Abstract
To evaluate the public health risk of exposure to microcystins in fish food in China, the distribution pattern of microcystin-LR and microcystin-RR in various organs (liver, intestine, kidney, muscle and lipid) of the dominant freshwater phytoplanktivorous fish Hypophthalmichthys molitrix in Hangzhou, China’s Tiesha River was investigated with the method of HPLC-ESI-MS analysis. The distribution of microcystins was different in the fish organs and the major total microcystins (microcystin-LR and microcystin-RR) were present in the intestines (6.49 μg/g fresh weight), followed by the livers (4.52 μg/g fresh weight) and the muscles (2.86 μg/g fresh weight). Microcystins were detected in kidneys (1.35 μg/g fresh weight), but not detected in lipid. The results suggested that the mean daily intake from fish was 0.03 μg/kg body weight which was very close to the recommended WHO tolerable daily intake (TDI) level of 0.04 μg/kg body weight per day, and local people were warned they may have health risk if they consumed fish from the river.
Keywords: Microcystin, Organ distribution, Bioaccumulation, Fish, Risk
INTRODUCTION
Cyanobacterial blooms are becoming an important water quality problem all over the world including China. Microcystins are the most widespread type of cyanobacterial toxins in freshwater systems, which represent a potential stress and hazard to aquatic animal population (Huynh-Delerme et al., 2005). Bioaccumulation of microcystins by fish has been reported by several authors (Tencalla et al., 1994; de Magalhães et al., 2001) and microcystins are also believed to be involved in the large-scale die-off of fish (Ibelings et al., 2005). Because fish is at the most important trophic level in the aquatic food chain, microcystins may pose a common health risk to human through a food-chain transfer process in the case of fish as an important human daily food source.
In 1998, heavy cyanobacterial blooms were observed in the Tiesha River, with Microcystis aeruginosa being a predominant species in the bloom material (Wang et al., 2003). It was reported that this bloom was toxic, with colorimetric protein phosphatases inhibition assay revealing that microcystin levels in surface water were higher than the WHO maximum allowable concentration for MCYST (microcystin) in drinking water of 1 μg/L (Falconer et al., 1994). Although some measures such as using the phytoplanktivorous freshwater fish to digest cyanobacteria have been applied to control these blooms in the river, recently we found trace level of microcystin-LR and microcystin-RR in the river. H. molitrix has been the dominant species in the Tiesha River and is often harvested by local fishermen for food. As phytoplanktivorous fish, H. molitrix are especially important to human because of its role in aquatic ecosystems as direct consumers of phytoplankton and potential for biological management of algal blooms. They may digest much more toxic cyanobacteria in normal diets compared with the herbivorous and carnivorous fish.
This study was aimed at examining the distribution patterns of microcystins in H. molitrix and at evaluating the effects of bioaccumulated microcystins in the organs and the risks for fish food chain in the Tiesha River.
MATERIALS AND METHODS
Chemicals
Microcystin-LR (CAS No. 101043-37-2, C49H74N10O12, molecular weight: 995.2, purity>95%) and microcystin-RR (CAS No. 111755-37-4, C49H75N13O12, molecular weight: 1038.2, purity>95%) were purchased from Sigma (St. Louis, MO, USA). Methanol and acetonitrile were HPLC grade, and all other reagents were analytical grade. Standard solutions were prepared in methanol and stored at −20 °C. The Oasis HLB (hydrophilic-lipophilic balance) solid phase extraction cartridges were purchased from Waters Corporation (Milford, MA, USA).
Cleaning-up method for fish samples
The analyzed fish (H. molitrix) harvested by local fishermen in the Tiesha River were dissected into five parts: intestine, liver, muscle, kidney and lipid, then immediately frozen at −20 °C by liquid nitrogen in the laboratory.
The purification of microcystins for analysis was based on de Magalhães et al.(2001) with some modification. Each fresh tissue sample (0.5 g) was extracted twice by 75% methanol (5 ml) with a supersonic instrument for 30 min. The methanol extract was centrifuged at 12 000 g/min for 10 min. Then the extract was mixed three times with 2 ml hexane. Hexane layers were discarded and the methanol extract was diluted with 10 ml water. The extract was concentrated and cleaned using an Oasis HLB solid phase extraction cartridge. The cartridges were washed with 2 ml of water after the adsorption of the targeted compounds. The cartridges were eluted with 1 ml of 90% methanol with 0.1% trifluoroacetic acid (TFA). The elution was evaporated dry, and the residue was dissolved in 0.2 ml of water. The final water solution was subjected to HPLC-ESI-MS analysis.
HPLC-ESI-MS analysis
An agilent 1100 HPLC system (Palo Alto, CA, USA) equipped with degasser, autosampler, a binary pump, and a mass spectrometer was used to analyze specific microcystins. The separation was performed using a 5 μm Zorbax SB-C18 column (150 mm×2.0 mm i.d.). The column temperature was set at 30 °C, controlled by temperature controller TC100 and HT-130 column heater. The mobile phase consisted of acetonitrile and water (0.5% formic acid) at a ratio of 35/65 (v/v). The flow rate was set at 0.3 ml/min. Sample injection volumes were typically 20 μl.
The mass spectrometer was a Quattro Micro (Micromass, Manchester, UK) triple quadrupole MS with electrospray ionization (ESI). Samples were introduced directly to the mass spectrometer via the HPLC column. The capillary voltage was set at 4.0 kV and the fragment voltage at 100 V. The desolvation gas (nitrogen) temperature and flow-rate were set at 350 °C and 11 L/min, respectively. The ion source temperature was set at 120 °C. The instrument was operated in the positive ion mode. In full-scan mode, mass spectra of 500 to 1200 m/z were obtained. In the selected-ion monitoring (SIM) mode, two ions, 519.9 (microcystin-RR, [M+2H]2+) and 995.6 (microcystin-LR, [M+H]+) were monitored.
During the HPLC-ESI-MS analysis we obtained a calculated limit of detection (LOD) of 0.02 μg/g fresh tissue. Recovery percentages were evaluated from spiked samples with recoveries of 74.2% for microcystin-LR and 88.4% for microcystin-RR, respectively.
RESULTS
Standards and samples mass spectrum
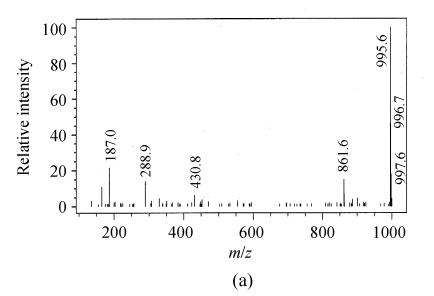
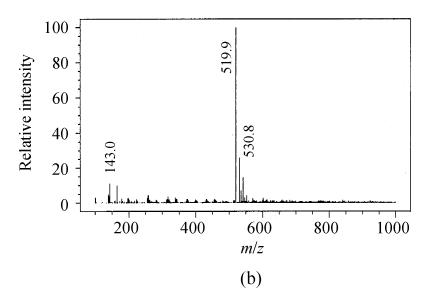
Depending on the amino acids in the microcystin structure, mono [M+H]+ or double charged [M+2H]2+ ions were obtained. The mass spectra of microcystin-LR showed mainly the single charged ions [M+H]+ at 995.6 (Fig.1a). But the main mass peak observed for microcystin-RR was that of the double charged ion [M+2H]2+ at 519.9 m/z, probably due to the presence of two arginine residues (Fig.1b). All ESI+mass spectra obtained were similar to those reported in the literature (Barco et al., 2002).
Fig. 1.
Full scan electrospray mass spectra of (a) microcystin-LR and (b) microcystin-RR with the base peaks at 995.6 m/z and 519.9 m/z of a standard solution
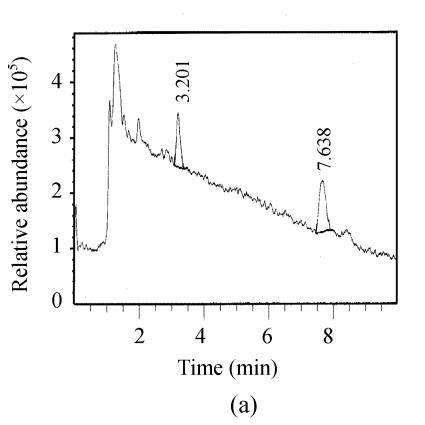
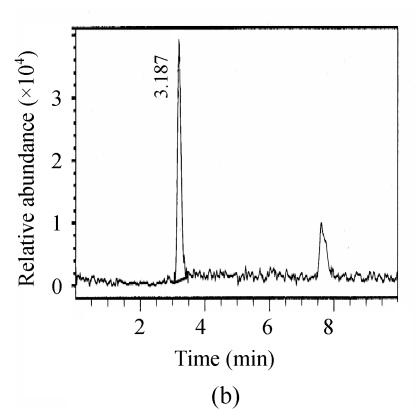
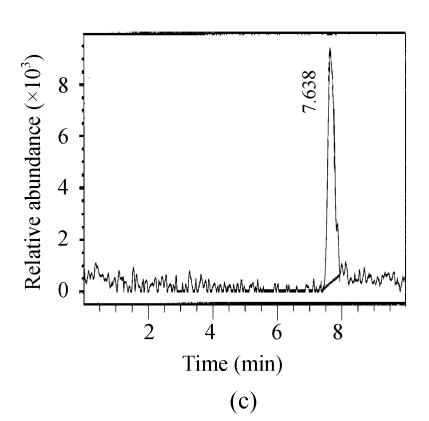
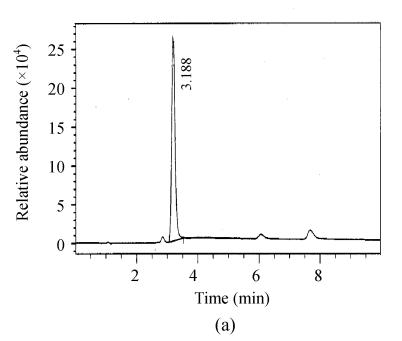
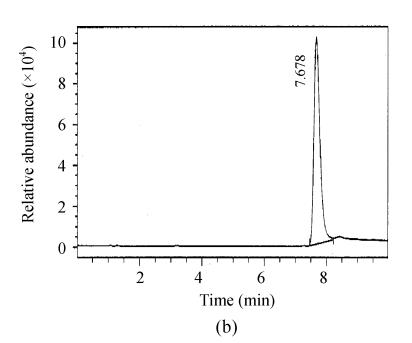
The chromatograms of the intestine of H. molitrix were measured in full scan mode (Fig.2) and SIM mode (Fig.3). We found that the SIM mode was over 8 times that of the full scan extraction ion mode for both two microcystins in HPLC-ESI-MS determination.
Fig. 2.
Full scan mass spectra of microcystin-RR and microcystin-LR in a fish intestine sample. (a) Total ion mass spectra of samples; (b) Extraction ion mass spectra of microcystin-RR; (c) Extraction ion mass spectra of microcystin-LR
Fig. 3.
SIM mass spectra of (a) microcystin-RR and (b) microcystin-LR in a fish intestine sample
Detected microcystins in different organs
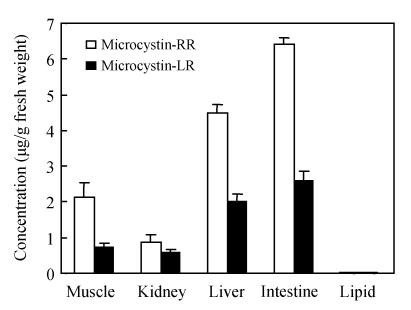
Fig.4 presents the distribution of microcystins in the organs of the fish H. molitrix. The highest level of microcystin-LR was found in the intestines, followed by the livers and kidneys. Small amounts of microcystin-LR were detected in the muscles. But microcystin-LR and microcystin-RR was not detected in the lipid. Microcystin-RR apparently showed the same distribution pattern as that of microcystin-LR. Furthermore, we found the concentration of microcystin-RR was about 2 times that of microcystin-LR.
Fig. 4.
Microcystin-LR and microcystin-RR in different tissues of Hypophthalmichthys molitrix samples collected from Tiesha River (n=3)
DISCUSSION
Bioaccumulation of microcystins in fish has been well documented (Malbrouck et al., 2003; Mohamed et al., 2003). Microcystins were often detected in livers, muscles and other tissues. Phytoplanktivorous, carnivorous, omnivorous and herbivorous fish have different microcystins patterns. In a sub-chronic toxicity experiment, Xie et al.(2004) found that microcystin-RR was detectable in the liver, muscle and blood samples of the phytoplanktivorous silver carp while no microcystin-LR was detectable in the muscle and blood samples in spite of the abundant presence of microcystin-LR in the intestines. Xie et al.(2005) found that no microcystins were detected in the intestines of the herbivorous Parabramis pekinensis, the carnivorous Culter illshaeformis and the Pseudobagrus fulvidraco, but in the phytoplanktivorous H. molitrix the highest amount of microcystins was detected in the intestines. In this study, the application of our proposed method showed the transfer of microcystins to different fish tissues (Fig.4). We found the maximal total microcystins (microcystin-LR and microcystin-RR) were present in the intestines, followed by the livers and the muscles, but microcystins detected in kidneys were minimal.
Dawson (1998) found microcystins are always bioaccumulated by the fish liver according to the bile transfer pathway. But in this study, the highest levels of microcystins were in intestines. We found a large number of Microcystis aeruginosa in the dissected fish samples intestines, within microcystins concentration was relatively high. Microcystins were included in the final analysis. Specziár et al.(1997) reported that cyanobacteria Microcystis aeruginosa was more abundant in phytoplanktivorous than omnivorous fish, since omnivorous fish normally consume zooplankton and detritus. So our finding also confirmed that H. molitrix could graze high numbers of toxic Microcystis cells.
de Maagd et al.(1999) found that log(n-octanol: water distribution ratio) [log(DOW)] of microcystin-LR was low, ranging from −1.76 to 2.18. These results suggested that microcystins could scarcely be accumulated through the epithelia of aquatic organisms. During this field study, there were no microcystins detected in the lipid tissues which further confirmed that the very low hydrophobicity and polar functions of microcystin-LR in neutral condition may inhibit their distribution into lipid tissues (Rivasseau et al., 1998). We also found microcystins in the internal tissues (liver, kidney, muscle and intestine) of H. molitrix, so ingestion can be proposed as the most probable route of toxin uptake in this wild fish.
For human health risk, two oral routes can lead to microcystins exposure: direct ingestion of water containing microcystins and the consumption of aquatic animals which have ingested cyanobacteria and accumulated microcystins (de Magalhães et al., 2001). Thus, transfer of microcystins to humans through consumption of contaminated fish is a potential threat to human health. The WHO has determined a tolerable daily intake (TDI) value of 0.04 μg/kg body weight per day for microcystin-LR (Chorus and Bartram, 1999). TDI is defined as the acceptable amount of a potentially toxic substance that can be consumed daily during a life period.
According to Gupta et al.(2003), the LD 50 in mice for microcystin-RR is about 5 times that for microcystin-LR, so coefficient of 0.2 was used to convert microcystin-RR into microcystin-LR equivalent. Based on our results, for a 60 kg adult, who ingests on the average 100 g of fish a day, the concentration of microcystin-LR eq in fish samples reaches 1.8 μg/g fresh weight, representing an estimated daily intake of 0.03 μg/kg body weight. This is very close to the TDI value suggested by WHO. Microcystins are chemically heat stable (Harada et al., 1996), and their structures are not broken down by cooking. So consuming toxic fishes in the Tiesha River is clearly a health risk to the local public. Therefore, the long-term impact of mirocystins to the local public health needs to be further investigated in the future.
Acknowledgments
The authors would like to thank Ph.D candidate Xiaodan Wu for having supported part of this work.
Footnotes
Project (No. Y505257) supported by the Natural Science Foundation of Zhejiang Province, China
References
- 1.Barco M, Rivera J, Caixach J. Analysis of cyanobacterial hepatotoxins in water samples by microbore reversed-phase liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 2002;959(1-2):103–111. doi: 10.1016/S0021-9673(02)00405-3. [DOI] [PubMed] [Google Scholar]
- 2.Chorus I, Bartram J. Toxic Cyanobacteria in Water. A Guide to Public Health Consequences, Monitoring and Management. London: E and FN Spon; 1999. pp. 416–417. [Google Scholar]
- 3.Dawson RM. The toxicology of microcystins. Toxicon. 1998;36(7):953–962. doi: 10.1016/S0041-0101(97)00102-5. [DOI] [PubMed] [Google Scholar]
- 4.de Maagd PGJ, Hendriks AJ, Seinen W, Sijm DTHM. pH-dependent hydrophobicity of the cyanobacteria toxin microcystin-LR. Water Res. 1999;33(3):677–680. doi: 10.1016/S0043-1354(98)00258-9. [DOI] [Google Scholar]
- 5.de Magalhães VF, Soares RM, Azevedo SMFO. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon. 2001;39(7):1077–1085. doi: 10.1016/S0041-0101(00)00251-8. [DOI] [PubMed] [Google Scholar]
- 6.Falconer LR, Burch MD, Steffensen DA, Choice M, Coverdale OR. Toxicity of the blue-green alga (cyanobacterium) Microcystis aeruginosa in drinking water to growing pigs, as an animal model for human injury and risk assessment. Environ Toxicol Water Qual. 1994;9(2):131–139. doi: 10.1002/tox.2530090209. [DOI] [Google Scholar]
- 7.Gupta N, Pant SC, Vijayaraghavan R, Lakshmana RPV. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystins variants (LR, RR, YR) in mice. Toxicology. 2003;188(2-3):285–296. doi: 10.1016/S0300-483X(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 8.Harada KI, Tsuji K, Watanabe MF, Kondo F. Stability of microcystins from cyanobacteria. III. Effect of pH and temperature. Phycologia. 1996;35(6):83–88. [Google Scholar]
- 9.Huynh-Delerme C, Edery M, Huet H, Puiseux-Dao S, Bernard C, Fontaine JJ, Crespeau F, de Luze A. Microcystin-LR and embryo-larval development of medaka fish, Oryzias latipes. I. Effects on the digestive tract and associated systes. Toxicon. 2005;46(1):16–23. doi: 10.1016/j.toxicon.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Ibelings BW, Bruning K, de Jonge J, Wolfstein K, Dionisio PLM, Postma J, Burger T. Distribution of microcystins in a lake foodweb: no evidence for biomagnification. Microbial Ecol. 2005;49(4):487–500. doi: 10.1007/s00248-004-0014-x. [DOI] [PubMed] [Google Scholar]
- 11.Malbrouck C, Trausch G, Devos P, Kestemont P. Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. Comp. Biochem Physiol Part C. 2003;135(1):39–48. doi: 10.1016/S1532-0456(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed ZA, Carmichael WW, Hussein AA. Estimation of microcystins in the freshwater fish Oreochromisniloticus in an Egyptian fish farm containing a Microcystis bloom. Environ Toxicol. 2003;18(2):137–141. doi: 10.1002/tox.10111. [DOI] [PubMed] [Google Scholar]
- 13.Rivasseau C, Martine S, Hennion MC. Determination of some physicochemical parameters of microcystins (cyanobacterial toxins) and trace level analysis in environmental samples using liquid chromatography. J Chromatogr A. 1998;799(1-2):155–169. doi: 10.1016/S0021-9673(97)01095-9. [DOI] [PubMed] [Google Scholar]
- 14.Specziár A, Tölg L, Biró P. Feeding strategy and growth of cyprinids in the littoral zone of Lake Balaton. J Fish Biol. 1997;51(6):1109–1124. doi: 10.1006/jfbi.1997.0514. [DOI] [PubMed] [Google Scholar]
- 15.Tencalla F, Dietrich D, Schlatter C. Toxicity of Microcystis aeruginosa peptide toxins to yearling rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 1994;30(3):215–224. doi: 10.1016/0166-445X(94)90059-0. [DOI] [Google Scholar]
- 16.Wang XF, Guo ZL, Wang Y. Detection of microcystin class compounds in water by colorimetric protein phosphatases inhibition assay. Acta Hydrobiologica Sinica. 2003;27(4):431–433. (in Chinese) [Google Scholar]
- 17.Xie LQ, Xie P, Ozawa K, Honma T, Yokoyama A, Park HD. Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut. 2004;127(3):431–439. doi: 10.1016/j.envpol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Xie LQ, Xie P, Guo LG, Li L, Miyabara Y, Park HD. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic lake Chaohu, China. Environ Toxicol. 2005;20(3):293–300. doi: 10.1002/tox.20120. [DOI] [PubMed] [Google Scholar]