Abstract
Response surface methodology (RSM) was used to optimize the fermentation medium for enhancing pyruvic acid production by Torulopsis glabrata TP19. In the first step of optimization, with Plackett-Burman design, ammonium sulfate, glucose and nicotinic acid were found to be the important factors affecting pyruvic acid production significantly. In the second step, a 23 full factorial central composite design and RSM were applied to determine the optimal concentration of each significant variable. A second-order polynomial was determined by the multiple regression analysis of the experimental data. The optimum values for the critical components were obtained as follows: ammonium sulfate 0.7498 (10.75 g/L), glucose 0.9383 (109.38 g/L) and nicotinic acid 0.3633 (7.86 mg/L) with a predicted value of maximum pyruvic acid production of 42.2 g/L. Under the optimal conditions, the practical pyruvic acid production was 42.4 g/L. The determination coefficient (R2) was 0.9483, which ensures adequate credibility of the model. By scaling up fermentation from flask to jar fermentor, we obtained promising results.
Keywords: Response surface methodology, Torulopsis glabrata, Pyruvic acid, Fermentation, Medium optimization
INTRODUCTION
Pyruvic acid, also known as 2-oxopropanoic acid, α-ketopropionic acid or acetylformic acid, is the most important α-oxocarboxylic acid. It plays a central role in energy metabolism in living organisms. Industrially, it is used mainly as a starting material in the biosynthesis of pharmaceuticals, such as L-tryptophan, L-tyrosine, L-dihydroxyphenylalanine (Uchio et al., 1976), L-phenylacetylcarbinol (Rosche et al., 2002), and N-acetyl-D-neuraminic acid (Mahmoudian et al., 1997). It is also employed in the production of crop protection agents, antioxidant, polymers, cosmetics and food additives. Calcium pyruvate also has a strong effect in reducing fat because it can accelerate the metabolism of fatty acids in the human body (Roufs, 1996), and presently it serves as a fat burner in the food industry. As it is widely used in drug, agrochemical, chemical and food industries, the commercial demand for pyruvic acid has been expanding (Yonehara and Miyata, 1994).
Basically, there are four different known approaches to produce pyruvic acid: (1) chemical synthesis and oxidation (Tsujino et al., 1992; Ai and Ohdan, 1995), (2) enzymes (Burdick and Schaeffer, 1987; Eisenberg et al., 1997), (3) resting cells (Izumi et al., 1982; Schinschel and Simon, 1993; Ogawa et al., 2001), and (4) fermentation processes (Yokota et al., 1994; Li et al., 2001a; 2001b; Causey et al., 2004). In comparison with these approaches, the fermentation processes is regarded as one of the most promising routes for the production of pyruvic acid.
Recent research efforts have focused on further process development (optimization) and scale-up of pyruvic acid production. As we all known, medium components play a very important role in enhancing the pyruvic acid accumulation, therefore, medium optimization study is very important. In general, medium optimization by the traditional ‘one-factor-at-a-time’ technique was used (Gokhade et al., 1991). This method is not only laborious and time consuming but also often leads to an incomplete understanding of the system behaviour, resulting in confusion and a lack of predictive ability.
Response surface methodology (RSM) is a powerful and efficient mathematical approach widely applied in the optimization of fermentation process, e.g. media components on enzyme production (Adinarayana and Ellaiah, 2002; Park et al., 2002; Puri et al., 2002), production of other metabolites (Zhang et al., 1996; Sunitha et al., 1998; Sadhukhan et al., 1999; Hujanen et al., 2001), spore production (Yu et al., 1997) and biomass production optimization (Lhomme and Roux, 1991). It can give information about the interaction between variables, provide information necessary for design and process optimization, and give multiple responses at the same time.
In this study, optimization of fermentation media of Torulopsis glabrata TP19 was investigated using RSM to increase the pyruvic acid production. In the first optimization step, a Plackett-Burman (PB) design was used to determine the likely effects of medium components on pyruvic acid production. In the second step, the factors that had significant effects were optimized using a central composite design (CCD) and response surface analysis. Based on optimal medium, scale-up was carried out in a 5-L jar fermentor.
MATERIALS AND METHODS
Microorganism
Torulopsis glabrata TG-06 wild type was obtained from Tianjin Key Lab of Industrial Microbiology, China. From this a mutation Torulopsis glabrata TP19 (NA−+Bio−+TPP−+Pdx−), which was used as a pyruvic acid producer in this research, was developed in the authors’ laboratory. It was maintained on agar slants containing (per liter): 30 g glucose, 10 g peptone, 20 g agar, 1 g KH2PO4 and 0.5 g MgSO4·7H2O. The organism was subcultured over the interval of 2 months and stored at 4 °C.
Medium
The seed medium consisted of (per liter): 30 g glucose, 5 ml corn steep liquor, 10 g peptone, 1 g KH2PO4 and 0.5 g MgSO4·7H2O. The components in the fermentation medium were added according to the design of each experiment. Trace element solution containing (per liter of 2 mol/L HCl): 2 g CaCl2·2H2O, 2 g FeSO4·7H2O, 5 g ZnCl2, 0.2 g MnCl2·4H2O and 0.5 g CuSO4·5H2O. The initial pH of all media was adjusted to 5.0. In the fermentor, the pH was automatically controlled at 5.0 with 8 mol/L NaOH solution. All vitamins were sterilized by microfiltration; and CaCO3 was sterilized by dry-heat sterilization at 160 °C for 30 min before being added to the medium.
Cultivation
A loopful of cells from the slant were transferred into a 250-ml conical flask containing 30-ml seed medium and incubated for 20 h on a rotary shaker operating at 200 r/min at 30 °C. It was then inoculated either into a 500-ml flask containing 50 ml fermentation medium or into a 5-L jar fermentor (Biostat M.B. Braun Co., Germany) containing 3 L fermentation medium. The inoculum size was 10% (v/v). In shake flask culture, it was incubated on a rotary shaker operating at 200 r/min at 30 °C. In fermentor culture, it was incubated at 30 °C, the agitation speed was controlled at 400 r/min. The air flow rate was 1.2 L/min. Foam formation was prevented by adding sterile polypropylene glycol solution (10% aqueous PPG 2000).
Sample preparation
After 60 h, fermentation broth was collected. The cells were removed by centrifugation at 12000 r/min for 10 min, the supernatant was collected and further diluted by a factor of 100 with Milli-Q water. This diluent was then filtered through a 0.45 μm millipore filter prior to injection into the chromatographic column.
HPLC
Pyruvic acid concentration was measured by HPLC (agilent 1100 series) using a Bio-Rad HPX-87H Aminex column 7.8 mm×300 mm and a UV detector at 210 nm with 0.005 mol/L H2SO4 as the eluent at a flow rate of 0.6 ml/min at 55 °C.
Experimental designs and data analysis
1. Plackett-Burman (PB) design
PB design, a very useful tool, was used to screen ‘n’ variables in just ‘n+1’ number of experiments (Plackett and Burman, 1946; Rama et al., 1999; Ghanem et al., 2000). There will be a tremendous decrease in total experiments. In this part, the PB design was used to evaluate the relative importance of various nutrients for pyruvic acid production in batch fermentation. This design does not consider the interaction effects among the variables and is used to screen the important variables affecting the pyruvic acid production. The experimental design for screening of medium components is shown in Tables 1 and 2. Each variable was set at two levels, that is, high level and low level. The high level of each variable was set far enough from the low level to identify which ingredients of the media have significant influence on the pyruvic acid production. Trace element solution was constantly maintained at 5 ml/L. The design matrix (Table 2) was developed using an SAS package, version 8.01.
Table 1.
Medium components for screening using a PB design
| Variables | Levels |
||
| Low (−1) | High (+1) | ||
| A | Glucose (g/L) | 80 | 100 |
| B | Ammonium sulfate (g/L) | 8 | 10 |
| C | KH2PO4 (g/L) | 1.0 | 1.5 |
| D | MgSO4·7H2O (g/L) | 0.5 | 1.5 |
| E | Nicotinic acid (mg/L) | 6.0 | 7.5 |
| F | Thiamine·HCl (µg/L) | 20 | 25 |
| G | Biotin (µg/L) | 10 | 15 |
| H | Pyridoxine·HCl (mg/L) | 0.4 | 0.6 |
Table 2.
The experimental design using the PB method for screening of medium components
| Run | Variables |
Pyruvic acid yield (g/L) | |||||||
| A | B | C | D | E | F | G | H | ||
| 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 32.5 |
| 2 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 36.3 |
| 3 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 36.9 |
| 4 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | 35.0 |
| 5 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 38.5 |
| 6 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | 40.2 |
| 7 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 36.8 |
| 8 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | 33.4 |
| 9 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 32.6 |
| 10 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 35.3 |
| 11 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 34.1 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 31.8 |
2. Central composite design (CCD)
CCD is one of the response surface methodologies (Chakravarti and Sahai, 2002). After the identification of the components affecting the pyruvic acid yield significantly, a CCD was adopted to optimize the major variables (ammonium sulfate, glucose and nicotinic acid), which were selected through PB design. Coded levels for independent variables are presented in Table 3.
Table 3.
Levels of the variables tested in CCD
| Variables | Coded levels |
||||
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Ammonium sulfate, X1 | 8.3 | 9.0 | 10.0 | 11.0 | 11.7 |
| Glucose, X2 | 83.2 | 90.0 | 100.0 | 110.0 | 116.8 |
| Nicotinic acid, X3 | 5.8 | 6.5 | 7.5 | 8.5 | 9.2 |
X 1=(x 1−10)/1; X 2=(x 2−100)/10; X 3=(x 3−7.5)/1
For statistical calculations the variables Xi were coded as xi according to Eq.(1)
| Xi=(xi−x0)/Δxi, i=1, 2, 3, …, K, | (1) |
where Xi is (dimensionless) coded value of the real variable xi, x 0 is the real value of Xi at the center point (zero) level, and the Δxi is the step change value.
A 23-factorial CCD, with six axial points (a=1.682) and six replications at the centre points (n 0=6) leading to a total number of 20 experiments was employed (Table 4) for the optimization of the three chosen medium variables. Pyruvic acid yield (Y, g/L) was used as the dependent output variable.
Table 4.
Experimental design matrix and results of CCD
| Run | Factor |
Pyruvic acid yield, Y (g/L) | ||
| X1 | X2 | X3 | ||
| 1 | −1 | −1 | −1 | 37.9 |
| 2 | −1 | −1 | 1 | 38.2 |
| 3 | −1 | 1 | −1 | 39.8 |
| 4 | −1 | 1 | 1 | 40.5 |
| 5 | 1 | −1 | −1 | 39.3 |
| 6 | 1 | −1 | 1 | 40.0 |
| 7 | 1 | 1 | −1 | 40.2 |
| 8 | 1 | 1 | 1 | 41.7 |
| 9 | −1.682 | 0 | 0 | 39.8 |
| 10 | 1.682 | 0 | 0 | 41.8 |
| 11 | 0 | −1.682 | 0 | 38.2 |
| 12 | 0 | 1.682 | 0 | 42.4 |
| 13 | 0 | 0 | −1.682 | 39.5 |
| 14 | 0 | 0 | 1.682 | 39.8 |
| 15 | 0 | 0 | 0 | 41.3 |
| 16 | 0 | 0 | 0 | 41.2 |
| 17 | 0 | 0 | 0 | 41.5 |
| 18 | 0 | 0 | 0 | 41.6 |
| 19 | 0 | 0 | 0 | 41.4 |
| 20 | 0 | 0 | 0 | 41.4 |
Second degree polynomials, Eq.(2), which includes all interaction terms, were used to calculate the predicted response.
 |
(2) |
where Y represents response variable, β 0 is the interception coefficient, βi, coefficient of the linear effect, βii, the coefficient of quadratic effect and βij, the coefficient of interaction effect.
The experiments were performed in duplicate with the mean values taken for analysis. An SAS package, version 8.01, was used for multiple regression analysis of the experimental data obtained. F-test was employed to evaluate the statistical significance of the quadratic polynomial. The multiple coefficients of correlation R and the determination coefficient of correlation R 2 were calculated to evaluate the performance of the regression equation. The optimum levels of the selected variables were obtained by solving the regression equation using a multi-stage Monte-Carlo optimization (Conley, 1984) program and also by analysing the response-surface plots (Khuri and Cornell, 1987).
RESULTS AND DISCUSSION
Plackett-Burman (PB) design
The first optimization step was using a 12-run PB design to identify the significant factors for pyruvic acid production by Torulopsis glabrata TP19. According to the resulting effects of these eight variables on pyruvic acid concentration and the associated significant levels presented in Table 5, it can be seen that with the increase in the concentration of ammonium sulfate, glucose, nicotinic acid, KH2PO4, thiamine·HCl and MgSO4·7H2O, all have positive effects on pyruvic acid production. With increase in the pyridoxine∙HCl levels, biotin has negative effects on pyruvic acid production. With the help of relative ranking, ammonium sulfate, glucose and nicotinic acid within the tested limits were selected for further optimization, which had the most significant effects on pyruvic acid production.
Table 5.
Coefficients, t values and significance levels calculated from the pyruvic acid yield obtained in the screening experiments
| Code | Coefficient | t value | P>|T| | Ranking |
| A | 1.0167 | 4.1157 | 0.0260 | 2* |
| B | 1.8500 | 7.4893 | 0.0049 | 1** |
| C | 0.5167 | 2.0916 | 0.1276 | 4 |
| D | 0.1500 | 0.6072 | 0.5866 | 8 |
| E | 0.8667 | 3.5085 | 0.0392 | 3* |
| F | 0.3833 | 1.5518 | 0.2185 | 6 |
| G | −0.1833 | −0.7422 | 0.5118 | 7 |
| H | −0.4333 | −1.7543 | 0.1777 | 5 |
Statistically significant at 95% of probability level
Statistically significant at 99% of probability level
The positive effects of ammonium sulfate and glucose were, probably, caused by the requirement of a large quantity of substrate to synthesize cells. It is possible that the high concentration of nicotinic acid could contribute to the improvement of the glucose consumption rate. This may be due to the fact that nicotinic acid is a component of NAD, a cofactor of glycolysis (Miyata and Yonehara, 1996), resulting in both good cell growth and satisfactory pyruvic acid production.
The PB design was proved to be a powerful tool to rapidly determine the effects of medium constituents on pyruvic acid production of Torulopsis glabrata TP19. However the optimal concentrations of medium components that significantly affect pyruvic acid production could not be obtained. Further work need to be done to find out this information.
Central composite design (CCD)
This is a very useful tool to determine the optimal level of medium constituents and their interaction. Based on the PB design, where ammonium sulfate, glucose and nicotinic acid were selected for their significant effects on the pyruvic acid production, a CCD was used for further optimization. Table 3 gave the variation levels at which these components were supplemented to pyruvic acid production. Other nutrients concentrations were set at their centre point tested in the PB design. Table 4 gives the design and results of experiments carried out by the CCD design. The results obtained were submitted to analysis of variance on SAS package, with the regression model given as
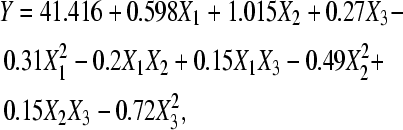 |
(3) |
where Y is the response value, that is, the pyruvic acid production, and X 1, X 2 and X 3 are the coded levels of ammonium sulfate, glucose and nicotinic acid, respectively.
The analysis of variance of the quadratic regression model demonstrated that Eq.(3) was a highly significant model, as was evident from the Fisher’s F-test with a very low probability value [(P model>F)=0.0001]. The model’s goodness of fit was checked by determination coefficient (R 2). In this case, the value of the determination coefficient (R 2=0.9483) indicated that only 5.17% of the total variations were not explained by the model. The value of the adjusted determination coefficient [Adj(R 2)=0.9019] was also very high in supporting the high significance of the model. Among the model terms, X 1, X 2, X 2 2 and X 3 2 were significant with a probability of 99%; X 3 and X 1 2 were significant with a probability of 95% (Table 6). The interaction between X 1, X 2 and X 3, however, had no significant influence on pyruvic acid production.
Table 6.
Analysis of variance for the fitted quadratic polynomial model
| Term | Effect | SS | DF | F ratio | P>F |
| X1 | 0.598 | 4.8799 | 1 | 28.6470 | 0.0003** |
| X2 | 1.015 | 14.0734 | 1 | 82.6170 | 0.0001** |
| X3 | 0.270 | 1.0049 | 1 | 5.8992 | 0.0355* |
| X12 | −0.310 | 1.4161 | 1 | 8.3134 | 0.0163* |
| X1X2 | 0.200 | 0.3200 | 1 | 1.8786 | 0.2005 |
| X1X3 | 0.150 | 0.1800 | 1 | 1.0567 | 0.3282 |
| X22 | −0.490 | 3.4637 | 1 | 20.3340 | 0.0011** |
| X2X3 | 0.150 | 0.1800 | 1 | 1.0567 | 0.3282 |
| X32 | −0.720 | 7.4721 | 1 | 43.8650 | 0.0001** |
| Model | − | 31.2741 | 9 | 20.3990 | 0.0001 |
| Error | − | 1.7034 | 10 | − | − |
| Total | − | 32.9775 | 19 | − | − |
Statistically significant at 95% of probability level
Statistically significant at 99% of probability level
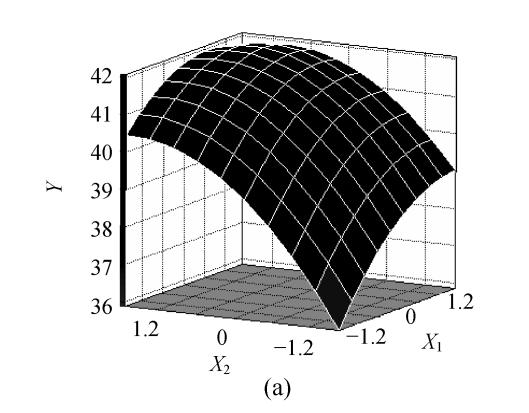
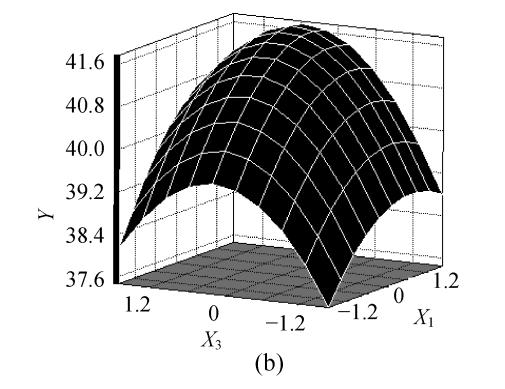
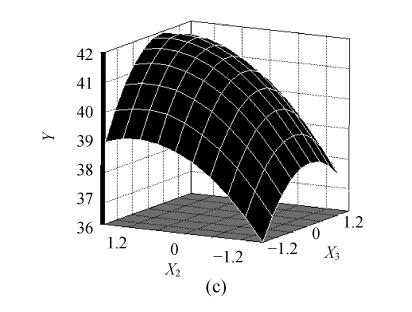
The fitted response for the above regression model was plotted in Fig.1. 3D graphs were generated for the pair-wise combination of the three factors while keeping the other one at its optimum levels for pyruvic acid production. Graphs are given here to highlight the roles played by various factors and also to emphasize the roles played by the physical constraints vis-a-vis the biosynthetic aspects in the final yield of the pyruvic acid.
Fig. 1.
Effects of (a) ammonium sulfate (X 1) and glucose (X 2), (b) ammonium sulfate (X 1) and nicotinic acid (X 3), (c) nicotinic acid (X 3) and glucose (X 2) and their interactive effect on the pyruvic acid production (Y) with other nutrient set at centre level
The predicted optimum levels of the tested variables, namely, ammonium sulfate, glucose and nicotinic acid were obtained by applying regression analysis on Eq.(3) using SAS package software, version 8.01. The optimal levels were as follows: X 1=0.7498 (10.75 g/L), X 2=0.9383 (109.38 g/L), X 3=0.3633 (7.86 mg/L) with the corresponding Y=42.2 g/L. Verification of the predicted values was conducted by using optimal medium in inoculation experiments. The practical corresponding response was 42.4 g/L. This result corroborated the validity and the effectiveness of this model.
In an attempt to approximate industrial conditions for pyruvic acid production, scale-up was carried out in a jar fermentor by using optimal medium. In this way, we achieved a volumetric productivity of 0.911 g pyruvate/(L·h), a pyruvate/glucose yield of 0.53 g/g, and pyruvic acid production of 54.6 g/L. These results are encouraging for optimization under pilot scale or industrial scale conditions.
CONCLUSION
RSM was performed to optimize the medium components for pyruvic acid production of Torulopsis glabrata TP19. A highly significant quadratic polynomial obtained by the CCD was very useful for determining the optimal concentrations of constituents that have significant effects on pyruvic acid production.
The optimal supplementary components (per liter) consisted of 109.38 g glucose, 10.75 g ammonium sulfate, 1.25 g KH2PO4, 1 g MgSO4·7H2O, 7.86 mg nicotinic acid, 22.5 μg thiamine·HCl, 12.5 μg biotin, 0.5 mg pyridoxine·HCl and 5 ml trace element solution. Under the optimal condition, 42.2 g/L pyruvic acid could be produced in theory and 42.4 g/L pyruvic acid in practical experiment. Scale-up in jar fermentor produced adequate results, although accurate moisture and temperature control, optimized O2 mass transfer, as well as fed batch studies, promise further process improvement.
References
- 1.Adinarayana K, Ellaiah P. Response surface optimization of the critical medium components for this production of alkaline protease by a newly isolated Bacillus sp. J Pharm Pharmaceut Sci. 2002;5(3):272–278. [PubMed] [Google Scholar]
- 2.Ai M, Ohdan K. Formation of pyruvic acid by oxidative dehydrogenation of lactic acid. Chem Lett. 1995;24(5):405. doi: 10.1246/cl.1995.405. [DOI] [Google Scholar]
- 3.Burdick BA, Schaeffer JR. Co-immobilized coupled enzyme systems on nylon mesh capable of gluconic and pyruvic acid production. Biotechnol Lett. 1987;9(4):253–258. doi: 10.1007/BF01027159. [DOI] [Google Scholar]
- 4.Causey TB, Shanmugam KT, Yomano LP, Inram LO. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci USA. 2004;101(8):2235–2240. doi: 10.1073/pnas.0308171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarti R, Sahai V. Optimization of compactin production in chemically defined production medium by Penicillium citrinum using statistical methods. Process Biochem. 2002;38(4):481–486. doi: 10.1016/S0032-9592(02)00138-3. [DOI] [Google Scholar]
- 6.Conley WC. Computer Optimization Techniques. Princeton, NJ: Petrocelli Books; 1984. pp. 147–163. [Google Scholar]
- 7.Eisenberg A, Seip JE, Gavagan JE, Payne MS, Anton DL, DiCosimo R. Pyruvic acid production using methylotrophic yeast transformants as catalyst. J Mol Catal B: Enzymatic. 1997;2(4-5):223–232. doi: 10.1016/S1381-1177(96)00021-5. [DOI] [Google Scholar]
- 8.Ghanem NB, Yusef HH, Mahrouse HK. Production of Aspergillus terreus xylanase in solid-state cultures: application of the Plackett-Burman experimental design to evaluate nutritional requirements. Bioresour Technol. 2000;73(2):113–121. doi: 10.1016/S0960-8524(99)00155-8. [DOI] [Google Scholar]
- 9.Gokhade DV, Patil SG, Bastawde KB. Optimization of cellulase production by Aspergillus niger NCIM 1207. Appl Biochem Biotechnol. 1991;30(2):99–109. doi: 10.1007/BF02922026. [DOI] [PubMed] [Google Scholar]
- 10.Hujanen M, Linko S, Linko YY, Leisola M. Optimization of media and cultivation conditions for L. (+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl Microbiol Biotechnol. 2001;56(1-2):126–130. doi: 10.1007/s002530000501. [DOI] [PubMed] [Google Scholar]
- 11.Izumi Y, Matsumura Y, Tani Y, Yamada H. Pyruvic acid production from 1,2-propanediol by thiamin-requiring Acinetobacter sp. 80-M. Agric Biol Chem. 1982;46(3):2673–2679. [Google Scholar]
- 12.Khuri AI, Cornell JA. Response Surfaces: Design and Analyses. New York: Dekker; 1987. [Google Scholar]
- 13.Lhomme B, Roux JC. Utilization of experimental designs for optimization of Rhizopus arrhizus culture. Bioresour Technol. 1991;35(3):301–312. doi: 10.1016/0960-8524(91)90129-8. [DOI] [Google Scholar]
- 14.Li Y, Chen J, Lun SY. Efficient pyruvate production by a multi-vitamin auxotroph of Torulopsis glabrata: key role and optimization of vitamin levels. Appl Microbiol Biotechnol. 2001;55(6):680–685. doi: 10.1007/s002530100598. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen J, Lun SY. Biotechnological production of pyruvic acid. Appl Microbiol Biotechnol. 2001;57(4):451–459. doi: 10.1007/s002530100804. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoudian M, Noble D, Drake CS, Middleton RF, Montgomery DS, Picrcey JE, Ramlakhan D, Todd M, Dawson M. An efficient process for production of N-acetylneuraminic acid using N-acetylneuraminic acid aldolase. Enzyme Microb Technol. 1997;20(5):393–400. doi: 10.1016/S0141-0229(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 17.Miyata R, Yonehara T. Improvement of fermentative production of pyruvate from glucose by Torulopsis glabrata IFO 0005. J Ferment Bioeng. 1996;82(5):475–479. doi: 10.1016/S0922-338X(97)86986-3. [DOI] [Google Scholar]
- 18.Ogawa J, Soong CL, Masashi I, Shimizu S. Enzymatic production of pyruvate from fumarate—an application of microbial cyclic-imide-transforming pathway. J Mol Catal B: Enzymatic. 2001;11(4-6):355–359. doi: 10.1016/S1381-1177(00)00024-2. [DOI] [Google Scholar]
- 19.Park YS, Kang SW, Lee JS, Hong SI, Kim SW. Xylanase production in solid state fermertation by Aspergillus niger mutant using statistical experimental designs. Appl Microbiol Biotechnol. 2002;58(6):761–766. doi: 10.1007/s00253-002-0965-0. [DOI] [PubMed] [Google Scholar]
- 20.Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33(4):305–325. doi: 10.2307/2332195. [DOI] [Google Scholar]
- 21.Puri S, Beg QK, Gupta R. Optimization of alkaline protease from Bacillus sp. by response surface methology. Curr Microbiol. 2002;44(4):286–290. doi: 10.1007/s00284-001-0006-8. [DOI] [PubMed] [Google Scholar]
- 22.Rama Mohan Reddy P, Reddy G, Seenayya G. Production of thermostble β-amylase and pullulanase by Clostridium thermosulfurogenes SV2 in solid-state fermentation: screening of nutrients using Plackett-Burman design. Bioprocess Eng. 1999;21(2):175–179. doi: 10.1007/s004490050659. [DOI] [Google Scholar]
- 23.Rosche B, Leksawasdi N, Sandford V, Breuer M, Hauer B, Rogers P. Enzymatic (R)-phenylacetylcarbinol production in benzaldehyde emulsions. Appl Microbiol Biotechnol. 2002;60(1-2):94–100. doi: 10.1007/s00253-002-1084-7. [DOI] [PubMed] [Google Scholar]
- 24.Roufs JB. Pyruvate: does it amp endurance and burn more fat? Muscle Fitness. 1996;57(2):195–197. [Google Scholar]
- 25.Sadhukhan AK, Ramana Murthy MV, Ajaya Kumar R, Mohan EVS, Vandana G, Bhar C, Venkateswara Rao K. Optimization of mycophenolic acid production in solid-state fermentation using response surface methodology. J Ind Microbiol Biotechnol. 1999;22(1):33–38. doi: 10.1038/sj.jim.2900597. [DOI] [Google Scholar]
- 26.Schinschel C, Simon H. Preparation of pyruvate from (R)-lactate with Proteus species. J Biotechnol. 1993;31(2):191–203. doi: 10.1016/0168-1656(93)90160-O. [DOI] [Google Scholar]
- 27.Sunitha I, Subba Rao MV, Ayyanna C. Optimization of medium constituents and fermentation conditions for the production of L-glutamic acid by the co-immobilized whole cells of Micrococcus glutamicus and Pseudomonas reptilivora . Bioprocess Eng. 1998;18(5):353–359. doi: 10.1007/PL00008995. [DOI] [Google Scholar]
- 28.Tsujino T, Ohigashi S, Sugiyama S, Kawashiro K, Hayashi H. Oxidation of propylene glycol and lactic acid to pyruvic acid in aqueous phase catalyzed by lead modified alladium-on-carbon and related systems. J Mol Catal. 1992;71(1):25–35. doi: 10.1016/0304-5102(92)80005-2. [DOI] [Google Scholar]
- 29.Uchio R, Kikuchi K, Hirose Y. Process for Producing Pyruvic Acid by Fermentation. No. 3 993 543. US Patent. 1976
- 30.Yokota A, Schimizu H, Terasawa Y, Takaoka N. Pyruvic acid production by a lipoic acid auxotroph of Escherichia coli W1485. Appl Microbiol Biotechnol. 1994;41(6):638–643. doi: 10.1007/BF00167278. [DOI] [Google Scholar]
- 31.Yonehara T, Miyata R. Fermentative production of pyruvate from glucose by Torulopsis glabrata . J Ferment Bioeng. 1994;78(2):155–159. doi: 10.1016/0922-338X(94)90255-0. [DOI] [Google Scholar]
- 32.Yu X, Hallet SG, Sheppard J, Watson AK. Application of the Plackett-Burman experimental design to evaluate nutritional requirements for the production of Colletotrichum coccodes spores. Appl Microbiol Biotechnol. 1997;47(3):301–305. doi: 10.1007/s002530050930. [DOI] [Google Scholar]
- 33.Zhang J, Marcin C, Shifflet MA, Salmon P, Brix T, Greasham R, Buokland B, Chartrain M. Development of a defined medium fermentation process for physotigmine production by Streptomyces griseofuscus . Appl Microbiol Biotechnol. 1996;44(5):568–575. doi: 10.1007/s002530050601. [DOI] [PubMed] [Google Scholar]