Abstract
Upon perception of a noxious stimulus, an organism executes defense mechanisms, such as escape responses. The molecular basis of these mechanisms is poorly understood. In this paper we show that upon exposure to noxious temperature, Caenorhabditis elegans reacts by a withdrawal reflex. To analyze this thermal avoidance behavior, we developed a laser-based assay to quantify the response. The escape reflex can be observed in 98% of the adult animals, but is not executed in animals in diapause. The thermal avoidance response differs significantly from the thermotaxis behavior that is based on the perception of physiological temperature. It involves different neurons and is influenced by mutations in distinct genes. As in mammals, the strength of the thermal avoidance response is increased by application of capsaicin, the pungent ingredient in chili peppers. We find that thermal avoidance is strongly reduced in mutants affecting the neural transmission modulated by glutamate and neuropeptides as well as in mutants affecting the structure and function of sensory neurons. We suggest that the study of this nociceptive behavior in C. elegans can be used to understand the genetic and molecular basis of thermal nociception.
Every organism depends on a set of regulatory, protective behaviors to ensure survival. Upon exposure to a noxious mechanical, chemical, or thermal stimulus, animals execute a protective withdrawal-reflex program to prevent cellular damage. In the central nervous system, aversive and protective reactions to these stimuli include the sensation of pain. Whereas the perception of pain may be silenced during anesthesia, nociceptive reflexes by the peripheral nervous system are still executed (1).
In vertebrates, the presence of tissue-damaging stimuli or the existence of tissue damage are sensed by primary afferent neurons. These, in general, respond only to high intensities of a stimulus and correspond either to a single sensory modality or are polymodal (are stimulated, for example, by mechanical or thermal stimuli). Several animal and in vitro models have been established to study the physiological and pharmacological parameters that interfere with pain perception and nociception. Recently, cell culture experiments suggested that the vanilloid receptor VR1 is activated both by capsaicin and by noxious heat (2, 3). VR1 belongs to the evolutionary conserved TRP (transient receptor potential) family of nonselective ion channels and is located in sensory nerve endings of the dorsal root ganglion. Despite this finding, little is known about the molecular mechanisms of nociception.
The nematode Caenorhabditis elegans is an excellent organism in which to study the behavioral responses to noxious environmental stimuli. Its powerful genetics and the simple nervous system have greatly facilitated the identification of the neural circuits and genes involved in various avoidance reactions such as its response to noxious chemicals, high osmolarities, acidic pH (4), and noxious mechanical stimuli (5).
We show here that C. elegans also responds to an acute heat stimulus with a reflexive withdrawal reaction. We report a robust assay to characterize the genetic and molecular background of this thermal avoidance behavior. We localized the receptive fields in the animals and tested mutants with defects in neuroanatomy, neurotransmission, and cell lineage. Our data indicate that most molecular aspects of the thermal avoidance response differ significantly from thermotaxis (6) and other described behaviors of C. elegans.
To evaluate whether C. elegans could be used as a new model to study the molecular basis of heat perception, we also tested the influence of chemical compounds used in pain research. Our results suggest that the response of C. elegans and vertebrates to noxious heat can be manipulated pharmacologically by the same chemicals and, therefore, might exhibit similarities at the molecular level.
MATERIALS AND METHODS
Strains and Genetic Procedures.
C. elegans strains were grown and maintained as described (7). The unc-86(n846)III; ttx-3(ks5)X double mutant strain was constructed as follows: unc-86(n846)III; vab-3(e648)X hermaphrodites (strain BR820) were mated with ttx-3(ks5) males. F2 animals homozygous for unc-86; ttx-3 were identified as mechanosensory, egg-laying defective, non-Vab (no deformed head) animals and then confirmed by complementation analysis. Dauer larvae were isolated by SDS treatment as described (8).
Behavioral Assays.
C. elegans was exposed to noxious heat by using either a pen with an electronically heated metal tip (Colwood Electronics, Eatontown, NJ) or a monochromatic laser diode (Schaefter & Kirchhoff, Hamburg). For the hot metal tip assays, the temperature of a heated platinum wire (0.8-mm diameter) was electronically controlled to produce a constant radial temperature gradient. The temperature at 3.0 mm from the tip was measured to be 33.0 ± 1.0°C by using an 818-UV Silicon Photodetector (Newport, Fountain Valley, CA). For the laser assays, a 50-mW laser diode was attached to a Leica MZ8 dissecting microscope and focused at the focal plane of the animals. The thermal avoidance (Tav) assays were performed in an air-conditioned room with a constant temperature of 20°C and a humidity of at least 45%. Before assaying, nonstarved populations of animals, or single animals, were given at least 1 min to become accustomed to the environmental conditions of the room. Then, the heat stimulus was presented in front of an animal and the initial response was scored. The response was classified according to the following scheme: class I, rapid reflexive withdrawal, backing for at least one body length followed by a heading change; class II, rapid reflexive withdrawal but only little backing; class III, slow backing; class IV, no response. Whenever mutant strains were tested, wild-type animals were tested as a reference. A minimum of 167 animals per mutant strain in at least two independent assays was analyzed. Mechanosensation (Mec) and nose touch (Not) response were analyzed as described (9, 10).
Pharmacological Studies.
Capsaicin (Sigma) was dissolved in 4% ethanol in M9 (7), and capsazepine (Research Biochemicals) was dissolved in 2% methanol in M9 (11). Animals from well-fed populations were washed twice with M9 and then incubated with different dilutions of the drugs. Samples were taken at various time points, transferred to NGM (7) plates without food, and, after a rest of 5 min, animals were assayed for their Tav, Not, or Mec phenotype. For the competition assays, animals were preincubated with 100 μM capsazepine for 30 min, and then 100 μM capsaicin was added. After another 30 min the Tav response was analyzed.
Mutational Analyses.
A complete list of mutants tested can be requested from the authors. The following mutants affecting the respective neurotransmitter synthesis and function were analyzed: γ-aminobutyric acid, unc-25(e156), unc-47(e307), unc-49(e382) (12); dopamine, cat-1(e1111), cat-2(e1112), cat-4(e1141), cat-6(e1861) (13); acetylcholine, ace-1(p1000), ace-2(g72), ace-3(dc2), cha-1(p1152) (14); octopamine and serotonin, (unc-86(n846), daf-10(e1387), che-3(e1810), osm-3(p802), cat-4(e1141), goa-1(n363, n1134) (15, 16); glutamate, glr-1(n2641), avr-15(ad1051), eat-4(n2472, ad572, ad819, ky5) (17–19); and neuropeptides, flp-1(yn2, yn4), npr-1(g320) (20, 21). Other mutants are described in Results and Discussion.
RESULTS AND DISCUSSION
The soil nematode C. elegans is fertile in a temperature range from about 13°C to 26°C. However, C. elegans not only responds to thermal cues within this physiologically tolerable range, but also reacts upon extreme temperature conditions such as cold or heat. A prolonged exposure at 30°C results in an induction of the heat-shock response, and the animals become sterile within a few hours (22). We noticed an additional behavioral response upon sudden exposure to extreme temperatures. When approached by a local heat source of at least 33°C, the animals try to escape the temperature stimulus by a nociceptive reflex. We termed the response to heat the Tav response.
A Laser-Based Assay to Analyze the Thermal Avoidance Behavior. We developed a laser-based assay to stimulate C. elegans by heat. We chose a laser diode that emits at a wavelength close to IR light (685 ± 0.5 nm). It has been reported previously that C. elegans reacts only weakly to light (23). Most notably, when exposed to monochromatic light ranging from 420 to 680 nm, wavelengths outside of 520–600 nm elicited no response (23). Because our laser diode does not emit below 680 nm, we ensured that the avoidance reaction we analyzed was caused by the response to heat rather than by photons. This was confirmed by the fact that the results we obtained in this study could be reproduced by a heated metal tip.
We focused the laser by collimator optics to a 30-μm spot visible under the dissecting scope to obtain a local temperature on the agar surface of 33.5 ± 1°C. For the thermal avoidance assays, the laser was pointed to the agar plates in front of the animals so that they moved nose-onward into the beam. We then recorded the initial response of each individual animal when it encountered the noxious heat stimulus for the first time. This parameter was chosen because it was not known whether the response would be modulated by experience, which could result in sensitization or habituation. Because the laser beam does not cause any permanent damage to the animals (data not shown), it is also an excellent tool for repetitive tests and allows the recovery of mutants with a defective thermal avoidance behavior.
Wild-Type Animals, but Not Stress-Tolerant Animals in Diapause, Respond to Noxious Heat. The thermal avoidance response that is executed by the animals is similar, but not identical, to their response to noxious mechanical stimuli reported previously (5, 10). The withdrawal reaction from a local heat source consists of three characteristic phases: the foraging animals first stop forward movement, then reverse for one to two and a half body lengths, reposition, and, finally, turn away from the heat source to resume forward movement in a new direction. In 83% of the trials, wild-type animals showed the full behavior and are included in class I (see Fig. 1a and Materials and Methods). Twelve percent of the animals stopped forward movement, but only reversed for about one body length and did not perform a heading change (class II). Three percent of the animals only responded slowly to the heat stimulus and were grouped into class III. Two percent of the animals did not show any response (class IV). When we tested individual nonresponding animals again, we found that they, in most cases, did respond upon repeated stimulation. This suggests some statistical variability of the response. We tested wild-type behavior of individual animals on separate cultivation plates and compared the results with the behavior of individual animals in dense populations under nonstarved conditions. As there was no detectable difference, the assay was based on testing individual animals in a population.
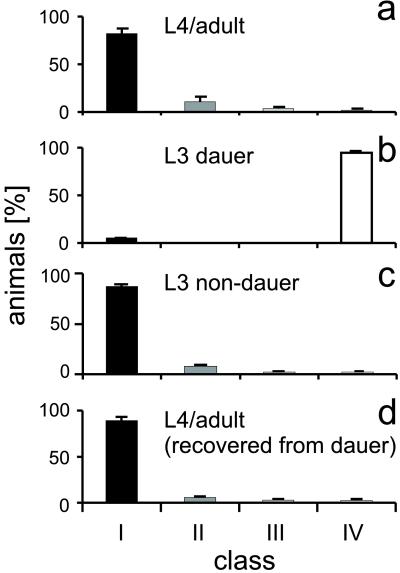
Figure 1.
Tav response of wild-type animals at different developmental stages. (a) The behavior of animals in a nonstarved population. Twelve hundred animals (L4 or adult animals) were tested over 15 days, and the Tav response was scored according to the behavioral classes defined in Materials and Methods (solid bars, class I; darkly shaded bars, class II; lightly shaded bars, class III; open bars, class IV). (b) Dauer animals do not respond to noxious heat. Two hundred and sixty-seven animals in the dauer stage were tested on 2 days. (c) In comparison, 122 animals of the alternative L3 non-dauer stage. (d) After exit from the dauer stage, animals exhibit a normal Tav response again. Two hundred and five adult animals recovered from dauer were tested on 2 days. Bars represent the percentage of animals within a particular response class. Error bars represent SE.
The thermal avoidance response of wild-type animals raised at 15°C, 20°C, and 25°C was indistinguishable. Therefore, unlike thermotaxis (6), the thermal avoidance response does not depend on the growth temperature.
In response to unfavorable conditions inadequate for reproduction, such as overcrowding or food deprivation, C. elegans larvae can enter a diapause, known as the dauer stage. Dauer larvae are more resistant to environmental stress, including heat shock conditions, and have a longer life span (8). Once dauer animals encounter more favorable conditions, they recover and will develop into fertile adults. We prepared dauer larvae from a starved population of wild-type animals (8). Most strikingly, Tav response is almost absent in those animals, because only 5 ± 0.3% of the dauer larvae reacted to the heat stimulus (Fig. 1b), whereas 97.5 ± 0.5% of well-fed animals that did not enter the dauer stage and developed into L3 larvae responded (Fig. 1c). Notably, this stress tolerance is reversible, because adult animals that recovered from the (nonresponsive) dauer stage exhibited a normal Tav response (Fig. 1d). During dauer formation, extensive anatomical modifications take place in the animals. We suggest that the profoundly reduced TAV response of dauer animals is caused by a remodeling of the sensory nervous system. The insensitivity for noxious thermal stimuli may increase the tolerance of dauer animals toward unfavorable environmental conditions and, therefore, may reduce the stress response and the energy consumption of dauer animals in the absence of food. We consider it unlikely that muscle defects or a reduced heat dissipation resulting from cuticle alterations causes the difference in response, because dauer animals did not exhibit movement defects and the Tav response was not improved in srf and dpy mutants that render the cuticle more permeable (ref. 11 and data not shown).
Taking these results together, we conclude that our thermal avoidance assay is highly reproducible and allows us to analyze the genetic and pharmacological parameters that modulate the response of C. elegans to noxious heat.
The Thermal Avoidance Response of C. elegans Is Modulated by Capsaicin. To evaluate whether the response of C. elegans to nociceptive heat is similar to that of vertebrates, we tested whether we could pharmacologically manipulate the animals’ response in comparable ways. Probably the best functional marker for C fiber nociceptors in vertebrates is their sensitivity to capsaicin, the pungent ingredient in a wide variety of chili peppers (24). Exposure to capsaicin provokes a sensation of burning pain. Capsaicin action manifests itself as a short-lasting stimulation of afferent neurons (25). Recently, it was suggested that its cellular target in rats is the VR1 ion channel that also can be activated by heat (2, 3).
We analyzed the Tav response in the presence of various concentrations of capsaicin. To control the uptake of the drugs, we applied them in liquid culture in 4% ethanol. Under these conditions, wild-type animals responded less well than on plates (69.1 ± 5.9% responding animals; Fig. 2). However, we observed a significantly increased reaction (95.3 ± 5.5% response) of C. elegans to the heat stimuli after only 30 min of exposure to 1–100 μM capsaicin. A total of 90.5 ± 10.5% of these animals responded with the full (class I) behavior. This hyperalgetic reaction could be induced for up to 1 hr after the start of the incubation. We find this result particularly surprising, because it had been suggested that most nonmammalian animal species appear to be poorly sensitive to capsaicin (25).
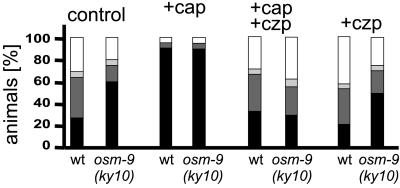
Figure 2.
Capsaicin-induced hyperalgesia is blocked by the competitive inhibitor capsazepine. Wild-type and osm-9(ky10) mutant animals were tested in liquid culture after the addition of the chemicals, as indicated. Bars represent the percentage of animals that responded (solid bars) or did not respond (open bars) in the Tav assays. Control, addition of 4% ethanol in M9; +cap, 100 μM capsaicin; +cap+czp, preincubation in 100 μM capsazepine, followed by addition of 100 μM capsaicin; +czp, 100 μM capsazepine. Shading of the bars is as in Fig. 1; for statistical data, see text.
After exposure to 10 nM–100 μM capsaicin for more than 60 min, we observed that other behavioral reactions of C. elegans, among them mechanosensation, became impaired. Whereas the control animals responded to body touch (Mec response) up to 15 times, capsaicin-treated animals already were adapted after the fifth stimulus. These nonselective depressions of excitability of sensory neurons after prolonged exposure or higher dosage also have been observed in other animal models (25).
Next, we investigated the specificity of the capsaicin response. For this purpose, we analyzed the effect of capzazepine, a potent and specific inhibitor of capsaicin activity. In dorsal root ganglion neurons, capsazepine reversibly antagonizes the excitatory action of capsaicin. It has been suggested that capsaicin and capsazepine compete for the same binding site on the capsaicin receptor (26). After preincubation of wild-type C. elegans animals in 100 μM capsazepine for 30 min, the following capsaicin exposure no longer resulted in a capsaicin-evoked hyperalgesia (70.9 ± 2.9% responding animals; Fig. 2). Incubation with 100 μM capsazepine alone had no significant effect on the behavior of the animals (57.6 ± 4.8% responding animals). This result indicates that the competitive capsaicin receptor antagonist capsazepine was able to selectively block capsaicin-induced hyperalgesia in C. elegans. This strongly suggests the presence of a capsaicin-sensitive receptor in C. elegans.
The recently finished C. elegans genome project revealed several genes with significant sequence similarities to the rodent VR1 capsaicin receptor (27). One of them is encoded by the gene osm-9 (28). The osm-9(ky10) mutation prevents expression of a functional OSM-9 protein and results in a failure to avoid noxious mechanical and chemical stimuli (28). However, we found that the thermal avoidance response of osm-9(ky10) animals was even somewhat stronger than that of wild type, although the difference was statistically not significant (80 ± 1.5% responding animals in liquid culture, P = 0.16, Fig. 2; for statistics in plate assays, see Table 1). As in wild type, osm-9 animals also displayed an increased Tav reactivity in the presence of capsaicin (94.8 ± 9.6% responding animals), and this response was selectively blocked by capsazepine (62.5 ± 4.5% response). Therefore, the OSM-9 protein most likely does not represent a bona fide vanilloid receptor homologue that can be activated by heat and capsaicin. We, however, cannot exclude the possibility that OSM-9 is part of a heteromeric channel complex and that the absence of one subunit does not affect the capsaicin binding or activation of the channel. In addition to osm-9, there are 10 other candidates for transient receptor potential-related channel genes in the C. elegans genome (27). There currently are no other mutants known in any of these genes that are available for thermal avoidance analyses.
Table 1.
Selected list of tested strains described in the text
| Strain | Genotype | % no avoidance | No. of animals | No. of assays | Different from wt (P < 0.05) |
|---|---|---|---|---|---|
| N2 | + | 1.7 ± 0.3 | 1,200 | 15 | — |
| CB648 | vab-3(e648) | 42.0 ± 7.8 | 280 | 3 | Yes |
| CX10 | osm-9(ky10) | 3.7 ± 0.9 | 252 | 3 | No |
| Thermotaxis mutants | |||||
| PR691 | tax-2(p694) | 4.7 ± 2.2 | 303 | 3 | No |
| PR678 | tax-4(p678) | 12.5 ± 4.5 | 152 | 2 | No |
| PR767 | ttx-1(p767) | 7.0 ± 1.2 | 199 | 2 | No |
| BR825 | unc-86(n846); ttx-3(ks5) | 8.8 ± 3.0 | 380 | 4 | No |
| Cilia structure mutants | |||||
| CB3323 | che-13(e1805) | 17.8 ± 4.3 | 297 | 4 | Yes |
| PR808 | osm-1(p808) | 33.5 ± 3.5 | 224 | 2 | Yes |
| PR813 | osm-5(p813) | 24.8 ± 6.4 | 387 | 4 | Yes |
| PR811 | osm-6(p811) | 36.5 ± 1.5 | 198 | 2 | Yes |
| PR802 | osm-3(p802) | 6.3 ± 2.0 | 267 | 3 | No |
| DR86 | daf-19(m86) | 5.0 ± 0.0 | 220 | 2 | No |
| CB1377 | daf-6(e1377) | 3.3 ± 0.3 | 197 | 3 | No |
| Neurotransmission mutants | |||||
| NY2 | flp-1(yn2) | 40.3 ± 8.7 | 379 | 4 | Yes |
| NY16 | flp-1(yn4) | 40.3 ± 9.3 | 278 | 4 | Yes |
| MT6319 | eat-4(n2474) | 62.0 ± 2.0 | 215 | 2 | Yes |
| KP4 | glr-1(n2461) | 4.3 ± 1.2 | 258 | 3 | No |
The Tav phenotype of more than 180 mutant strains was analyzed. Percentage of animals (% no avoidance) showing no thermal avoidance response (=class IV response), the number of animals tested, and the number of assays are given. The last column displays a statistical analysis (Student’s t test) to determine whether the Tav response of a given mutant strain is different (yes) or indistinguishable (no) from the wild type (wt).
Thermal Nociceptors Are Located in the Head and Tail Ends of the Animals. In vertebrates, the response to external thermonociceptive stimuli is mediated by specialized nociceptors or polymodal sensory neurons. To determine whether analogous neurons exist in C. elegans, we scanned different body parts of the animals for their responsiveness to heat. The small size of the laser spot (30 μm) compared with the size of the animals [1.3 mm in length and 80 μm in diameter (29)] allowed us to target different body parts (Fig. 3a). Five easily identifiable body regions were defined from anterior to posterior: the tip of the nose, the region enclosed by the pharyngeal bulbs (where most of the animals neuronal cell bodies are located), anterior midbody (between the posterior bulb and vulva), posterior midbody (between vulva and the anus), and the tail. For each body region, the response of 70 animals was tested. We identified the anterior and posterior ends of the animals as the only heat-sensitive body regions. Stimulation of the tip of the nose up to the neurons anterior to the nerve ring resulted in the most significant withdrawal reaction. Stimulation of the tail also induced a reaction similar to the tap-withdrawal response of C. elegans (9). A resting animal initiated backward movement, whereas a forward-moving animal accelerated its forward movement. It is noteworthy that the strongest response was obtained when we stimulated the tips of either nose or tail in a region where no neural cell bodies are located. Therefore, the most likely candidate neurons involved in heat perception are those that possess neurites extending into the tips of either nose or tail. Neurons of this class localized in the head have been implicated in a variety of sensory behaviors (30). Only two types of tail neurons have been correlated functionally with any behavior, namely, mechanosensation and chemosensation (5, 29).
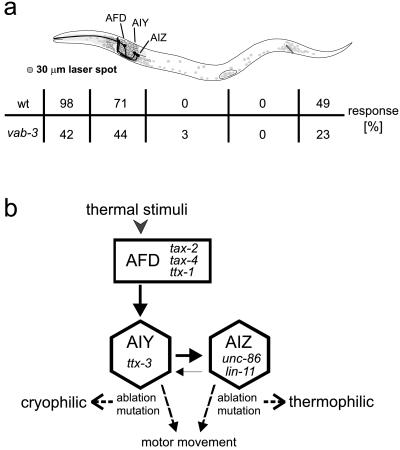
Figure 3.
Thermoreceptive regions/neurons in C. elegans. (a) The schematic drawing shows an adult animal with the anterior/tip of the nose to the left and the posterior/tail to the right. Somata of neurons are darkly shaded. The solid areas are the somata and axonal processes of AFD, AIY, and AIZ neurons that mediate thermotaxis response (34). The table below the drawing shows the percentage of wild-type (wt) and vab-3(e648) animals that responded to the heat stimulus in the indicated body region. The size of the laser is indicated to the left. (b) Neural circuit for thermotaxis (modified after ref. 34) showing the connectivity of AFD, AIY, and AIZ. Both ablation of the sensory neuron AFD or the interneuron AIY and mutations eliminating their functions lead to a cryophilic phenotype, whereas ablation or mutations abolishing the function of AIZ lead to a thermophilic phenotype (34). Neither mutant affected the thermal avoidance response.
The gene PAX6/vab-3 is required for correct head and tail sensory neuron development in C. elegans and has similar functions in humans and mouse (31, 32). Because vab-3 null mutations are lethal, we tested the nonnull allele vab-3(e648) in our studies (32). vab-3(e648) mutant animals are able to move backward, but develop severe defects in the gross head morphology and also in the head and tail nervous system anatomy. These include malpositioning and malfunction of sensory neurons in both head and tail (33). vab-3(e648) revealed a partially defective Tav response (Table 1). This suggests that the morphogenesis defects of vab-3 mutants include cells that are involved in the perception or transmission of the heat signal.
Nociceptive Heat Response Uses a Different Neural Circuit Than Thermotaxis. Previous studies have shown that C. elegans perceives and responds to small temperature changes (<0.1°C) in its environment (6). When wild-type animals are placed on a thermal gradient, they seek the temperature at which they were raised. This taxis behavior has been studied in detail by mutational and laser-ablation analyses (34). The neural circuit of thermotaxis suggests that temperature in the range of between 15°C and 25°C is perceived by a pair of sensory neurons (the AFD neurons or AFD) that are connected to a pair of interneurons (two AIY) (29, 34). Ablation of either the AFD or AIY neurons leads to a cryophilic (cold-seeking) phenotype. Most synapses of the AIY neurons connect to the two AIZ interneurons. Ablation of the AIZ neurons leads to a thermophilic (warmth-seeking) phenotype, suggesting that AIY and AIZ direct two reciprocal behavioral components required for thermotaxis to the preferred temperature (for location and connectivity of the neurons, see Fig. 3).
Our analysis suggested that the thermal avoidance response is significantly different from the animals’ thermotaxis behavior. First, thermal avoidance response can be observed only upon exposure to nonphysiological temperature. Second, a different motor program is initiated, resulting in a reflexive withdrawal and not a targeted taxis behavior. To substantiate these differences further, we tested the thermal avoidance response of mutants defective in thermotaxis. Mutations in the genes tax-2, tax-4, and ttx-1 result in a severe thermotaxis defect, most likely as a consequence of functional defects in the AFD neurons (34, 35). However, mutations in these genes had no effect on the Tav response (Table 1). This implies that either the AFD neurons are not involved in the sensation of noxious heat or that AFD functions other than the ones required for thermotaxis are involved in noxious heat sensation.
To test whether the interneurons AIY and AIZ are involved in the thermal avoidance response, we analyzed mutants that affect the development or function of AIY and AIZ. The AIZ neurons are presumably nonfunctional in lin-11(n389) mutants (36) and are absent in unc-86(n846) mutants (37). These mutant animals display the same thermophilic phenotype as animals in which the AIZ neurons were removed by laser microsurgery (35). ttx-3 is required for the function of AIY. ttx-3(ks5) mutant animals display a severe axonal outgrowth defect (38) and a cryophilic behavior indistinguishable from that of animals in which the AIY neurons were ablated (34). Laser ablation of both pairs of interneurons results in a total absence of thermotaxis behavior (34). We found that lin-11, unc-86, and ttx-3 mutant animals responded to nociceptive heat like wild-type animals (data not shown), suggesting that neither AIY nor AIZ are involved in the transmission of heat sensation. This argument is corroborated by the analysis of a double mutant unc-86(n846); ttx-3(ks5) that lacks both AIY and AIZ function. Our data show that the double mutant had a wild-type thermal avoidance behavior (Table 1), indicating that the neural circuit necessary for Tav behavior is different from that involved in thermotaxis.
In summary, we find that neither the genes nor the neurons necessary for thermotaxis are required for thermal avoidance response.
Furthermore, as described below, several mutants that are defective for thermal avoidance response display a wild-type thermotaxis behavior. This suggests that genes required for Tav response are not required for the execution of thermotaxis and that sensory neurons other than the AFD neurons function in the perception of noxious heat.
We conclude from these data that C. elegans senses noxious heat differently than it senses temperature within its physiological temperature range.
The Thermal Avoidance Response Is Modulated by Glutamate and Neuropeptides. To begin a molecular and genetic analysis of the thermal avoidance behavior, we tested more than 180 C. elegans strains with mutations in genes affecting neural transmission, sensation, and behaviors. In addition, we also assayed mutants that cause lineage defects or morphological abnormalities or are involved in cell death (see Materials and Methods).
Several of the mutants we analyzed display a locomotion defect that is not specific for the thermal avoidance phenotype. Although we used nose-touch and body-touch responses as a differential avoidance behavior, it was not always possible to discriminate whether a particular mutant responded only weakly to thermal stimuli or was unable to reverse because of a severe movement defect. Therefore, to allow the comparison of the Tav behaviors of various mutants, we used criteria similar to criteria simpler than the ones described above to group the heat responses. We placed all animals from classes I–III into one group representing the responding animals and compared them with animals in class IV that did not exhibit any response to the laser heat. Table 1 shows the results for mutant strains that will be discussed in the text.
We tested mutants involved in neurotransmission by γ-aminobutyric acid, acetylcholine, glutamate, dopamine, octopamine, and serotonin (for a list of mutants tested, see Materials and Methods). These mutants affect genes involved in the synthesis of the respective neurotransmitters as well as genes encoding candidate receptors. Mutants in cha-1 have a reduced choline acetyltransferase activity. These mutants showed a very weak response in our assays (data not shown). The animals twitched their head upon stimulation, but did not move backward. This suggests that the temperature signal was received by the sensory neurons, but was not transmitted to body muscles. The uncoordinated phenotype of cha-1 mutants is not specific for the TAV response. The neurotransmitter mutants affecting γ-aminobutyric acid, dopamine, octopamine, and serotonin all showed thermal avoidance response indistinguishable from wild type (data not shown), suggesting that these neurotransmitters play no or only a minor role in the modulation of the noxious heat response.
In contrast, all mutant alleles of the eat-4 gene we tested displayed a significantly reduced Tav response [data for allele eat-4(n2474) are presented in Table 1]. eat-4 encodes a protein similar to the mammalian brain-specific Na+/Pi-cotransporter BNPI (19). Mutants in this gene have defects in multiple behaviors that depend on glutamatergic neurotransmission [feeding (Eat), thermotaxis (Ttx), avoidance of high osmolarities (Osm), and nose touch (Not)], but appear to have little effect on other neuronal functions (39). Therefore, glutamate most likely plays an important role in the response of C. elegans to noxious temperature. Glutamate, a fast, excitatory neurotransmitter in the vertebrate central nervous system, is also one of the major neurotransmitters in nociceptive afferents in mammals (40). In pain transmission it is often cotransmitted with neuropeptides modulating nociceptive processes, e.g., tachykinins (substance P, neurokinin A), cholecystokinin, and bombesin (41). There is immunochemical evidence for the presence of these neuropeptides in nematodes (42), which might suggest that a related mechanism is used in C. elegans.
Glutamatergic modulation of the thermal avoidance response is most likely not mediated by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor gene glr-1 (17), because glr-1(n2641) mutants respond as wild-type in our Tav assay. In contrast, glr-1(n2641) mutants are defective for mechanical avoidance responses (43). Therefore, although the EAT-4 Na+/Pi-cotransporter is used in all of these avoidance behaviors, distinct glutamate receptors are involved in thermal vs. mechanical or chemical avoidance. In vertebrates, it has been shown that the N-methyl-d-aspartate (NMDA)-type glutamate receptors, rather than the AMPA-type receptors, are involved in thermal nociception (44). There currently are no characterized mutants in a NMDA-type receptor available.
We next tested whether neuropeptides contribute to the Tav response. For this purpose, we examined two alleles of the flp-1 locus that encodes seven distinct neuropeptides of the FMRFamide-related family (20). Both mutants show a strongly impaired thermal avoidance response (Table 1). FMRFamide-related neuropeptides have been implicated in the modulation of nociception in vertebrates, as intrathecally administered FMRFamide antagonize opioid-induced analgesia (45) and morphine-induced analgesia (46). Therefore, it seems likely that FMRFamide-related neuropeptides have a similar modulatory function in C. elegans.
Sensory Neurons Embedded in the Cuticle Mediate the Thermal Avoidance Response. Free nerve endings are regarded as the morphological correlates of vertebrate nociceptors (47). We therefore examined mutants that affect the structure and function of sensory endings. Many of these mutants display defects in chemosensation and mechanosensation. From our laser-scanning experiments we had concluded that the sensory endings of heat-receptive nociceptor cells have to be located in the most anterior part of the head and the posterior tip of the tail. Several mutants (dyf-1 to dyf-13 and daf-6) that do not take up dyes have defects in the exposed sensory neurons or their support cells (48). All of these mutants showed wild-type thermal avoidance behavior (for daf-6, see Table 1), indicating that the thermal nociceptor cells do not have to be exposed to the environment. This result correlates well with reports from vertebrate thermonociceptors of the skin that are also embedded in the cutis and do not have access to the surface (47). This result is corroborated by the finding that most of the chemosensory mutants we tested also have a wild-type thermal avoidance behavior.
Mutations in the genes osm-1, osm-5, osm-6, and che-13 displayed a significantly reduced response of the respective animals to the heat stimulus (Table 1). These mutants have shortened axonemes and defects in the ectopic assembly of ciliary structures and microtubules in almost all sensory neurons (49). osm-6 is expressed in 56 of 60 ciliated neurons. These data indicate that the thermonociceptors in C. elegans are most likely ciliated neurons.
Mutations in osm-3 and che-12 affect only a subset of ciliated neurons (28/60), the amphid and phasmid ciliary structures in the chemosensory organs of the animals. Both mutants displayed a wild-type Tav response (for osm-3 see Table 1), suggesting again that the ciliated neurons that detect heat do not have to be exposed to the environment and also that the thermal nociceptor is not part of the amphid or phasmid neurons.
The ultrastructural analysis of thermonociceptors in the skin of mammals revealed that their thermoreceptive endings are fine, branched, tree-like structures (48). Several ciliated endings in C. elegans have similarly complex structures, including the AFD neurons, the sensory neurons for thermotaxis, and the AWB neurons, which function in chemotaxis (29). Mutants in the gene daf-19 lack all cilia except the cilia rootlets (49), but the mutant animals still respond to noxious heat (Table 1). Because the complex, branched ciliary structures of AWB and AFD, among others, are not affected by daf-19 mutations (N. Dwyer and C. I. Bargmann, personal communication, and ref. 49), the most likely explanation for this finding is that in C. elegans, as in mammals, specialized sensory structures are needed for perceiving noxious heat stimuli.
CONCLUSION
We established C. elegans as a model for the molecular analysis of heat perception. The response of C. elegans to noxious heat is mediated by sensory neurons that do not have to be exposed to the outside and is modulated by glutamate and by the neuropeptides encoded in the flp-1 locus. In addition, capsaicin-induced hyperalgesia is inhibited by its competitive inhibitor capsazepine. Therefore, the nociceptive response of C. elegans to heat resembles, in several aspects, that of higher organisms. The many genetic tools available for C. elegans now may be exploited to identify novel genes and the pathways involved in this behavior.
Acknowledgments
We thank L. Bloom and B. Horvitz for suggestions and P. Nef and R. Plasterk for providing mutants before publication. We also thank S. Wicks, C. Bargmann, R. Lee, L. Avery, J. Burr, B. Conradt, and Bernard Lakowski and the other members of the Baumeister lab for stimulating discussions. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work is supported by a grant from the Deutsche Forschungsgemeinschaft to R.B.
ABBREVIATION
- Tav
thermal avoidance defective
References
- 1.Campbell J N, Meyer R A. In: Neurobiology of Nociceptors. Belmonte C, Cervero F, editors. Oxford: Oxford Univ. Press; 1996. pp. 117–145. [Google Scholar]
- 2.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga M, Caterina M, Malmberg A, Rosen T, Gilbert H, Skinner K, Raumann B, Basbaum A, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 4.Dusenbery D B, Sheridan R E, Russell R L. Genetics. 1975;80:297–309. doi: 10.1093/genetics/80.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalfie M, Sulston J E, White J G, Southgate E, Thomson J N, Brenner S. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedgecock E M, Russell R L. Proc Natl Acad Sci USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassada R C, Russell R L. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 9.Chalfie M, Sulston J. Dev Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan J M, Horvitz H R. Proc Natl Acad Sci USA. 1993;90:2227–2231. doi: 10.1073/pnas.90.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rand J B, Johnson C D. In: Methods in Cell Biology. Epstein H F, Shakes D C, editors. Vol. 48. San Diego: Academic; 1995. pp. 187–204. [DOI] [PubMed] [Google Scholar]
- 12.McIntire S L, Jorgensen E, Kaplan J, Horvitz H R. Nature (London) 1993;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- 13.Sulston J, Dew M, Brenner S. J Comp Neurol. 1975;163:215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- 14.Hosono R, Sassa T, Kuno S. J Neurochem. 1987;49:1820–1823. doi: 10.1111/j.1471-4159.1987.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 15.Horvitz H R, Chalfie M, Trent C, Sulston J E, Evans P. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 16.Sègalat L, Elkes D A, Kaplan J M. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- 17.Hart A C, Sims S, Kaplan J M. Nature (London) 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 18.Dent J A, Davis M W, Avery L. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R Y N, Sawin E R, Chalfie M, Horvitz H R, Avery L. J Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson L S, Rosoff M L, Li C. Science. 1998;281:1686–1690. doi: 10.1126/science.281.5383.1686. [DOI] [PubMed] [Google Scholar]
- 21.de Bono M, Bargmann C. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 22.Lithgow G, White T, Melov S, Johnson T. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burr A H. Photochem Photobiol. 1985;41:577–582. doi: 10.1111/j.1751-1097.1985.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 24.Szolcsanyi J, Anton F, Reeh P, Handwerker H. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- 25.Holzer P. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 26.Bevan S, Hothi S, Hughes G, James I F, Rang H P, Shah K, Walpole C S J, Yeats J C. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bargmann C I. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 28.Colbert H A, Smith T L, Bargmann C I. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White J G, Southgate E, Thomson J N, Brenner S. Philos Trans R Soc London. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 30.Bargmann C I. Annu Rev Neurosci. 1993;16:47–71. doi: 10.1146/annurev.ne.16.030193.000403. [DOI] [PubMed] [Google Scholar]
- 31.Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. Nat Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- 32.Chisholm A D, Horvitz H R. Nature (London) 1995;377:52–55. doi: 10.1038/377052a0. [DOI] [PubMed] [Google Scholar]
- 33.Lewis J A, Hodgkin J A. J Comp Neurol. 1977;172:489–510. doi: 10.1002/cne.901720306. [DOI] [PubMed] [Google Scholar]
- 34.Mori I, Ohshima Y. Nature (London) 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 35.Coburn C M, Bargmann C I. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 36.Hobert O, D’Alberti T, Liu X, Ruvkun G. J Neurosci. 1998;18:2084–2096. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finney M, Ruvkun G. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- 38.Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 39.Avery L. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jessel T, Kelly D. In: Principles of Neural Science. Kandel E, Schwatz J, Jessel T, editors. Norwalk, CT: Appelton and Lange; 1991. pp. 385–399. [Google Scholar]
- 41.Duggan A, Weihe F. In: Toward a New Pharmacotherapy of Pain. Basbaum A, Besson J, editors. New York: Wiley; 1991. pp. 35–67. [Google Scholar]
- 42.Brownlee D J A, Fairweather I, Holden-Dye L, Walker R J. Parasitol Today. 1996;12:343–351. doi: 10.1016/0169-4758(96)10052-1. [DOI] [PubMed] [Google Scholar]
- 43.Maricq A V, Peckol E, Driscoll M, Bargmann C I. Nature (London) 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 44.Neugebauer V, Lucke T, Grubb B, Schaible H. Neurosci Lett. 1994;170:237–240. doi: 10.1016/0304-3940(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 45.Gouarderes C, Sutak M, Zajac J M, Jhamanadas K. Eur J Pharmacol. 1993;237:73–81. doi: 10.1016/0014-2999(93)90095-y. [DOI] [PubMed] [Google Scholar]
- 46.Kavaliers M. Neurosci Lett. 1990;115:307–312. doi: 10.1016/0304-3940(90)90473-m. [DOI] [PubMed] [Google Scholar]
- 47.Kruger L, Halata Z. In: Neurobiology of Nociceptors. Belmonte C, Cervero F, editors. Oxford: Oxford Univ. Press; 1996. pp. 37–72. [Google Scholar]
- 48.Starich T A, Herman R K, Kari C K, Yeh W H, Schackwitz W S, Schuyler M W, Collet J, Thomas J H, Riddle D L. Genetics. 1995;139:171–188. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkins L A, Hedgecock E M, Thomson J N, Culotti J G. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]