Pohl J, Wen J, Li L. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325(2):453–460. doi: 10.1016/j.bbrc.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 2.Bainter JJ, Boos A, Kroll KL. Neural induction takes a transcriptional twist. Dev Dyn. 2001;222(3):315–327. doi: 10.1002/dvdy.1210. [DOI] [PubMed] [Google Scholar]
- 3.Blondheim NR, Levy YS, Zur TB, Burshtein A, Cherlow T, Kan I, Barzilai R, Bahat-Stromza M, Barhum Y, Bulvik S, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15(2):141–164. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- 4.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Mao T, Ding G. Experimental study on the chondrogenesis potentiality of marrow stromal cell under the induction of transforming growth factor-β. West China J Stomoatol. 2003;21(2):92–94. (in Chinese) [PubMed] [Google Scholar]
- 6.Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1α. Stem Cells. 2005;23(3):383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 7.Chu Q, Wang Y, Fu X, Zhang S. Mechanism of in vitro differentiation of bone marrow stromal cells into neuron-like cells. J Huazhong Univ Sci Technol Med Sci. 2004;24(3):259–261. doi: 10.1007/BF02832006. [DOI] [PubMed] [Google Scholar]
- 8.Collas P, Hakelien AM. Teaching cells new tricks. Trends Biotechnol. 2003;21(8):354–361. doi: 10.1016/S0167-7799(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 9.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113(12):1701–1710. doi: 10.1172/JCI200420935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107(2):275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 11.Guo ZK, Liu XD, Hou CM, Li XS, Mao N. Human bone marrow mesenchymal stem cells differentiate into neuron-like cells in vitro. J Exp Hematol Sinica. 2001;9(1):91–92. (in Chinese) [PubMed] [Google Scholar]
- 12.Hayflick L. Antecedents of aging research. Exp Gerontol. 1989;24(5-6):355–365. doi: 10.1016/0531-5565(89)90043-0. [DOI] [PubMed] [Google Scholar]
- 13.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320(3):914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 16.Jori FP, Napolitano MA, Melone MA, Cipollaro M, Cascino A, Altucci L, Peluso G, Giordano A, Galderisi U. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem. 2005;94(4):645–655. doi: 10.1002/jcb.20315. [DOI] [PubMed] [Google Scholar]
- 17.Kleber M, Lee HY, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol. 2005;169(2):309–320. doi: 10.1083/jcb.200411095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korbling M, Estrov Z. Adult stem cells for tissue repair–a new therapeutic concept? N Engl J Med. 2003;349(6):570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 19.Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28(1):33–39. doi: 10.1111/j.1525-1594.2004.07320.x. [DOI] [PubMed] [Google Scholar]
- 20.Kotobuki N, Hirose M, Machida H, Katou Y, Muraki K, Takakura Y, Ohgushi H. Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng. 2005;11(5-6):663–673. doi: 10.1089/ten.2005.11.663. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Kim YH, Kim SH, Han SH, Hahn SB. Chondrogenic differentiation of mesenchymal stem cells and its clinical applications. Yonsei Med J. 2004;45(Suppl.):41–47. doi: 10.3349/ymj.2004.45.Suppl.41. [DOI] [PubMed] [Google Scholar]
- 22.Lisignoli G, Cristino S, Piacentini A, Toneguzzi S, Grassi F, Cavallo C, Zini N, Solimando L, Maraldi NM, Facchini A. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials. 2005;26(28):5677–5686. doi: 10.1016/j.biomaterials.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77(2):174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]
- 24.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 25.Meisler MH, Howard G. Effects of insulin on gene transcription. Ann Rev Physiol. 1989;51(1):701–714. doi: 10.1146/annurev.ph.51.030189.003413. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21(4):437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- 27.Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fisher I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004;77(2):192–204. doi: 10.1002/jnr.20147. [DOI] [PubMed] [Google Scholar]
- 28.Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA. β-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131(15):3545–3557. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Poh M, Boyer M, Solan A, Dahl SLM, Pedrotty D, Banik SSR, McKee JA, Klinger RY, Counter CM, Niklason LE. Blood vessels engineered from human cells. Lancet. 2005;365(9477):2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 31.Qiu LY, Wang JF, Shen D, Jin J. Expansion and chondrogenic induction of human bone marrow mesenchymal stem cells. J Zhejiang Univ (Sci Ed) 2004;31(2):337–342. (in Chinese) [Google Scholar]
- 32.Ren T, Cao Y, Zhao Q, Zhou C, Liao L, Jia M, Zhao Q, Cai H, Han ZC, Yang R, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347(1):12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 34.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20(6):587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 36.Suon S, Jin H, Donaldson AE, Caterson EJ, Tuan RS, Deschennes G, Marshall C, Iacovitti L. Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells Dev. 2004;13(6):625–635. doi: 10.1089/scd.2004.13.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23(8):1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Luo CJ, Guo CH, Zhang Y. Mesenchymal stem cells and related factors. J Exp Hematol Sinica. 2002;10(5):468–471. (in Chinese) [PubMed] [Google Scholar]
- 39.Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 40.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 41.Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19(4):738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 42.Xiang P, Xia WJ, Zhang LR, Chen ZG, Zhang XM, Li Y, Li SN. Human mesenchymal stem cells differentiate into neuron-like cells. Chin J Pathophysiol. 2001;17(4):385–387. (in Chinese) [Google Scholar]
- 43.Xiang P, Zhang LR, Chen ZG, Xia WJ, Zhang XM, Li Y, Li SN. Adult human mesenchymal stem cells differentiate into adipocytes. Chin J Pathophysiol. 2001;17(6):598–601. (in Chinese) [Google Scholar]
- 44.Yamaguchi M, Hirayama F, Kanai M, Sato N, Fukazawa K, Yamashita K, Sawada K, Koike T, Kuwabara M, Ikeda H, et al. Serum-free coculture system for ex vivo expansion of human cord blood promitive progenitors and SCID mouse-reconstituting cells using human bone marrow primary stromal cells. Exp Hematol. 2001;29(2):174–182. doi: 10.1016/S0301-472X(00)00653-6. [DOI] [PubMed] [Google Scholar]
- 45.Yin ZH, Liu M, Wang JT, Cao JL, Zheng J. Inducing rabbit bone marrow mesenchymal stem cells (MSCs) to express chondrogenic protential in vitro. Orthop J China. 2002;9(6):586–589. (in Chinese) [Google Scholar]
- 46.Zhang Y, Wang C, Liao W, Li Z, Guo X, Zhao Q, Duan C, Xia R. In vitro chondrogenic phenotype differentiation of bone marrow-derived mesenchymal stem cells. J Huazhong Univ Sci Technol Med Sci. 2004;24(3):275–278. doi: 10.1007/BF02832011. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Wang JZ, Sun HY, Zhang JN, Yang SY. The effects of GM1 and bFGF synergistically inducing adult rat bone marrow stromal cells to form neural progenitor cells and their differentiation. Chin J Traumatol. 2004;7(1):3–6. (in Chinese) [PubMed] [Google Scholar]