Abstract
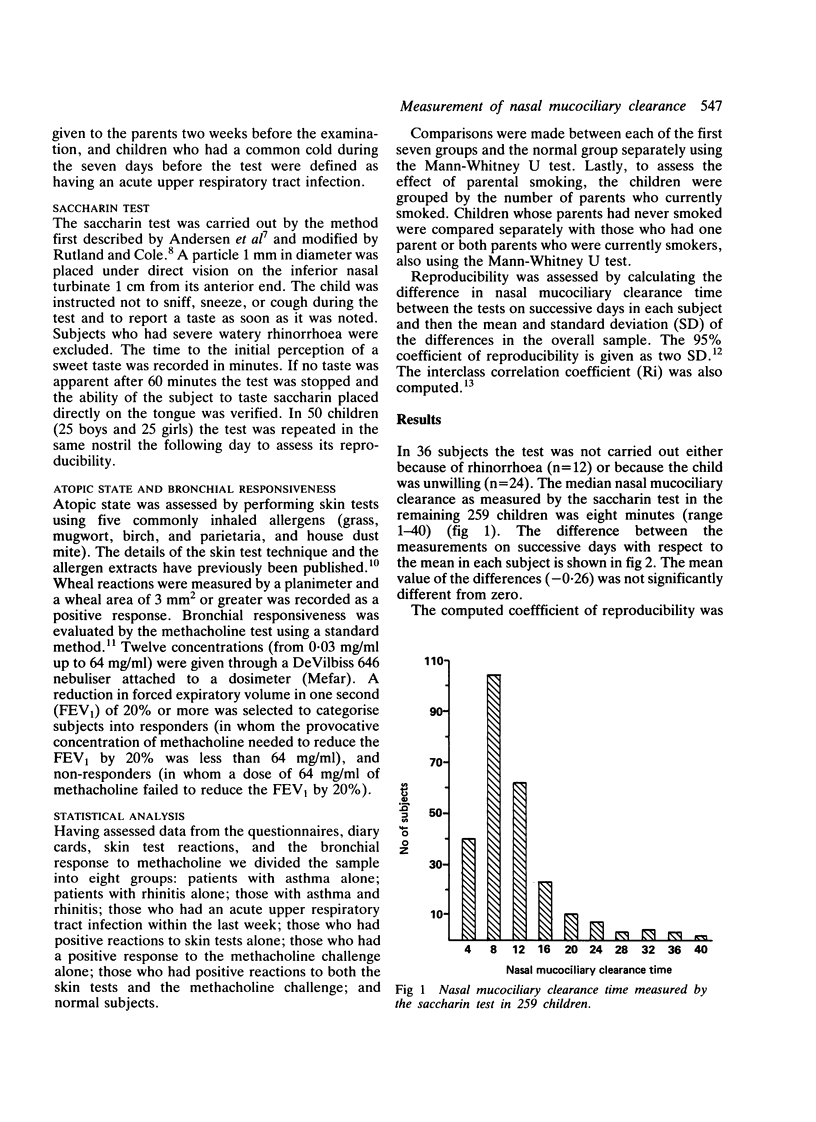
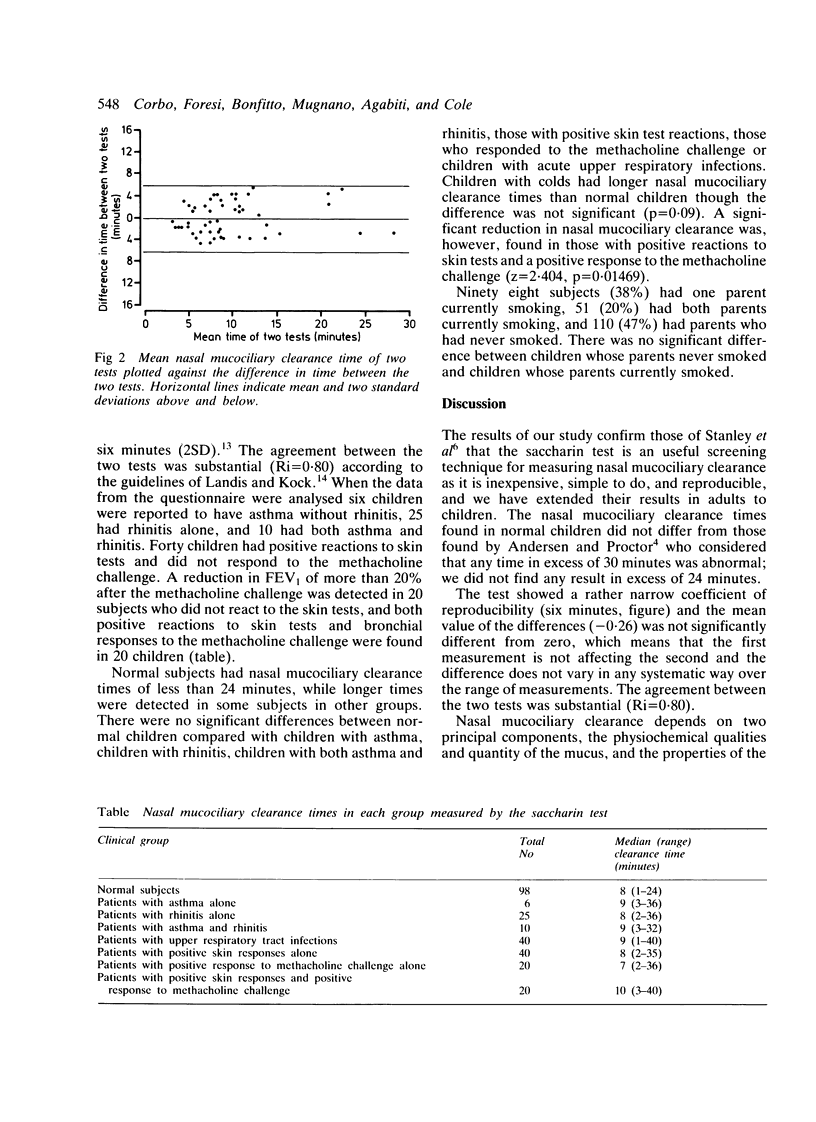
The saccharin test was carried out in a randomly selected sample of schoolchildren (142 boys and 153 girls, age range 11-14 years) to determine the variability and reproducibility of the test and to assess whether it could be used as a screening test for nasal mucociliary clearance. Nasal mucociliary clearance times were analysed according to clinical history (asthma, rhinitis, asthma with rhinitis, and acute upper respiratory tract infections), laboratory findings (positive skin test responses and bronchial hyper-reactivity assessed by methacholine challenge), and parental smoking. Nasal mucociliary clearance times showed a narrow coefficient of repeatability (six minutes) in 50 subjects and there was substantial agreement between the two tests. Nasal mucociliary clearance times were less than 40 minutes in all the children. Normal children had nasal mucociliary clearance times of less than 24 minutes while significantly impaired nasal mucociliary clearance was detected in those with positive skin reactions and a positive response to methacholine challenge. We were unable to show that passive smoking had any consistent effect on nasal mucociliary clearance. We suggest that a time response between 30-60 minutes should be checked again at least two weeks later, and that children in whom repeated saccharin tests show a nasal mucociliary clearance of greater than 30 minutes should have ciliary beat frequency estimated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzelius B. A., Camner P., Mossberg B. Acquired ciliary defects compared to those seen in the immotile-cilia syndrome. Eur J Respir Dis Suppl. 1983;127:5–10. [PubMed] [Google Scholar]
- Andersen I., Camner P., Jensen P. L., Philipson K., Proctor D. F. A comparison of nasal and tracheobronchial clearance. Arch Environ Health. 1974 Nov;29(5):290–293. doi: 10.1080/00039896.1974.10666589. [DOI] [PubMed] [Google Scholar]
- Andersen I., Camner P., Jensen P. L., Philipson K., Proctor D. F. Nasal clearance in monozygotic twins. Am Rev Respir Dis. 1974 Sep;110(3):301–305. doi: 10.1164/arrd.1974.110.3.301. [DOI] [PubMed] [Google Scholar]
- Andersen I., Proctor D. F. Measurement of nasal mucociliary clearance. Eur J Respir Dis Suppl. 1983;127:37–40. [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. S. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N Engl J Med. 1985 Feb 21;312(8):463–468. doi: 10.1056/NEJM198502213120802. [DOI] [PubMed] [Google Scholar]
- Corbo G. M., Foresi A., Verga A. E., Mattoli S., Polidori G., Ciappi G. Allergy indices based on allergen dose-response curve in a randomly selected sample of schoolchildren. Allergy. 1987 Apr;42(3):230–237. doi: 10.1111/j.1398-9995.1987.tb02204.x. [DOI] [PubMed] [Google Scholar]
- Dulfano M. J., Luk C. K. Sputum and ciliary inhibition in asthma. Thorax. 1982 Sep;37(9):646–651. doi: 10.1136/thx.37.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Mossberg B., Camner P., Afzelius B. A. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med. 1977 Jul 7;297(1):1–6. doi: 10.1056/NEJM197707072970101. [DOI] [PubMed] [Google Scholar]
- Greenstone M., Cooper P., Warner J., Cole P. J. Effect of acute antigenic challenge on nasal ciliary beat frequency. Eur J Respir Dis Suppl. 1983;128(Pt 2):449–450. [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. M. The effect of tobacco smoke, with or without phenylmethyloxadiazole (PMO), on rat bronchial epithelium: a light and electron microscopic study. J Pathol. 1981 Apr;133(4):341–359. doi: 10.1002/path.1711330406. [DOI] [PubMed] [Google Scholar]
- Kaminski E. J., Fancher O. E., Calandra J. C. In vivo studies of the ciliastatic effects of tobacco smoke. Absorption of ciliastatic components by wet surfaces. Arch Environ Health. 1968 Feb;16(2):188–193. doi: 10.1080/00039896.1968.10665042. [DOI] [PubMed] [Google Scholar]
- Kollerstrom N., Lord P. W., Whimster W. F. A difference in the composition of bronchial mucus between smokers and non-smokers. Thorax. 1977 Apr;32(2):155–159. doi: 10.1136/thx.32.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. S., Feinstein A. R. Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther. 1981 Jan;29(1):111–123. doi: 10.1038/clpt.1981.18. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Mezey R. J., Cohn M. A., Fernandez R. J., Januszkiewicz A. J., Wanner A. Mucociliary transport in allergic patients with antigen-induced bronchospasm. Am Rev Respir Dis. 1978 Oct;118(4):677–684. doi: 10.1164/arrd.1978.118.4.677. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981 Sep;36(9):654–658. doi: 10.1136/thx.36.9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G., Dolovich M. B., Roberts R. S., Frith P. A., Juniper E. F., Hargreave F. E., Newhouse M. T. Standardization of inhalation provocation tests: two techniques of aerosol generation and inhalation compared. Am Rev Respir Dis. 1981 Feb;123(2):195–199. doi: 10.1164/arrd.1981.123.2.195. [DOI] [PubMed] [Google Scholar]
- Sakakura Y. Changes of mucociliary function during colds. Eur J Respir Dis Suppl. 1983;128(Pt 1):348–354. [PubMed] [Google Scholar]
- Sleigh M. A. Primary ciliary dyskinesia. Lancet. 1981 Aug 29;2(8244):476–476. doi: 10.1016/s0140-6736(81)90811-4. [DOI] [PubMed] [Google Scholar]
- Stanley P. J., Wilson R., Greenstone M. A., MacWilliam L., Cole P. J. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax. 1986 Jul;41(7):519–523. doi: 10.1136/thx.41.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., MacWilliam L., Greenstone M., Mackay I., Cole P. Efficacy of a saccharin test for screening to detect abnormal mucociliary clearance. Br J Dis Chest. 1984 Jan;78(1):62–65. [PubMed] [Google Scholar]
- Wanner A. Allergic mucociliary dysfunction. J Allergy Clin Immunol. 1983 Oct;72(4):347–350. doi: 10.1016/0091-6749(83)90498-0. [DOI] [PubMed] [Google Scholar]
- Wasserman S. J. Ciliary function and disease. J Allergy Clin Immunol. 1984 Jan;73(1 Pt 1):17–19. doi: 10.1016/0091-6749(84)90478-0. [DOI] [PubMed] [Google Scholar]
- Wihl J. A., Mygind N. Studies on the allergen-challenged human nasal mucosa. Acta Otolaryngol. 1977 Sep-Oct;84(3-4):281–286. doi: 10.3109/00016487709123968. [DOI] [PubMed] [Google Scholar]
- Wilson R., Alton E., Rutman A., Higgins P., Al Nakib W., Geddes D. M., Tyrrell D. A., Cole P. J. Upper respiratory tract viral infection and mucociliary clearance. Eur J Respir Dis. 1987 May;70(5):272–279. [PubMed] [Google Scholar]
- Yamatake Y., Sasagawa S., Yanaura S., Kobayashi N. Allergy induced asthma with Ascaris suum administration to dogs. Jpn J Pharmacol. 1977 Apr;27(2):285–293. doi: 10.1254/jjp.27.285. [DOI] [PubMed] [Google Scholar]


