Abstract
Female mice lacking the gene encoding the prostaglandin (PG) E2 receptor subtype EP2 (EP2−/−) become pregnant and deliver their pups at term, but with a much reduced litter size. A decrease in ovulation number and a much reduced fertilization rate were observed in EP2−/− females without difference of the uterus to support implantation of wild-type embryos. Treatment with gonadotropins induced EP2 mRNA expression in the cumulus cells of ovarian follicles of wild-type mice. The immature cumuli oophori from wild-type mice expanded in vitro in response to both follicle-stimulating hormone and PGE2, but the response to PGE2 was absent in those from EP2−/− mice. Cumulus expansion proceeded normally in preovulatory follicles but became abortive in a number of ovulated complexes in EP2−/− mice, indicating that EP2 is involved in cumulus expansion in the oviduct in vivo. No difference in the fertilization rate between wild-type and EP2−/− mice was found in in vitro studies using cumulus-free oocytes. These results indicate that PGE2 cooperates with gonadotropin to complete cumulus expansion for successful fertilization.
Keywords: gene targeting, fertilization, ovulation, prostanoids, gonadotropins
Ovulation and fertilization are key processes in mammalian female reproduction, which is highly regulated by pituitary gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone. These hormones induce a number of preovulatory processes, including follicular development, oocyte maturation, cumulus expansion, and rupture of antral follicles (1). The ovulated eggs move to the oviducts, and timely interaction between an egg and a sperm then leads to successful fertilization (2). Undoubtedly, these processes are initiated by the gonadotropins, but how gonadotropins regulate these processes remains unclear. It is known that some gonadotropin actions are mediated by other mediators. Prostanoids, the cyclooxygenase (COX)-metabolites of arachidonate, likely mediate the ovulatory actions of gonadotropins (3) because aspirin-like drugs that inhibit COX have been reported to inhibit spontaneous and gonadotropin-primed ovulation in many species (4). Prostaglandin E2 (PGE2), a dominant prostanoid in the ovary, can reverse the inhibitory effect of aspirin-like drugs, when administered simultaneously. These results suggest that some of the steps of ovulation are mediated by this prostanoid (5). Indeed, luteinizing hormone surge leads to high expression of COX-2 in granulosa cells (6, 7), and a large amount of PGE2 is produced and released into the antral fluid (8). Recently, the importance of prostanoids in early pregnancy has been definitely shown in mice deficient in COX-2. The COX-2−/− animals showed a reduction in ovulation number and severe failure in fertilization as well as defects in the ability of the uterus to receive implants and to undergo decidualization (9). The result that not only ovulation but fertilization was also severely affected indicates that prostanoids play a role in one of a series of preovulatory processes that are required for both ovulation and fertilization.
One of the processes in which PGE2 possibly plays a role is the cumulus expansion, which also is triggered by the endogenous preovulatory surge of gonadotropins (10). The cumulus oophorus begins to expand dramatically when the cumulus cells, consisting of a few layers of granulosa cells closely surrounding the oocyte, are induced to synthesize hyaluronic acid, which aggregates in a viscous intercellular matrix (11). This expansion facilitates the extrusion of the oocyte through the ruptured follicle wall during ovulation and assists its capture by the oviductal fimbria and its entry into the oviduct (12). This process can be mimicked in vitro by treating an immature mouse cumulus oophorus with FSH and cAMP analogues (13). Although PGE2 elicits expansion by itself and enhances FSH-induced expansion, it remains unclear whether endogenous PGE2 truly contributes to these steps because indomethacin failed to inhibit FSH-induced expansion (14).
Actions of PGE2 are mediated by cell-surface receptors specific to this prostanoid (15, 16), and, to date, four receptor subtypes, EP1, EP2, EP3 and EP4, have been identified and cloned (reviewed in ref. 17). To examine the roles of PGE2 in the reproductive and other systems, we disrupted the gene encoding the EP2 by homologous recombination. Our data show that PGE2 cooperates with gonadotropin to complete cumulus expansion for successful fertilization.
MATERIALS AND METHODS
Gene Targeting of the EP2 Gene.
A 10.9-kilobase KpnI-EcoRV genomic fragment spanning the area starting 8.2-kilobases 5′-upstream of the EP2 transcription initiation site to 1.7-kilobases 3′-downstream of the end of exon 1 was subcloned into pBluescript SK(+) (Stratagene). A 1.8-kilobase fragment containing the coding region from Asp-2 in the N-terminal region to Thr-281 in the sixth transmembrane domain (18) was replaced by the neomycin-resistant gene. The MC1-herpes simplex virus thymidine kinase gene was inserted at the 3′ end of the homologous region. Two lines of resultant embryonic stem (E14–1) cells injected into C57BL/6 blastocysts gave rise to chimeric offspring, which in turn were mated with C57BL/6 females as described (19, 20). In the following experiments, mice homozygous for the mutated or wild-type allele in the background of (129/Ola × C57BL/6) F2 were examined. For Southern blot analysis, 10 μg of genomic DNA was digested with ScaI, subjected to hybridization analysis. Analysis of RNA prepared from the uterus at day 5 of pregnancy of wild-type and EP2−/− with EP2-specific cDNA probes verified the loss of EP2 expression in EP2−/− mice. Trachea were isolated from adult animals and were subjected to bioassay by the method of Magnus (21). Carbachol (0.2 μM) was used to induce contraction. PGE2 (0.1 μM) and butaprost (0.1 μM) were used to induce relaxation.
Litter Size, Ovulation, and Fertilization.
Female mice were mated with male mice in various combination of genotypes, and their litter sizes and the number of corpora lutea were examined on day 19 of pregnancy. The estrous cycle was monitored by vaginal smears. To examine normal ovulation and fertilization, wild-type and EP2−/− mice were bred with fertile wild-type C57BL/6 males. To induce superovulation, female mice (3 weeks old) were injected intraperitoneally with pregnant mare serum gonadotropin (PMSG) (5 units/mouse) and with human chorionic gonadotropin (hCG) (5 units/mouse) 48 h later. These mice then were housed with males overnight. The day when a vaginal plug was found in the morning was designated as day 1 of pregnancy. Mice were killed on day 1; oviducts were flushed with saline to recover eggs or embryos, and their morphology was examined under a microscope. The eggs with pronuclei or in the two-cell stage were taken as fertilized. The number of preovulatory follicles was examined by sectioning of the ovaries isolated from mice 10 h after hCG injection. The significance of difference was evaluated statistically by using the Student’s t test.
Embryo Transfer.
Pseudopregnancy in wild-type or EP2−/− recipients was induced by mating with wild-type vasectomized males. Day-4 wild-type blastocysts were transferred into the uteri of these recipients on day 4. On day 14, the number of implants were recorded. Embryo transfer experiments were independently repeated twice, and similar results were obtained.
In Situ Hybridization.
In situ hybridization was performed as described (22). Antisense 35S-labeled cRNA probes were generated by the appropriate polymerases from mouse cDNAs specific to EP2 and COX-2 for in situ hybridization (23). The probes had specific activities of ≈2 × 109 dpm/μg. The specificity of the signals for each probe was verified by its disappearance when excess amount of unlabeled probe was added (data not shown). In situ hybridizations were repeated three times for each time point studied.
In Vitro Expansion of the Cumuli Oophori.
Wild-type or EP2−/− females, 24 days old, were primed with PMSG. Forty-eight hours later, the cumuli oophori were isolated by puncturing the large Graafian follicles with 27-gauge needles in PBS containing 30 mg/ml BSA (24). After extensive washing, the cumuli oophori were transferred into 50 μl of Eagle’s minimum essential medium containing 5% fetal bovine serum. The cumulus–oocyte complexes were dispersed with micropipets. FSH (500 ng/ml), PGE2 (1 μM), butaprost (1 μM), or dibutyryl cyclic AMP (2 mM) were added into the media, and the complexes were incubated at 37°C under 5% CO2 for 18–20 h. The extent of expansion was evaluated morphologically by using a differential interference contact microscope, and physically as loss of adhesion to plastic dishes. In vitro expansion experiments were independently repeated five times. The cumulus expansion in vivo was assessed by morphological examination of the postovulatory cumulus–oocyte complexes before fertilization, which were recovered from the oviducts of superovulated mice in 12 and 14 h post-hCG treatment. The experiments were independently repeated three times for each time point.
In Vitro Fertilization.
In vitro fertilization was essentially performed as described elsewhere (25, 26). Wild-type male mice were killed, and their epididymides were resected and punctured with a needle. A white drop of sperm was introduced into α-modification of Eagle’s medium containing 30 mg/ml of BSA covered by mineral oil, followed by incubation for 2 h at 37°C under 5% CO2. Cumulus–oocyte complexes were collected from wild-type or EP2−/− oviducts 14 h after hCG treatment and then were introduced into a drop of α-modification of Eagle’s medium equilibrated under the same conditions as for sperm. Cumulus-free oocytes were prepared by 0.1% hyaluronidase treatment and by removal of the cumulus layer as described (27). Medium containing sperm was added to a drop containing oocytes to give a final concentration of 200 sperm/μl. After 6 and 12 h of incubation, the oocytes were fixed with 4% formalin. Fertilized eggs were identified by the formation of pronuclei.
RESULTS
Disruption of EP2 Receptor Gene.
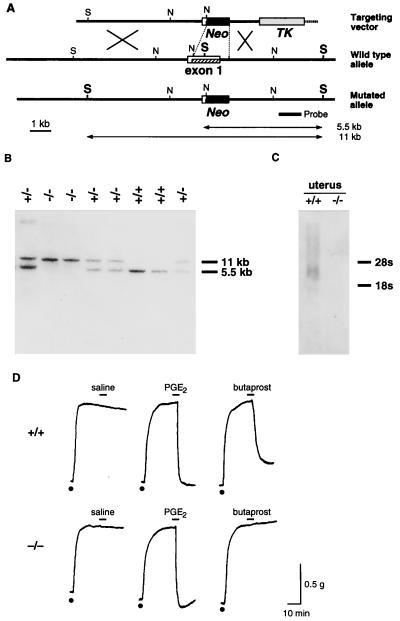
A targeting vector was constructed so that the coding region of the first exon of the EP2 receptor gene could be replaced with the neomycin-resistant gene (Fig. 1A). Crossing these mice yielded homozygous animals as determined by Southern blot analysis (Fig. 1B) with a frequency of 26.7% (n = 101), indicating that mice lacking EP2 receptors can develop normally to birth. Disruption of the EP2 receptor gene was verified in uterine tissue by Northern blot analysis; mRNA expression disappeared in the homozygous mice (Fig. 1C). Disruption of EP2 receptor expression also was verified functionally by examining butaprost-induced relaxant activity in trachea. No relaxant response was observed by butaprost, an EP2-specific agonist, in the trachea obtained from homozygous mice (Fig. 1D).
Figure 1.
Disruption of the gene encoding the EP2 receptor. (A) Strategy used for EP2 gene targeting. Construct of the targeting vector, organization of the EP2 gene, and the structure of the targeted genome are shown. Restriction sites are indicated: S, ScaI; N, NcoI. The thick line indicates the DNA probe used in Southern hybridization. TK, thymidine kinase gene; Neo, neomycin-resistant gene. (B) Southern blot analysis of ScaI-digested genomic DNA from a representative litter of 8 pups. +/+, wild-type; +/−, heterozygote; −/−, homozygote. (C) Northern blot analysis. Total RNA was isolated from a mouse uterus on day 5 of pregnancy and was used for hybridization with an exon 1-specific probe. (D) Loss of butaprost-induced relaxation of the trachea in EP2−/− mice. Carbachol (dots), 0.2 μM, was used to induce contraction. PGE2, 0.1 μM, and butaprost, 0.1 μM, were used to induce relaxation.
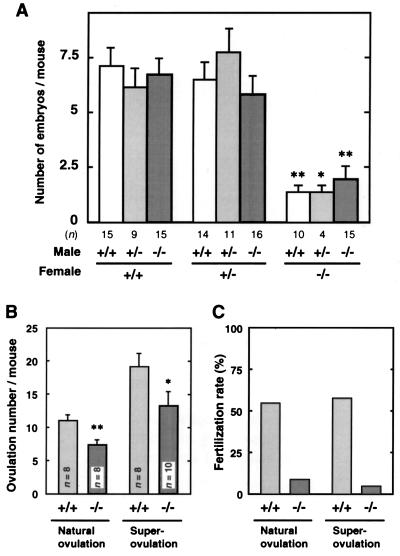
Impaired Fertility in EP2−/− Mice. Heterozygous mice appeared normal and, when interbred, yielded litters of normal size with a Mendelian genetic distribution. EP2−/− mice develop normally, gain weight at a rate equal to that of wild-type animals and have lifespans of >1 year. Male EP2−/− mice had normal fertility when crossed with wild-type or heterozygous females. Female EP2−/− mice are normal in their estrous cycle, become pregnant and deliver their pups at term, but their litter sizes are always small irrespective of the genotypes of the mating males. Very few pups (≈1.0 per pregnancy, on average) were born alive and grew normally. Such reduction in litter size was observed when we examined live fetuses in utero on day 19; the average litter number in EP2−/− was 1.5 whereas that in wild-type was 7.1 (Fig. 2A). In addition, this decrease was observed in the number of implants at day 7 of pregnancy. Hence, the decreased number of pups born to EP2−/− mothers is attributable to maternal problems affecting ovulation, fertilization, implantation, or decidualization.
Figure 2.
Impaired ovulation and fertilization in EP2−/− mice. (A) Decrease in litter size in EP2−/− female mice. Female mice were mated with male mice in the indicated combination of genotypes, and their litter sizes were examined on day 19 of pregnancy. The results are shown as the mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01 (vs. wild-type females). (B and C) Ovulation and fertilization rates in natural and super-ovulation. Mature (8-week-old) or immature (5-week-old) superovulated mice were mated with wild-type male mice. Eggs were recovered from their oviducts, and the eggs with pronuclei or in the two-cell stage were taken as fertilized. The number of ovulated eggs (B) and the fertilization rate (C) are expressed as the mean ± SEM and as a percent of the sum of fertilized eggs vs. total ovulated eggs, respectively.
Previously, we found that EP2 mRNA is expressed exclusively in the luminal epithelium on day 5 of pregnancy, when blastocyst implantation occurs (22). From these results, we suspected that EP2-deficiency may affect implantation and the following processes, and we investigated blastocyst implantation in EP2−/− mice. We transferred wild-type blastocysts to day-4 pseudopregnant mice (Table 1) and counted the number of implants on day 14 of pregnancy. However, no significant difference was observed in the number of implants in the EP2−/− mice, suggesting that implantation and decidualization occurred normally. We then examined the number of ovulated eggs and fertilization rate during natural ovulation. As shown in Fig. 2 B and C, 55% (44/88, n = 8) of eggs recovered from wild-type females were fertilized. In contrast, slightly reduced number of eggs (59, n = 8) were recovered from EP2−/− females. Only 8.5% (5/59) of them were fertilized. Because we could not find any differences in the extrusion of the first polar body, fertilization failure was not attributable to a developmental abnormality in the eggs. These results indicate that EP2−/− females have slightly impaired ovulation and severely impaired fertilization. When EP2−/− females were further subjected to superovulation with exogenous gonadotropins, severe failures of fertilization with slightly impaired ovulation again were observed (Fig. 2 B and C). These results establish that the reduced rate of ovulation was not the result of a deficiency in pituitary gonadotropins. No significant difference was found in the number of preovulatory follicles in ovaries 10 h after hCG treatment (wild-type, 27.6 ± 2.6; EP2−/−, 29.5 ± 3.0; means ± SEM, n = 6), and a slight reduction again was observed in the number of corpora lutea in pregnant EP2−/− mice [wild-type, 8.4 ± 0.68; EP2−/−, 6.0 ± 0.87; means ± SEM, n = 8, P < 0.05 (t test)]; follicular development and corpora lutea formation occur normally in EP2−/− mice. Collectively, these results suggest that defective ovulation and fertilization appear to be the major cause of reduced fertility in EP2−/− females.
Table 1.
Implantation of wild-type blastocysts transferred to pseudopregnant wild-type or EP2−/− mice
| Genotype | No. of blastocysts transferred | No. of recipients | No. of implants (%) |
|---|---|---|---|
| EP2+/+ | 40 | 4 | 18 (45) |
| EP2−/− | 40 | 4 | 18 (45) |
Day-4 wild-type blastocysts were transferred into the uteri of wild-type (EP2+/+) or EP2−/− mice on day 4 of pseudopregnancy. Recipients were killed on day 14 to examine the implants.
Expression of EP2 mRNA in Ovarian Tissues.
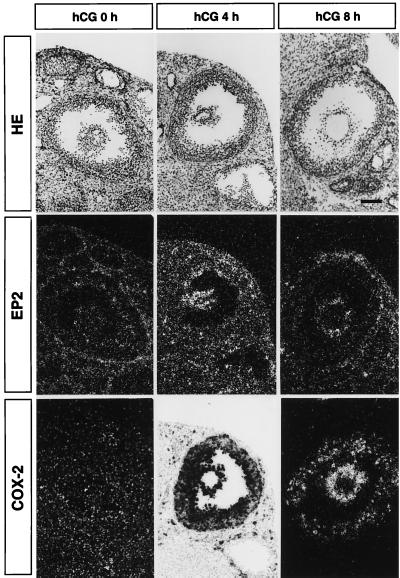
It has been demonstrated that high levels of COX-2 transcripts are induced in preovulatory follicles (6, 7). We investigated the expression of EP2 mRNA in ovarian tissues from wild-type gonadotropin-primed females, and compared it to the expression of COX-2 mRNA (Fig. 3). Faint expression of EP2 mRNA could be detected in theca cells in ovaries obtained at 48 h after PMSG treatment (hCG-0h) whereas expression of COX-2 mRNA was not detected. In the ovaries obtained from mice 4 h after injection of hCG, extremely high levels of COX-2 expression were observed in the inner layer of the follicles and also in cumulus cells. Although expression of COX-2 mRNA in granulosa cells decreased 8 h after hCG treatment, expression in cumulus cells was still high. EP2 mRNA was induced in cumulus cells but not in the other cells. This expression was still observed 8 h after hCG treatment, and also 12 h after treatment, at the time of ovulation (data not shown). Cumulus cells thus showed time-dependent expression of EP2 similar to that of COX-2. We therefore focused on the function of cumulus cells in EP2−/− mice.
Figure 3.
Expression of EP2- and COX-2-mRNA in the developing follicle detected by in situ hybridization. The photomicrographs show hematoxylin-eosin-staining (top panels), signals for EP2-mRNA (middle panels), and signals for COX-2-mRNA (bottom panels). Wild-type 4-week old females were primed with PMSG, and ovaries were isolated before hCG injection (left panels; hCG 0 h), 4 h after hCG injection (center panels; hCG 4 h), or 8 h after hCG injection (right panels; hCG 8 h). The photomicrographs showing hybridization signals were presented in a dark field-manner except for that of the COX-2 signals at 4 h post-hCG, in which extremely strong signals were seen in the bright field. (Bar = 200 μm.)
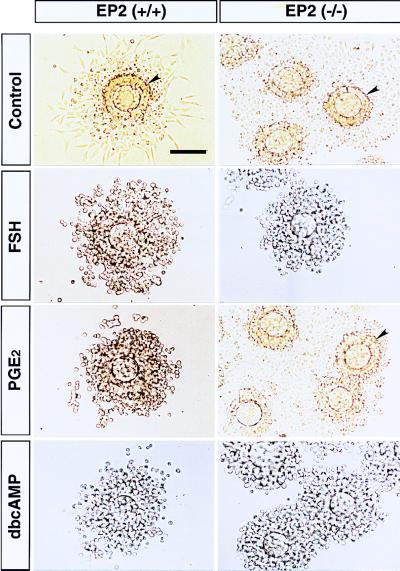
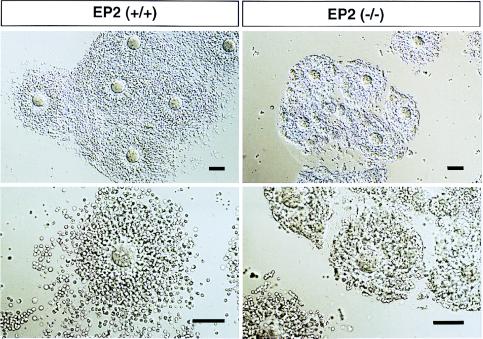
EP2 Deficiency Affects Cumulus Cell Function. One of the well known events of cumulus cells during ovulation and fertilization is expansion, which is important in both natural and gonadotropin-primed ovulation (10). Cumulus expansion is proceeded by the secretion of hyaluronic acid, a constituent of the expanded cumulus matrix, triggered in vivo by the endogenous preovulatory surge of gonadotropins (11). This process can be mimicked in vitro by treating an immature mouse cumulus oophorus with cyclic AMP analogues, FSH (10), or PGE2 (14, 24). Because ovulation is sensitive to aspirin-like drugs, PGE2 has been implicated in in vivo cumulus expansion elicited by endogenous gonadotropins (28). We first investigated the in vitro expansion of cumuli oophori isolated from EP2−/− mice (Fig. 4). In cumuli oophori from wild-type females, PGE2, as well as FSH and membrane-permeable dibutyryl cAMP, elicited in vitro expansion. However, in cumuli oophori from EP2−/− mice, PGE2 failed to elicit expansion, although expansion was seen on treatment with both FSH and dibutyryl cAMP. Butaprost also elicited expansion in cumuli oophori isolated from wild-type mice but not in those from EP2−/− mice (data not shown). These results showed that in vitro cumulus expansion elicited by PGE2 is mediated by EP2. The results also showed that cumuli oophori from EP2−/− mice are able to expand in response to gonadotropins. Indeed, the ovulation process was not severely affected in the EP2−/− mice. We next examined the morphology of ovulated cumulus–oocyte complexes recovered from the oviducts of EP2−/− mice before fertilization (Fig. 5). The complexes from wild-type animals were scattered, and some distance away from each other. These cumulus cells showed a representative radial extension. On the other hand, complexes from EP2−/− mice were irregularly aggregated, and the cumulus cells were less expanded, suggesting that EP2 deficiency prevents cumulus expansion in vivo.
Figure 4.
In vitro expansion in immature cumuli oophori from EP2−/− mice. Immature cumuli oophori were isolated from PMSG-treated wild-type (left panels) and EP2−/− mice (right panels). The isolated complexes were incubated with media only (Control), or media containing FSH (FSH), PGE2 (PGE2), or dibutyryl cAMP (dbcAMP). The expanded complexes were recovered and processed onto slides (PGE2 in wild-type, and FSH and dbcAMP in both animals). Complexes that did not expand adhered to the dishes and were processed directly (PGE2 in EP2−/−, and control in both animals). The surfaces of the complexes are indicated by arrowheads. (Bar = 100 μm.)
Figure 5.
Abortive cumulus expansion in EP2−/− females. Differential interference contrast photomicrographs of cumulus–oocyte complexes in the postovulatory period are shown. The oviductal postovulatory cumulus–oocyte complexes were isolated from wild-type (left panels) and EP2−/− (right panels) superovulated mice in 12–14 h post-hCG treatment. (Bars = 100 μm.)
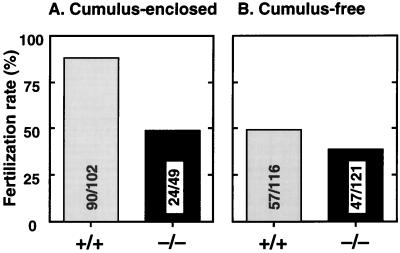
Cumulus cells are required for oocyte maturation, which is accompanied by cumulus expansion (29, 30). The less expanded cumulus cells indicate defects in cumulus cell function, and consequently oocyte maturation, in EP2−/− mice. To explore this possibility, we performed in vitro fertilization in the presence or absence of cumulus cells (Fig. 6). In cumulus-enclosed oocytes incubated with sperm for 6 h, 88.2% of the wild-type eggs were fertilized whereas only 49.0% of the EP2−/− complexes were fertilized. The removal of cumulus cells did not restore but, rather, slightly reduced the fertilization rate of EP2−/− eggs (38.8% were fertilized); EP2−/− oocytes are not fully mature for fertilization in the absence of PGE2-induced cumulus cell function. Consistently, when wild-type oocytes were stripped of cumulus cells during this assay, only 49% of the cells were successfully fertilized. Similar results were obtained in an incubation for 12 h. These results demonstrate the importance of EP2-induced cumulus expansion in final stage of oocyte maturation.
Figure 6.
In vitro fertilization in the presence (A) or absence (B) of cumulus cells. Cumulus–oocyte complexes were collected from oviducts of wild-type and EP2−/− mice 14 h after hCG injection. Cumulus-free oocytes were prepared by 0.1% hyaluronidase treatment. After incubation with sperm for 6 h, fertilized eggs were identified by the formation of pronuclei. Numbers within the bars indicate the number of fertilized eggs/total number of eggs.
DISCUSSION
One of the most remarkable findings in EP2−/− mice is reduced fertility, which is partly attributable to reduction in ovulation and predominantly attributable to severe failure of fertilization. EP2 is expressed in cumulus cells in response to gonadotropins in wild-type animals, and in vitro cumulus expansion is elicited by FSH but not by PGE2 in cumuli oophori isolated from EP2−/− mice, indicating that EP2 is involved in cumulus expansion in vitro. The EP2−/− cumulus cells of the complexes in oviducts just before fertilization were less expanded compared with wild-type cumulus cells, indicating that EP2 is involved in cumulus expansion in vivo. The in vitro fertilization experiments using cumulus-free oocytes demonstrated that impaired fertility is caused by impaired cumulus cell functions represented by incomplete expansion. Collectively, we concluded that EP2 contributes to ordered cumulus expansion required for successful fertilization.
Slightly reduced ovulation and severely impaired fertilization in EP2−/− mice are in good agreement with the results obtained with COX-2-deficient mice (9). Therefore, failures in COX-2−/− mice in early pregnancy is at least in part caused by impaired cumulus function, as found in this study. In earlier studies, indomethacin treatment was found to inhibit luteinizing hormone-induced ovulation, and the addition of PGE2 was shown to reverse its effects. These investigations have suggested that some of the ovulatory effects of luteinizing hormone surge is mediated by the actions of this prostanoid (4, 24). However, little is known about the contribution of prostanoids to fertilization. The present study demonstrated that PGE2 via EP2 contributes partly to ovulation and mainly to successful fertilization.
Previous in vitro studies reported that PGE2 not only stimulates cumulus expansion by itself but also enhances gonadotropin-induced expansion (14). However, it remains unknown how important this action of PGE2 on cumulus expansion is during and after ovulation. The results obtained in this study indicate that gonadotropins, without the actions of PGE2 via EP2, is enough for the appearance of ovulation. This is supported by the normal histology and number of preovulatory follicles, and also by the result that cumulus–oocyte complexes were sensitive to hyaluronidase treatment. In addition, the cumulus cells from EP2−/− mice have the ability to expand in response to gonadotropins. From these results, it is likely that EP2−/− mice are normal in primary events of ovulation processes, including proteolysis, but are impaired in later stage. Because quality and quantity of the preovulatory cumulus expansion influence the extrusion of the oocyte (12), it is possible that PGE2-induced expansion contributes to effective detachment of cumulus–oocyte complexes. Once ovulated, the cumulus–oocyte complexes are no longer under the control of blood-borne gonadotropins. In contrast, it was shown that oviductal cumulus cells still showed COX-2 expression and secreted PGE2 after ovulation. It is therefore suggested that ovulated cumulus cells might be regulated by PGE2 but not by gonadotropins. In this respect, the severe failure of fertilization in EP2−/− mice is possibly the result of the loss of PGE2-induced expansion in vivo. EP2 appears to be responsible for the continuous expansion of cumulus cells in an autocrine manner for successful fertilization. Impaired fertilization in EP2−/− mice is presumably the result of incomplete oocyte maturation because cumulus-oocyte interactions are considered important for the production of fertilization-competent eggs (2, 29). The disordered expansion may interfere with these interaction. Thus, these results established that PGE2 and EP2 have a crucial role in the completion of cumulus-oocyte maturation under the local condition that gonadotropin is not able to control.
During the preparation of this manuscript, generation of EP2−/− mice with reduced fertility was reported (31). However, this report only stated that impaired ovulation and fertilization on normal ovulation occurred but did not address the mechanistic aspects of this failure. In addition to the roles in ovulation and fertilization clarified in the present study, prostanoids are considered to participate in implantation and decidualization (32). EP2 has been thought to participate in implantation or the following processes because of its specific expression in the luminal epithelium during the peri-implantation period (22). However, no abnormalities were found in these processes in EP2−/− mice. One possibility is that EP4 showing expression in the luminal epithelium compensates for the role of EP2 in implantation. Double-knockouts of the EP2 and EP4 genes may help to address this issue.
Acknowledgments
We appreciate Dr. Y. Masuda and Dr. K. Nakanishi, Shiga University of Medical Science, for their assistance with embryonic transfer techniques and fundamental procedures in female reproduction biology. We thank Dr. Y. Aze, Ono Pharmaceutical Company, for his support of histological analysis. We are grateful to Miss H. A. Popiel for careful reading. This work was supported by grants from the Ministry of Education, Science and Culture, Japan.
ABBREVIATIONS
- PG
prostaglandin
- COX
cyclooxygenase
- FSH
follicle-stimulating hormone
- hCG
human chorionic gonadotropin
- PMSG
pregnant mare serum gonadotropin
References
- 1.Tsafriri A, Chun S-Y. In: Reproductive Endocrinology, Surgery, and Technology. Adashi E Y, Rock J A, Rosenwaks Z, editors. Philadelphia: Lippincott; 1996. pp. 235–249. [Google Scholar]
- 2.Wassarman P M, Albertini D F. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 79–122. [Google Scholar]
- 3.Goldberg V J, Ramwell P W. Physiol Rev. 1975;55:325–351. doi: 10.1152/physrev.1975.55.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch W J, Hansen T R, McPherson L A. Prostaglandins. 1993;46:85–115. doi: 10.1016/0090-6980(93)90037-8. [DOI] [PubMed] [Google Scholar]
- 5.Wallach E E, Wright K H, Hamada Y. Am J Obstet Gynecol. 1978;132:728–738. doi: 10.1016/s0002-9378(78)80007-6. [DOI] [PubMed] [Google Scholar]
- 6.Sirois J, Simmons D L, Richards J S. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- 7.Sirois J. Endocrinology. 1994;135:841–848. doi: 10.1210/endo.135.3.8070377. [DOI] [PubMed] [Google Scholar]
- 8.Brown C G, Poyser N L. J Reprod Fertil. 1984;72:407–414. doi: 10.1530/jrf.0.0720407. [DOI] [PubMed] [Google Scholar]
- 9.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 10.Eppig J J. Nature (London) 1979;281:483–484. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- 11.Tsafriri A. Adv Exp Med Biol. 1995;377:121–140. doi: 10.1007/978-1-4899-0952-7_8. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Russell P T, Larsen W J. Mol Reprod Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- 13.Salustri A, Yanagishita M, Hascall V C. Dev Biol. 1990;138:26–32. doi: 10.1016/0012-1606(90)90173-g. [DOI] [PubMed] [Google Scholar]
- 14.Eppig J J. Biol Reprod. 1981;25:191–195. doi: 10.1095/biolreprod25.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Moncada S, Flower R J, Vane J R. In: The Pharmacological Basis of Therapeutics. Gilman A G, Goodman L S, Rall T W, Murad F, editors. New York: McGraw–Hill; 1985. pp. 660–673. [Google Scholar]
- 16.Coleman R A, Kennedy I, Humphrey P P A, Bunce K, Lumley P. In: Comprehensive Medicinal Chemistry. Emmett J C, editor. Vol. 3. Oxford: Pergamon; 1990. pp. 643–714. [Google Scholar]
- 17.Coleman R A, Smith W L, Narumiya S. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 18.Katsuyama M, Nishigaki N, Sugimoto Y, Morimoto K, Negishi M, Narumiya S, Ichikawa A. FEBS Lett. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 20.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tsuboi K, Katsuyama M, Ichikawa A, et al. Nature (London) 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner P J. Br J Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsuyama M, Sugimoto Y, Mmorimoto K, Hasumoto K, Fukumoto M, Negishi M, Ichikawa A. Endocrinology. 1997;138:344–350. doi: 10.1210/endo.138.1.4885. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 24.Salustri A, Petrngaro S, Siracusa G. Biol Reprod. 1985;33:229–234. doi: 10.1095/biolreprod33.1.229. [DOI] [PubMed] [Google Scholar]
- 25.Hogan B, Beddington R, Costantini F, Lucy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, New York: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 26.Kuno N, Kadomatsu K, Fan Q-W, Hagihara M, Senda T, Mizutani S, Muramatsu T. FEBS Lett. 1998;425:191–194. doi: 10.1016/s0014-5793(98)00213-0. [DOI] [PubMed] [Google Scholar]
- 27.Hashlamoun L A, Killian G J. Arch Androl. 1985;15:159–171. doi: 10.3109/01485018508986906. [DOI] [PubMed] [Google Scholar]
- 28.Downs S M, Longo F J. J Exp Zool. 1983;228:99–108. doi: 10.1002/jez.1402280111. [DOI] [PubMed] [Google Scholar]
- 29.Eppig J J. BioEssays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- 30.Cecconi S, D’Aurizio R, Colonna R. J Reprod Fertil. 1996;107:207–214. doi: 10.1530/jrf.0.1070207. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy C R J, Zhang Y, Brandon S, Guan Y, Coffee K, Funk C D, Magnuson M A, Oates J A, Breyer M D, Breyer R M. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty I, Das S K, Wang J, Dey S K. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]