Abstract
Muscarinic acetylcholine receptors (M1–M5) regulate many key functions of the central and peripheral nervous system. Primarily because of the lack of receptor subtype-selective ligands, the precise physiological roles of the individual muscarinic receptor subtypes remain to be elucidated. Interestingly, the M4 receptor subtype is expressed abundantly in the striatum and various other forebrain regions. To study its potential role in the regulation of locomotor activity and other central functions, we used gene-targeting technology to create mice that lack functional M4 receptors. Pharmacologic analysis of M4 receptor-deficient mice indicated that M4 receptors are not required for muscarinic receptor-mediated analgesia, tremor, hypothermia, and salivation. Strikingly, M4 receptor-deficient mice showed an increase in basal locomotor activity and greatly enhanced locomotor responses (as compared with their wild-type littermates) after activation of D1 dopamine receptors. These results indicate that M4 receptors exert inhibitory control on D1 receptor-mediated locomotor stimulation, probably at the level of striatal projection neurons where the two receptors are coexpressed at high levels. Our findings offer new perspectives for the treatment of Parkinson’s disease and other movement disorders that are characterized by an imbalance between muscarinic cholinergic and dopaminergic neurotransmission.
Members of the muscarinic acetylcholine receptor family (M1–M5) are widely expressed in the central nervous system and in the body periphery (1–7). Central muscarinic receptors are known to play key roles in memory and learning as well as in the regulation of many sensory, motor, and autonomic processes (1–3). In the body periphery, muscarinic receptors mediate the well known activities of acetylcholine released from parasympathetic nerves (1–3).
Based on the overlapping expression patterns of the different muscarinic receptor subtypes (4–7) and the lack of ligands that display a high degree of receptor subtype selectivity (8, 9), the precise functional roles of the individual muscarinic receptor species remain to be determined. To address this issue, we therefore decided to use gene-targeting technology to generate mouse lines deficient in individual muscarinic receptor subtypes. In this study, we present an initial pharmacologic analysis of a mouse line that lacks functional M4 muscarinic receptors.
At the molecular level, the M4 receptor subtype, like the structurally closely related M2 receptor, couples to G proteins of the Gi/Go family (8, 9). M4 receptors are expressed abundantly in the striatum (caudate-putamen) and are also present, though at lower levels, in several other brain regions including cerebral cortex and hippocampus (4–7, 10, 11). In the striatum, a region known to be critically involved in extrapyramidal motor control, the M4 as well as other muscarinic receptor subtypes are coexpressed with D1 and D2 dopamine receptors on striatal projection neurons (12–16). Considerable evidence suggests that complex interactions between these two neurotransmitter receptor systems are critical for the proper regulation of motor control (2, 17, 18). Consistent with this notion, the severe motor deficits observed in patients suffering from Parkinson’s disease and other extrapyramidal motor disorders are thought to reflect an imbalance between muscarinic cholinergic and dopaminergic tone in the striatum (2, 17). To shed light on the potential interactions of M4 muscarinic receptors with the different dopamine receptor subtypes, we have investigated systematically the locomotor effects induced by administration of D1- and D2-selective ligands in wild-type and M4 receptor knockout (KO) mice.
Interestingly, recent pharmacologic studies suggested that the M4 receptor subtype may be responsible for mediating muscarinic receptor-dependent antinociception (19, 20). Thus, another main focus of the present study was to study muscarinic agonist-induced analgesic effects in the M4 receptor KO mice. In addition, we also examined the potential involvement of the M4 receptor subtype in muscarinic receptor-mediated tremor, salivation, and hypothermia, three of the most striking effects caused by administration of centrally acting muscarinic agonists (21).
We demonstrate that M4 receptor-deficient mice show an increase in basal locomotor activity and are hypersensitive to the stimulatory locomotor effects of D1 receptor activation, indicative of a functional interaction between these two receptor systems. Moreover, we show that M4 receptors do not play a pronounced role in mediating muscarinic receptor-dependent analgesia, tremor, hypothermia, and salivation.
MATERIALS AND METHODS
Gene Targeting. A murine M4 muscarinic receptor clone was isolated from a 129SvJ mouse genomic library (Genome Systems) by hybridization with a PCR fragment encoding the central portion of the third intracellular loop. The M4 targeting vector was derived from pPN2T (ref. 22), which is characterized by the presence of two copies of the herpes simplex virus thymidine kinase gene (HSV-TK) (Fig. 1a). The M4 receptor coding sequence was disrupted by replacing a 0.55-kb BglII-XhoI genomic fragment (which encodes the region between the middle of the second transmembrane domain and the N terminus of the third intracellular loop) with the PGK-neomycin resistance gene (PGK-neo).
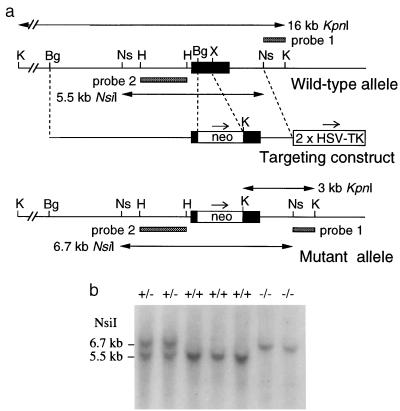
Figure 1.
Disruption of the mouse M4 muscarinic receptor gene. (a) Knockout strategy showing restriction maps of wild-type receptor locus, targeting construct, and targeted allele. Only relevant restriction sites are shown. The receptor coding region is represented by a solid bar. The probes used for Southern analysis and the sizes of the restriction fragments detected with these probes are indicated. Bg, BglII; H, HindIII; Ns, NsiI; K, KpnI; X, XhoI. (b) Genotyping of F2 offspring via Southern blot analysis of NsiI-digested mouse tail DNA (probe 2). The 5.5- and 6.7-kb bands represent the wild-type and mutant M4 receptor alleles, respectively.
The targeting construct was linearized at a unique NotI site and introduced into TC1 (129 SvEv) mouse embryonic stem (ES) cells (23) by electroporation, followed by selection in G418 and gancyclovir. Colonies that survived the double-selection procedure were isolated and screened by Southern blotting for homologous recombination. This analysis showed that 13 of a total of 140 screened clones had been targeted properly. Positive ES cell clones were microinjected into C57BL/6 blastocysts to generate chimeric offspring. Male chimeras were crossed with female CF-1 mice (Charles River Breeding Laboratories) to generate F1 offspring. F1 animals heterozygous for the M4 receptor mutation then were intermated to produce homozygous M4 receptor KO mice. All mice used in the present study were F2 hybrids.
In Situ Hybridization.
Twelve-micrometer-thick horizontal brain sections were processed for mRNA in situ hybridization histochemistry as described (24). A 35S-labeled ribonucleotide antisense probe was synthesized from the 0.55-kb BglII-XhoI genomic fragment (cloned into pBluescript) that was deleted during the construction of the M4 targeting vector (Fig. 1a).
Radioligand-Binding Studies.
Mouse brains were removed, dissected, frozen immediately on dry ice, and stored at −70°C until use. To measure muscarinic receptor densities in different brain regions, radioligand-binding studies were carried out with membrane preparations essentially as described (21, 25). All experiments were carried out by using a saturating concentration (2 nM) of the nonselective muscarinic antagonist, [3H]quinuclidinyl benzilate ([3H]QNB; 45.0 Ci/mmol; New England Nuclear). To determine dopamine receptor densities in mouse striatal homogenates, radioligand-binding studies were carried out by using saturating concentrations of the selective D1-type antagonist ligand, [3H]SCH 23390 (4 nM; 81.4 Ci/mmol; New England Nuclear), or the selective D2-type antagonist ligand, [3H]spiperone (2 nM; 15 Ci/mmol; New England Nuclear). Dopamine receptor-binding assays were carried out essentially as described (26).
Immunoprecipitation Assays.
Muscarinic receptor subtype-specific rabbit polyclonal antisera were raised against nonconserved regions of the third cytoplasmic loops of the mouse receptor proteins in a fashion similar to that described by Levey et al. (7). For immunoprecipitation studies, membranes derived from different mouse brain regions were incubated with 2 nM [3H]QNB to label M1–M5 muscarinic receptors. 3[H]QNB-labeled receptors were solubilized with 1% digitonin and subjected to immunoprecipitation by M2 or M4 receptor-specific antisera by following a procedure described by Yasuda et al. (10).
Neurochemistry.
Mice were killed by decapitation, and striatal tissue was quickly dissected from brain and frozen on dry ice. Striatal acetylcholine, dopamine, and 3,4-dihydroxyphenyl acetic acid (DOPAC) levels were determined via HPLC with electrochemical detection by following a previously published procedure (27).
Pharmacologic and Behavioral Studies. All studies were carried out with animals that were at least 3 months old. Mice were housed in a temperature- and humidity-controlled vivarium, which was kept on a 12-hr dark/12-hr light cycle. Experiments were carried out with mice of either sex. Because the observed pharmacologic and behavioral responses (see below) were similar in male and female mice, data generally were pooled.
Oxotremorine (OXO)-induced tremor, salivation, and hypothermia were measured simultaneously in the same group of mice. Mice from each genotype were injected s.c. with vehicle (distilled water) or different doses of OXO. Body temperature, salivation, and tremor were assessed immediately before and 30 min after injections. Core body temperature was measured by a rectal thermometer (model BAT 8; Bailey Instruments, Saddle Brook, NJ). Salivation and tremor were scored by a trained observer on a scale of 0 (no salivation or tremor), 1 [moderate salivation (moisture on face only) or tremor (intermittent head and body tremor)], and 2 [marked salivation (moisture on face and chest) or tremor (nearly continuous whole body tremor)]. The data were expressed as percent effect, where the score for each mouse was expressed as a percentage of the maximum possible score (i.e., 2).
The tail-flick test was performed by immersing mouse tails in a 55°C water bath and measuring withdrawal latencies immediately before (baseline) and 30 min after OXO or vehicle injection (cut-off time: 10 sec). Data were expressed as percentage of maximum possible effect (MPE), where % MPE = 100× [(postdrug latency − baseline)/(10 − baseline)].
The hot-plate test was carried out by using an electronically controlled hot-plate analgesia meter (model HP/fj; Omnitech Electronics, Columbus, OH). The nociceptive threshold was defined as response latency to lick the front or hind paws after being placed on a 55°C hot plate, immediately before (baseline) and 30 min after OXO or vehicle injection (cut-off time: 30 sec). Data were expressed as percentage of MPE (= 100× [(postdrug latency − baseline)/(30 − baseline)]).
To assess spontaneous locomotor activity, mice from each genotype were placed in polypropylene cages [24 × 45 × 15 cm (height)], and the number of photocell beam interruptions were counted for 60 min to monitor horizontal activity by using a Photobeam Activity System (San Diego Instruments, San Diego). In experiments involving drug injections, mice were placed into the locomotion boxes for a 30-min acclimatization period, followed by the s.c. administration of drugs and immediate monitoring of locomotor activity for the next 60 min. All experiments were performed during the light cycle between 9 a.m. and 12 p.m.
Drugs.
The following drugs were used for injection experiments: apomorphine hydrochloride (Sigma), (±)-SKF 39383 hydrochloride (Research Biochemicals, Natick, MA), haloperidol (a gift from Janssen), SCH 23390 hydrochloride (a gift from Schering-Plough), oxotremorine sesquifumarate (Sigma), and (−)-quinpirole hydrochloride (Research Biochemicals). All drugs were dissolved in distilled water and injected s.c. in a volume of 10 ml/kg.
RESULTS
Generation of M4 Muscarinic Receptor-Deficient Mice. The M4 muscarinic receptor gene was inactivated in mouse TC1 (129SvEv) ES cells by replacing a segment of the receptor coding sequence (encoding the region between the middle of the second transmembrane domain and the N terminus of the third intracellular loop) with a neomycin-resistance cassette (Fig. 1a). Properly targeted ES cell clones were used to generate chimeric mice. These animals then were crossed with CF-1 mice to generate F1 offspring heterozygous (+/−) for the M4 receptor mutation. Male and female F1 heterozygotes were intercrossed to obtain wild-type (+/+) as well as heterozygous (+/−) and homozygous (−/−) M4 receptor mutant mice, as demonstrated by Southern blotting (Fig. 1b). M4−/− mutant mice (n = 143) were obtained with the expected Mendelian frequency (25%).
M4 receptor-deficient mice appeared healthy, did not show any obvious morphological abnormalities, were fertile, and bred normally. They did not display any postural abnormalities, ataxia, tremor, or catalepsy. However, adult M4−/− mutant mice weighed approximately 5% less than their wild-type littermates (P < 0.05).
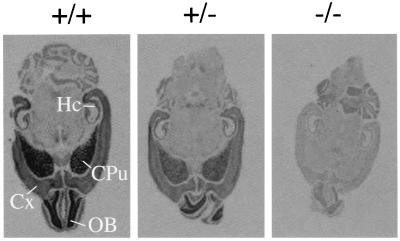
mRNA Hybridization and [3H]QNB-Binding Studies. Initially, in situ hybridization experiments were carried out to study the distribution of M4 receptor mRNA in mouse brain and to confirm the absence of functional M4 receptor transcripts in the M4 receptor mutant mice. For these experiments, a 35S-labeled antisense riboprobe corresponding in sequence to the segment of the receptor coding region deleted in the M4KO mice (Fig. 1a) was used as probe. Studies with brains derived from wild-type mice showed that M4 transcripts were particularly abundant in higher brain regions such as cerebral cortex, striatum (caudate-putamen), hippocampus, and olfactory bulb (Fig. 2). As expected, only background staining was found when brains derived from M4−/− mutant mice were analyzed, indicative of the absence of functional M4 receptor transcripts.
Figure 2.
In situ hybridization analysis of M4 receptor mRNA expression in wild-type and M4 receptor mutant mice. Horizontal brain sections obtained from mice of the indicated genotypes were analyzed. A 35S-labeled ribonucleotide antisense probe corresponding in sequence to the genomic fragment that was deleted during the construction of the M4 targeting vector (see Fig. 1a) was used as a probe. CPu, caudate-putamen; Cx, cerebral cortex; Hc, hippocampus; OB, olfactory bulb.
In addition, radioligand-binding studies were carried out by using a saturating concentration (2 nM) of the nonselective muscarinic antagonist, [3H]QNB, which labels all five muscarinic receptor subtypes. These studies showed that the number of specific [3H]QNB-binding sites was reduced considerably, by about 25–40% (P < 0.001), in cerebral cortex, striatum, and olfactory bulb of M4−/− mutant mice (n = 3–4; data not shown).
Immunoprecipitation Assays.
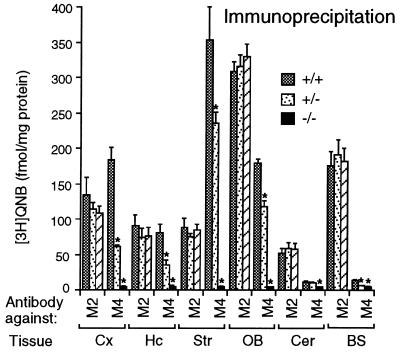
Immunoprecipitation studies were carried out to confirm the absence of functional M4 receptors in the M4 receptor mutant mice and to exclude the possibility that loss of M4 receptors may have led to compensatory changes in the expression of the functionally closely related M2 receptor subtype (8, 9). For these studies, M2 and M4 receptor-selective antisera were raised against nonconserved regions of the third cytoplasmic loops of the mouse M2 and M4 receptor proteins (21). Membranes derived from different brain regions of wild-type and M4 receptor mutant mice first were incubated with [3H]QNB, followed by immunoprecipitation of solubilized, radiolabeled receptors with anti-M4 or anti-M2 antisera. Studies with the anti-M4 antiserum confirmed that M4 receptors are highly expressed in several different forebrain regions in wild-type mice and that M4−/− mutant mice lacked functional M4 receptors (Fig. 3). Use of the anti-M2 antiserum demonstrated that M2 receptor densities remained virtually unchanged in the M4 receptor mutant mice, as compared with their wild-type littermates (Fig. 3).
Figure 3.
Immunoprecipitation analysis of muscarinic receptor expression. Immunoprecipitation studies were carried out as described in Materials and Methods, using [3H]QNB-labeled receptors solubilized from the indicated brain regions of wild-type and M4 receptor mutant mice. M2 or M4 muscarinic receptors were immunoprecipitated with subtype-specific anti-M2 or anti-M4 rabbit antisera, respectively. Cx, cerebral cortex; Hc, hippocampus; Str, striatum; OB, olfactory bulb; Cer, cerebellum; BS; brain stem. Data are given as means ± SD (n = 3–4 for each dose and genotype). ∗, P < 0.001 (Student’s t test).
Tremor, Salivation, and Body Temperature Responses.
To examine the potential involvement of the M4 receptor subtype in mediating muscarinic receptor-dependent tremor, salivation, and hypothermia, wild-type and M4 receptor mutant mice were injected with increasing doses of the centrally active, nonselective muscarinic agonist, OXO (0.01–0.3 mg/kg s.c.). OXO administration resulted in dose-dependent whole-body tremor, salivation, and hypothermia (21). Maximum responses usually were observed after administration of 0.3 mg/kg OXO. In general, OXO dose-response curves for tremor, salivation, and hypothermia responses remained largely unaffected in the M4 receptor-deficient mutant mice, as compared with their wild-type littermates (n = 16–20 for each dose and genotype) (data not shown).
Muscarinic Receptor-Mediated Analgesia.
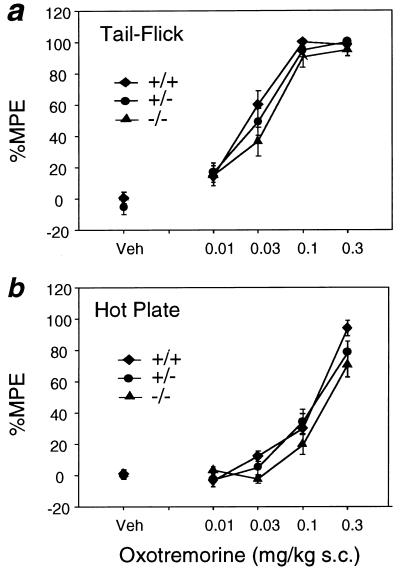
The tail-flick and hot-plate tests were used to examine the potential involvement of the M4 receptor subtype in muscarinic receptor-mediated analgesia (Fig. 4). Injection of OXO (0.01–0.3 mg/kg s.c.) induced strong dose-dependent analgesic effects in wild-type control mice in both tests. As shown in Fig. 4, M4 receptor mutant mice displayed OXO-dependent analgesic responses that were very similar to those seen with their wild-type littermates.
Figure 4.
OXO-induced antinociceptive responses in M4 receptor mutant mice. (a) Tail-flick test. (b) Hot-plate assay. Mice of the indicated genotypes were injected s.c. with vehicle (Veh) or increasing doses of the nonselective muscarinic agonist, OXO. Analgesia measurements were carried out as described in Materials and Methods. Data are presented as means ± SEM (n = 18–20 for each dose and genotype) and are expressed as the percentage of MPE. Analgesic responses were not significantly different among the various genotypes (ANOVA).
Locomotor Activity Measurements. Coordinated locomotor activity depends on the proper balance of muscarinic cholinergic and dopaminergic neurotransmission in the striatum (2, 17, 18). Because the M4 receptor subtype is particularly abundant in the striatum, we initially examined whether the loss of M4 receptors had any effect on spontaneous locomotor activity determined in an “open-field” test. We found that M4 receptor-deficient M4−/− mutant mice showed a significant increase in basal locomotor activity (by about 30% as compared with their wild-type littermates), as measured by the total number of consecutive photobeam interruptions during a 60-min test period (Fig. 5a). Examination of the time course of this effect showed that the M4 receptor KO mice were more active than their wild-type littermates during each 10-min interval of the 60-min observation period (Fig. 5b).
Figure 5.
Basal locomotor activity of M4−/− receptor mutant mice and their wild-type littermates. (a) Number of total photobeam breaks during a 1-hr observation period (n = 115) studied with vehicle-injected mice. (b) Time course of basal locomotor activity studied with vehicle-injected mice (n > 100 for each data point). Number of total photobeam breaks were measured in 10-min intervals. Locomotor activity measurements were carried out as described in Materials and Methods. Data are given as means ± SEM. ∗, P < 0.05 (Newman–Keuls post hoc comparison).
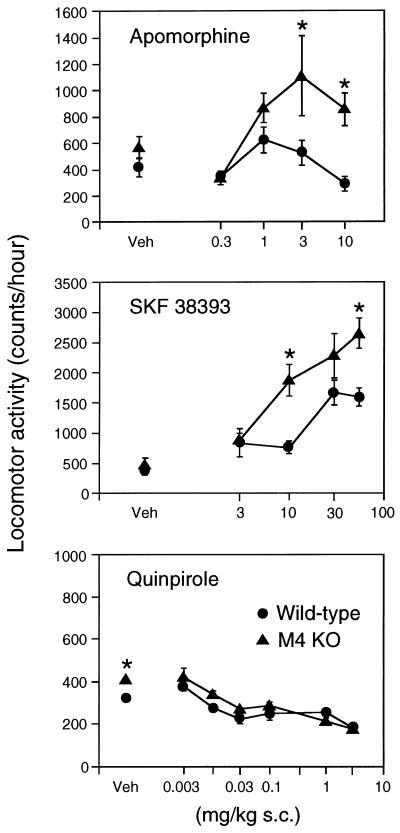
Previous studies have shown that the M4 receptor subtype is colocalized with D1 and D2 dopamine receptors on striatal projection neurons (12–16). We therefore wanted to study whether locomotor responses induced by injection of dopamine receptor agonists and antagonists were altered in M4−/− mutant mice. In wild-type mice, injection of the nonselective dopaminergic agonist, apomorphine (0.3–10 mg/kg s.c.), resulted in a bell-shaped dose-response curve, characterized by a modest increase in locomotor activity at the two intermediate doses (1 and 3 mg/kg) (Fig. 6). Strikingly, the stimulatory locomotor effects of apomorphine were enhanced greatly in M4 receptor KO mice, an effect that was most pronounced at the two highest apomorphine doses used (Fig. 6).
Figure 6.
Effect of dopamine receptor agonists on the locomotor activity of M4−/− receptor mutant mice and their wild-type littermates. Mice of the indicated genotypes were injected s.c. with vehicle (Veh) or increasing doses of the following dopamine receptor agonists: apomorphine (nonselective), SKF 38393 (D1-type selective), and quinpirole (D2-type selective). Locomotor activity measurements were carried out as described in Materials and Methods. Data (number of total photobeam breaks during a 1-hr observation period) are given as means ± SEM (n ≥ 14 for each dose and genotype). ∗, P < 0.05 (Newman–Keuls post hoc comparison).
To examine whether the apomorphine-dependent hypermotility observed with the M4−/− mutant mice involved the activation of D1 or D2 dopamine receptors, we also measured locomotor responses after administration of SKF 38393, an agonist selective for dopamine receptors of the D1 class (D1, D5), and of quinpirole, an agonist selective for dopamine receptors of the D2 class (D2, D3, and D4). In wild-type animals, SKF 38393 (3–56 mg/kg s.c.) produced a dose-dependent increase in locomotor activity, whereas administration of quinpirole (0.003–3 mg/kg s.c.) resulted in a dose-dependent inhibition of locomotor responses (Fig. 6). Notably, the stimulatory responses to injection of the D1-type agonist SKF 38393 were found to be enhanced greatly in the M4 receptor KO mice (Fig. 6). In contrast, the inhibitory locomotor responses induced by the D2-type agonist, quinpirole, were similar in wild-type and M4−/− mutant mice (Fig. 6). Taken together, these data indicate that D1 receptor-mediated increases in locomotor activity are potentiated in the absence of functional M4 receptors.
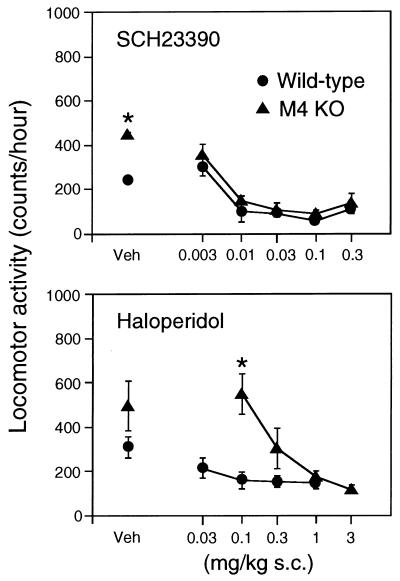
We next measured locomotor responses induced by administration of the D1-type antagonist, SCH 23390 (0.003–0.3 mg/kg s.c.), and of the D2-type antagonist, haloperidol (0.03–3 mg/kg s.c.). Consistent with previous studies (28–30), injection of either antagonist into wild-type mice resulted in a reduction in locomotor activity (Fig. 7). SCH 23390-injected M4−/− mutant mice displayed inhibitory locomotor responses similar to those observed with their wild-type littermates. On the other hand, haloperidol-dependent reduction of locomotor activity required higher haloperidol doses (≥0.3 mg/kg) in the M4 receptor KO mice (Fig. 7), probably because the locomotor-depressing effect of haloperidol was counteracted more effectively by stimulatory D1 receptors in the mutant mice lacking inhibitory M4 receptors.
Figure 7.
Effect of dopamine receptor antagonists on the locomotor activity of M4−/− receptor mutant mice and their wild-type littermates. Mice of the indicated genotypes were injected s.c. with vehicle (Veh) or increasing doses of SCH 23390, a D1-type receptor antagonist, or haloperidol, a D2-type receptor antagonist. Locomotor activity measurements were carried out as described in Materials and Methods. Data (number of total photobeam breaks during a 1-hr observation period) are given as means ± SEM (n = 13–16 for each dose and genotype). ∗, P < 0.05 (Newman–Keuls post hoc comparison).
Determination of Striatal Dopamine Receptor Densities and Monoamine Levels. Radioligand-binding studies were carried out to exclude the possibility that the hyperlocomotor effects in M4−/− mutant mice were caused by altered striatal dopamine receptor densities. Striatal membrane preparations derived from M4−/− mutant mice and their wild-type littermates were incubated with saturating concentrations of the D1-type antagonist, [3H]SCH 23390 (4 nM), or the D2-type antagonist, [3H]spiperone (2 nM). These studies showed that the number of specific [3H]SCH 23390- and [3H]spiperone-binding sites were similar in striatal preparations from wild-type and M4 receptor mutant mice. Specifically, the following receptor densities were determined (fmol/mg; n = 10): [3H]SCH 23390 (+/+, 4,560 ± 890; −/−, 5,200 ± 890) and [3H]spiperone (+/+, 2,610 ± 390; −/−, 2,690 ± 330).
In addition, striatal acetylcholine, dopamine, and DOPAC (a major dopamine metabolite) levels were determined via HPLC-coupled electrochemical detection. These studies did not reveal any significant differences in the tissue levels of these three monoamines (nmol/g tissue; n = 6): acetylcholine (+/+, 11.3 ± 0.9; −/−, 9.8 ± 0.9), dopamine (+/+, 86.8 ± 6.9; −/−, 89.7 ± 4.1), and DOPAC (+/+, 6.95 ± 0.59; −/−, 5.95 ± 0.34).
DISCUSSION
Centrally acting muscarinic agonists produce pronounced analgesic effects (31–35), suggesting that such agents are of potential clinical usefulness as potent analgesic drugs. It has been proposed, based on in vivo pharmacologic studies, that muscarinic receptor-mediated analgesia is mediated by the M4 subtype (19, 20). However, we found that OXO-induced analgesic effects were affected little in M4 receptor-deficient mice (Fig. 4), suggesting that the M4 subtype does not play a major role in mediating these effects in mice. Consistent with this observation, we reported recently that OXO-induced antinociception was reduced greatly in mice lacking functional M2 muscarinic receptors (21), demonstrating that M2 receptor stimulation is primarily responsible for muscarinic receptor-dependent analgesia. These findings highlight the usefulness of muscarinic receptor KO mice in assigning, in an unambiguous fashion, specific pharmacologic activities to defined muscarinic receptor subtypes.
OXO-injection experiments also showed that muscarinic receptor-mediated salivation, tremor, and hypothermia responses were similar in M4 receptor mutant mice and their control littermates (data not shown). Similarly, muscarinic agonist-induced tremor and salivation also were observed in M1 receptor-deficient mice (36). Interestingly, pharmacologic analysis of M2 receptor KO mice showed that muscarinic agonist-dependent hypothermia and tremor are primarily (hypothermia) or exclusively (tremor) mediated by the M2 subtype (21). Moreover, pharmacologic evidence suggests that muscarinic receptors mediating salivary (glandular) secretion are predominantly of the M3 subtype (1–3, 8).
The M4 muscarinic receptor is expressed abundantly in the striatum (4–7, 10, 11), a region known to be critically involved in the regulation of extrapyramidal motor activity. A proper balance between striatal muscarinic cholinergic and dopaminergic neurotransmission is required for coordinated locomotor control (2, 17, 18). Consistent with this notion, both dopamine receptor agonists and muscarinic receptor antagonists are clinically useful in the treatment of Parkinson’s disease, a movement disorder caused by the loss of striatal dopamine (2, 17). In situ hybridization histochemical studies (12–16) have shown that D1 and D2 dopamine receptors are expressed abundantly in the striatum, where they are localized on different subpopulations of spiny projection neurons that comprise more than 90% of striatal neurons. Whereas the D1 subtype is localized to striatal neurons that project directly to the substantia nigra (striatonigral pathway), the D2 subtype is expressed primarily by striatal neurons that project to the globus pallidus (striatopallidal pathway). Interestingly, almost all D1 receptor-expressing striatal projection neurons also express M4 (as well as M1) muscarinic receptors, whereas less than half of the D2 receptor-expressing striatal projection neurons express the M4 subtype (13, 16). The source of acetylcholine acting on these striatal muscarinic receptors are the large, striatal cholinergic interneurons that are known to be tonically active (reviewed in ref. 18). To study the potential modulatory role of the M4 receptor subtype in dopamine receptor-dependent regulation of locomotor activity, we therefore examined locomotor responses to administration of different dopamine receptor agonists and antagonists in wild-type and M4 receptor-deficient mice.
We initially demonstrated that M4 receptor KO mice showed a significant increase in basal locomotor activity (Fig. 5). Moreover, the locomotor stimulatory effects of apomorphine, a nonselective dopamine receptor agonist, and SKF 38393, a selective D1-type receptor agonist, were enhanced greatly in M4 receptor-deficient mice (Fig. 6). Given the high abundance of D1 dopamine and M4 muscarinic receptors in the striatum, their colocalization on striatonigral projection neurons, and the known importance of these neurons in the regulation of locomotor activity, it is likely that the increased locomotor responses observed with the M4 receptor KO mice are, in fact, a result of altered striatal activity.
At a cellular level, D1 dopamine and M4 muscarinic receptors mediate opposing biochemical effects, with D1 receptor stimulation leading to an increase in adenylyl cyclase activity (37) and M4 receptor activation resulting in an inhibition of adenylyl cyclase (8, 9). Moreover, Onali and coworkers (38, 39) demonstrated that D1 receptor-mediated increases in cAMP levels in striatal membrane preparations can be inhibited by concomitant stimulation of muscarinic receptors, which are probably of the M4 subtype. These observations suggest a possible molecular mechanism by which M4 receptors located on striatonigral projection neurons can counteract the increase in neuronal activity after D1 receptor activation (18). It is likely that the increased locomotor responses displayed by the M4 receptor-deficient mice are caused by an increased activity of the striatonigral pathway because of the lack of inhibitory striatal M4 receptors.
It should be noted that all mice analyzed in this study (as well as in most other studies using gene-targeted mice) were of a mixed genetic background (129SvEv/CF-1 hybrids). As discussed by Gerlai (40), caution must be exercised in properly interpreting the phenotypical changes observed in KO mice of such genetic background. However, it is known that the 129SvEv mice, from which the targeted ES cells were derived, display reduced rather than increased locomotor activity (40, 41). This observation virtually excludes the possibility that the observed increases in locomotor activity observed with the M4 receptor-deficient mice are caused by 129SvEv genes that are linked to the KO locus.
Studies with pirenzepine and other muscarinic antagonists of limited receptor subtype selectivity suggest that M1 receptors may play a role in higher cognitive functions such as memory and learning (1, 3). However, virtually all antagonists that display high affinity for M1 receptors also show high affinity for M4 receptors (8). The availability of the M4 receptor KO mice therefore will offer the opportunity to study the possible involvement of M4 receptors in memory and learning processes.
Taken together, our findings suggest that the clinical usefulness of muscarinic antagonists in improving locomotor deficits in patients suffering from Parkinson’s disease and other extrapyramidal motor disorders is due, at least in part, to the blockade of inhibitory striatal M4 muscarinic receptors. The development of centrally acting, selective M4 receptor antagonists therefore remains an attractive therapeutic goal, because such agents are predicted to lack most of the bothersome side effects associated with the use of classical nonselective muscarinic antagonists, including dry mouth, blurred vision, and cognitive impairment (2). The M4 receptor-deficient mice described here should serve as a valuable tool for studying the role of the M4 muscarinic receptor in striatal function and the regulation of extrapyramidal motor control.
Acknowledgments
We thank G. Aronson, A. Cummins, and C. R. Gerfen for carrying out the in situ hybridization studies; S. C. Peters and C. Li for expert technical assistance; C. Paszty for providing the pPN2T targeting vector; M. Weinstein and D. Accili for advice and helpful discussions; and A. M. Spiegel for generous support of this work.
ABBREVIATIONS
- ES cells
embryonic stem cells
- KO
knockout
- MPE
maximum possible effect
- OXO
oxotremorine
- QNB
quinuclidinyl benzilate
References
- 1.Levine R R, Birdsall N J M, editors. Life Sci. 1995;56:801–1050. [Google Scholar]
- 2.Brown J H, Taylor P. In: The Pharmacological Basis of Therapeutics. 9th Ed. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 141–160. [Google Scholar]
- 3.Levine R R, Birdsall N J M, editors. Life Sci. 1997;60:963–1207. [Google Scholar]
- 4.Levey A I. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 5.Vilaro M T, Mengod G, Palacios J M. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe B B, Yasuda R P. Ann N Y Acad Sci. 1995;757:186–193. doi: 10.1111/j.1749-6632.1995.tb17474.x. [DOI] [PubMed] [Google Scholar]
- 7.Levey A I, Kitt C A, Simonds W F, Price D L, Brann M R. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caulfield M P. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 9.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda R P, Ciesla W, Flores L R, Wall S J, Li M, Satkus S A, Weisstein J S, Spagnola B V, Wolfe B B. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 11.Hersch S M, Gutekunst C-A, Rees H D, Heilman C J, Levey A I. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerfen C R, Engber T M, Mahan L C, Susel Z, Chase T N, Monsma F J, Jr, Sibley D R. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 13.Weiner D M, Levey A I, Brann M R. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Moine C, Normand E, Guitteny A F, Fouque B, Teoule R, Bloch B. Proc Natl Acad Sci USA. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Moine C, Normand E, Bloch B. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard V, Normand E, Bloch B. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornykiewicz O. In: Movement Disorders. Marsden D C, Fahn S, editors. Boston: Butterworth; 1981. pp. 41–58. [Google Scholar]
- 18.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 19.Shannon H E, Womer D E, Bymaster F P, Calligaro D O, Delapp N W, Mitch C H, Ward J S, Whitesitt C A, Swedberg M D, Sheardown M J, et al. Life Sci. 1997;60:969–976. doi: 10.1016/s0024-3205(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 20.Ellis J L, Harman D, Gonzalez J, Spera M L, Liu R, Shen T Y, Wypij D M, Zuo F. J Pharmacol Exp Ther. 1999;288:1143–1150. [PubMed] [Google Scholar]
- 21.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paszty C, Mohandas N, Stevens M E, Loring J F, Liebhaber S A, Brion C M, Rubin E M. Nat Genet. 1995;11:33–39. doi: 10.1038/ng0995-33. [DOI] [PubMed] [Google Scholar]
- 23.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 24.Gerfen C R, Keefe K A, Gauda E B. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann M R. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 26.Farrell C B, Lawlor M, Dunne A, O’Boyle K M. J Neurochem. 1995;65:1124–1130. doi: 10.1046/j.1471-4159.1995.65031124.x. [DOI] [PubMed] [Google Scholar]
- 27.Sacaan A I, Bymaster F P, Schoepp D D. J Neurochem. 1992;59:245–251. doi: 10.1111/j.1471-4159.1992.tb08897.x. [DOI] [PubMed] [Google Scholar]
- 28.Starr B S, Starr M S. Neuropharmacology. 1986;25:455–463. doi: 10.1016/0028-3908(86)90168-1. [DOI] [PubMed] [Google Scholar]
- 29.Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. Psychopharmacology. 1991;105:335–339. doi: 10.1007/BF02244427. [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Moratalla R, Gold L H, Hiroi N, Koob G F, Graybiel A M, Tonegawa S. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 31.Yaksh T L, Dirksen R, Harty G J. Eur J Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- 32.Green P G, Kitchen I. Prog Neurobiol. 1986;26:119–146. doi: 10.1016/0301-0082(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 33.Hartvig P, Gillberg P G, Gordh T, Jr, Post C. Trends Pharmacol Sci. 1989;10,Suppl.:75–79. [PubMed] [Google Scholar]
- 34.Iwamoto E T, Marion L. J Pharmacol Exp Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- 35.Swedberg M D, Sheardown M J, Sauerberg P, Olesen P H, Suzdak P D, Hansen K T, Bymaster F P, Ward J S, Mitch C H, Calligaro D O, et al. J Pharmacol Exp Ther. 1997;281:876–883. [PubMed] [Google Scholar]
- 36.Hamilton S E, Loose M D, Qi M, Levey A I, Hille B, McKnight G S, Idzerda R L, Nathanson N M. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibley D R, Monsma F J., Jr Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 38.Olianas M C, Adem A, Karlsson E, Onali P. Br J Pharmacol. 1996;118:283–288. doi: 10.1111/j.1476-5381.1996.tb15400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olianas M C, Onali P. Br J Pharmacol. 1996;118:827–828. doi: 10.1111/j.1476-5381.1996.tb15474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 41.Kelly M A, Rubinstein M, Phillips T J, Lessov C N, Burkhart-Kasch S, Zhang G, Bunzow J R, Fang Y, Gerhardt G A, Grandy D K, Low M J. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]