Abstract
Striatal lesions disrupt both motor and cognitive performance in rats, many aspects of which can be restored by striatal transplants. Because the normal striatum is involved in the formation and maintenance of motor habits, it has been hypothesized that grafted animals may require explicit retraining to relearn previously established habits that have been disrupted by the lesions. We have used a lateralized-discrimination task to reproduce this “learning to use the transplant” effect, combined with a transfer-of-training paradigm to demonstrate that recovery requires relearning specific lateralized stimulus–response associations and cannot be explained simply by a generalized training-dependent improvement in motor skill. These results have clear implications for developing appropriate strategies for the rehabilitation of Huntington’s disease patients participating in clinical transplantation programs.
Embryonic striatal transplants can restore many aspects of cognitive as well as motor function in rats (1–4) and monkeys (5, 6) after striatal damage, findings that provide the experimental basis for recently commenced clinical trials of striatal transplantation in Huntington’s disease (4, 7, 8).
One of the main functions of the neostriatum is understood to be the learning and mediating of stimulus–response (S-R) habits (9, 10), and striatal lesions induce deficits on a range of motor learning and complex motor initiation and response selection paradigms (9–13). Consequently, it has been hypothesized that, for functional recovery to be apparent after transplantation, explicit retraining may be necessary to reestablish previously learned habits within the reconstructed graft–host striatal circuitry, a phenomenon that we have termed “learning to use the transplant”(14). A similar requirement for retraining has been found necessary for rats to be able to use restored visual inputs mediated by a retinal graft (15). However, previous experimental explorations of this learning effect (14–16) failed to establish exactly what is being relearned by grafted animals, because either a general improvement in motor skills or the learning of specific S-R associations could account for behavioral recovery.
In the present study, we have endeavored to demonstrate the specificity of the retraining that is required for the restitution of function after striatal transplantation. We exploit the laterality of function present within the striatum (11, 12) by combining a lateralized visual-discrimination task (11) with a “transfer of training” procedure to dissociate specific S-R associations from more general aspects of responding.
METHODS
The experiments were conducted in accordance with the regulations and licensing of the United Kingdom Animals (Scientific Procedures) Act of 1986.
Surgery.
Forty-six young-adult male Hooded Lister rats (Harlan Olac, Bichester, U.K.) were used. All stereotaxic surgery was conducted under halothane gaseous anesthesia. Lesions were made by injection of 2 × 0.5 μl of 0.09 M quinolinic acid (Sigma) in 0.1 M PBS, pH 7.4, delivered over 4 min each via a 30-gauge stainless steel cannula attached to a microdrive pump into the neostriatum at stereotaxic coordinates A = 0.0 mm, L = 3.5 mm, V = −4.5 mm and A = 1.2 mm, L = 2.8 mm, V = −4.5 mm, with the nose bar set 2.3 below the interaural line. Surgery was performed contralateral to the side of better preoperative performance as measured by response bias. Graft tissue was dissected from the whole ganglionic eminence of embryonic day 15 (E15) embryos of the same strain and prepared as a dissociated cell suspension according to a standard protocol (17). Suspensions were prepared at a final concentration of 1 ganglionic eminence per 2 μl, yielding final cell counts of 8.5 × 105 cells/μl with 97.5% viability (17). Graft tissue was transplanted to rats that had received lesions 10 days previously by stereotaxic injection of 1 × 2 μl of cell suspension via a 10-μl glass microsyringe (Scientific Glass Engineering, Ringwood, Australia) into the host neostriatum at A = 0.6, L = 3.2, V = −4.5. No special postoperative care was required after either surgery.
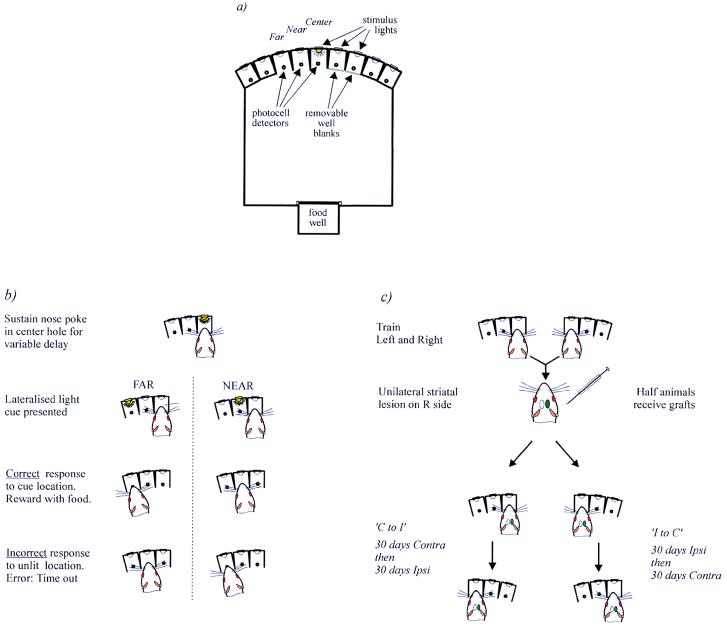
Behavioral Task. Testing was conducted in the nine-hole box apparatus (Fig. 1a), and this apparatus has been described fully elsewhere (18). All rats were trained to perform a lateralized choice-reaction time task (11) that was designed to assess performance on each side of the rat’s body. In this task, only three holes were exposed for any one session—the center hole and two lateralized response holes, which were either immediately to the left of the center hole or to the right. The lateralized response holes were designated “near” and “far,” accordingly (Fig. 1b). On each trial, the rat was required to poke and to hold its nose in the central hole until a brief stimulus light (200 ms) appeared in one of the two response holes. Immediately after the presentation of the stimulus, the rat was required to respond rapidly by withdrawing its nose from the center hole (reaction time) and poking its nose into the response hole in which the stimulus had appeared (movement time). Sessions were composed of 160 trials.
Figure 1.
Schematic diagram of the nine-hole box apparatus (a) and task requirements when response holes are configured to the left (b). The rat must sustain a nose poke in the center hole. After a variable delay, a brief light flash appeared in one of the two holes to the side of the rat. The rat then had to poke its nose into the same hole in which the light had appeared to obtain food reward (delivered at the rear of the chamber). (c) Experimental protocol. All animals were required to learn to perform the task to both the left and right sides. After surgery, animals in each of the three treatment groups (sham, lesion, graft) received one of two postoperative training regimes. Half of each group received 30 daily sessions on the ipsilateral side and then 30 daily sessions on the contralateral side (“Ipsi to Contra”) whereas the remaining animals received 30 contralateral sessions and then 30 ipsilateral sessions (“Contra to Ipsi”). Thus, performance on the contralateral side could be compared between the animals in each training regime to assess whether prior training on the ipsilateral side “transferred” to the contralateral side. All postoperative training commenced 4 months after graft surgery. The diagram outlines this procedure as if all surgery was performed on the right striatum, although the side of surgery was determined by the preoperative performance of each animal.
The primary measure of interest was response bias, because previous studies have revealed that the deficits induced by unilateral striatal lesions are the result of disrupting the spatial organization of responding (5, 6). Response bias was expressed as the number of correct and incorrect responses made to the near hole as a percentage of the total number of correct and incorrect responses made to either hole. Thus, a bias score of 50% represents no bias, >50% denotes a bias toward the near hole, and <50% denotes a bias in responding toward the far hole. The reaction time and movement time for each response also were recorded.
The location of the two response holes alternated daily between left and right sides of the central hole. Thus, on each day, the ability to detect, discriminate, and react accurately to stimuli on just one side of the body is tested. Because sensory and motor pathways cross from the periphery to the opposite hemisphere, reactions to left stimuli are preferentially under the control of the right hemisphere and reactions to right stimuli are under the control of the left hemisphere.
During periods of behavioral testing, all rats were maintained on a food-deprivation schedule; they were fed 15–17 g lab chow per day to maintain body weight at approximately 90% of free-feeding level. For the presurgical training phase, rats were trained on the left and right sides on alternative days over approximately 40 days. Once trained to an asymptotic level of performance, animals were divided into three matched groups to receive (i) unilateral striatal lesions (“lesion” group), (ii) unilateral lesions plus striatal grafts, or (iii) sham lesions and grafts (“control” group), made by stereotaxic injection of 0.1 M PBS into the striatum on each occasion. After surgery rats were given free access to food and water over 4 months before the food-deprivation regime was reintroduced and they were retested on the task for a further 60 days.
Experimental Design. Testing in the lateralized discrimination task resumed 16 weeks after surgery, a period sufficient to allow not only complete graft maturation but also to maximize anatomical integration between the graft and the host brain (19, 20). To address the specificity of retraining necessary for functional recovery, we used two alternative postoperative training regimes. In one condition, all animals were trained for 30 sessions exclusively on the contralateral side, followed by 30 ipsilateral sessions (“Contra to Ipsi” groups). In the second condition, this pattern was reversed (“Ipsi to Contra” groups; see Fig. 1c). Because unilateral striatal lesions impair performance principally on the side contralateral to the lesion, it was possible to determine whether extensive retraining on the ipsilateral side would “transfer” to the impaired contralateral side.
If graft-derived recovery related only to general task demands (including general motor and motivational factors as well as the overall contingency to respond to the location of a light stimulus), then training on either side should prove beneficial and ipsilateral practice could be expected to “transfer” to the contralateral side. Alternatively, grafted animals may have to relearn the specific S-R associations in contralateral space within the reconstructed graft–host circuitry. If specific S-R associations must be relearned, then one would expect minimal transfer of ipsilateral practice, and a similar amount of retraining on the contralateral side would be required irrespective of any prior ipsilateral training.
Statistics.
All behavioral measures were analyzed by multifactorial, split-plot ANOVAs with between-subject factors of Group (Control, Lesion, Graft) and Condition (“I to C,” “C to I”) and within-subject factors of Side (ipsilateral, contralateral), Distance (near hole, far hole), and Blocks of Days of testing (30 days subdivided into 6 × 5-day blocks). The locus of significant interactions was analyzed by restricted post hoc comparisons between groups by using Newman–Keuls tests. Preoperative performance was relatively consistent between animals, so analysis of postoperative performance with preoperative performance as a covariate did not change the results and is not presented.
Histology.
At the completion of behavioral testing the rats were deeply anesthetized with Euthatal (May & Baker, Dagenham, U.K.) and perfused through the heart with 100 ml of PBS followed by 250 ml of 4% paraformaldehyde in buffered saline. The brains were removed, postfixed for 4 hr in fixative, immersed in 30% sucrose until they sank, and then sectioned at 60 μm on a freezing sledge microtome. Parallel series of sections were stained with cresyl violet to reveal cell bodies, for acetylcholinesterase histochemistry, by using the conventional thiocholine reaction with 0.25% silver nitrate to enhance the sulfide reaction product and 0.01% ethoproprazine to inhibit nonspecific esterases and, immunohistochemically, by using the streptavidin-biotin-peroxidase complex reaction and using an ABC kit (Dako) and primary antibodies directed against tyrosine hydroxylase (TH; Institut Jacques Boy, Reims, France; 1:3,000) and dopamine- and adenosine-regulated phosphoprotein (DARPP-32, a kind gift of H. Hemmings and P. Greengard; 1:20,000). Sections were photographed digitally in a Leica microscope and processed in Adobe photoshop 3.0.
RESULTS
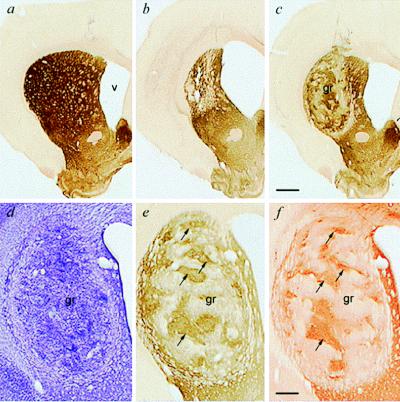
The lesions and grafts were confirmed at post mortem histology to have been accurate in their placement and effect (see Fig. 2). The lesions produced extensive cell loss and atrophy in the ipsilateral striatum (loss of striatal volume, 45 ± 4%; enlargement of ventricular volume, 217 ± 19%), whereas the grafts survived well in all cases, were rich in healthy-appearing neurons with little overt gliosis, and exhibited the patchy staining in acetylcholinesterase, TH, and DARPP-32 immunohistochemistry that is characteristic of such transplanted tissue (1, 19, 21).
Figure 2.
Photomicrographs of striatal graft survival and integration. (a) Intact neostriatum in a control rat. (b) Unilateral quinolinic acid lesions induce massive neostriatal cell loss, striatal atrophy, and enlargement of the lateral ventricle. (c) Striatal transplant surviving in the lesioned striatum, increasing total striatal volume and reducing ventricular expansion. (a–c) All acetylcholinesterase histochemistry at low magnification. (d–f) Higher-magnification view of the same striatal graft visualized in adjacent sections with cresyl violet to visualize cell bodies (d), acetylcholinesterase to visualize the characteristic cholinergic neuropil of the striatum (e), and DARPP-32, a receptor marker predominantly located on striatal neurons (f). Note the good survival and distinctive patchy internal organization of the grafts, with the striatal-like compartment being aligned in adjacent sections (arrows). [Bars = 1 mm (c) and 250 μm (f).]
All animals obtained a high level of performance accuracy preoperatively (accuracy, 73.3 ± 1.2%). All subsequent data presented are for postoperative performance only.
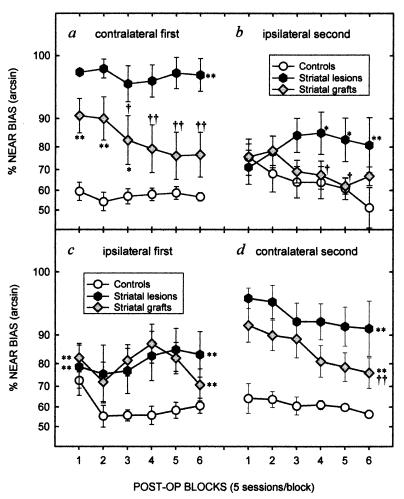
In animals tested first on the contralateral side (“C to I” groups), the lesions induced a decline in choice accuracy because of a marked bias of responding to the nearer of the two contralateral response holes (Fig. 3a). This deficit was stable over 30 days of testing. The grafted rats initially also were severely impaired, with more than 90% of responses biased toward the near hole. However, whereas the lesion deficit remained stable, the grafted group showed a progressive improvement in task performance (Groups, F2,20 = 19.37, P < 0.001; Groups × Days, F10,100 = 1.99, P < 0.05). Indeed, by the last 10 days of contralateral testing the grafted animals were completing sufficient responses to the previously neglected contralateral far hole that their response bias did not differ from control rats (see Fig. 3a). Thus, even after extensive anatomical growth of the grafts over 4 months, transplanted rats were unable to perform the task on the contralateral side when first retested, yet exhibited a marked recovery in the ability to perform complex S-R habits with daily retraining over a 30-day period.
Figure 3.
Postoperative percentage bias scores (100 × responses to near hole/responses to both near and far holes) for the 6 × 5-day blocks of testing on the contralateral side (a) and then the ipsilateral side (b) in the “C to I” subgroup and on the ipsilateral side (c) followed by the contralateral side (d) in the “I to C” subgroup. Asterisks indicate a significant difference between the lesion or graft group and controls, daggers indicate a significant difference between the lesion and graft groups (∗,†, P < 0.05; ∗∗,††, P < 0.01). When only the main effect of group was significant, ∗∗ or †† are shown against the end of the row of blocks (c and d), whereas when the Group × Day interaction was significant, significant differences between groups is shown separately for each block of days (a and b). Data were subject to an arcsin transformation for homogeneity of variance and plotted on an arcsin scale with corresponding percentage bias scores.
Comparison of performance on the contralateral side for animals that either received (Fig. 3d) or did not receive (Fig. 3a) prior ipsilateral retraining demonstrates graphically that this relearning is specific for contralateral retraining. When rats that first had been retested on the ipsilateral side were switched to testing on the contralateral side (“I to C” subgroup, Fig. 3d), the lesion animals again indicated a marked response bias, a deficit that was alleviated significantly by the grafts (Groups, F2,20 = 14.89, P < 0.001). In particular, when the order of testing is included in the analysis, there is no significant differences in the profile of recovery on the contralateral side between the grafted rats tested first on that side (Fig. 3a) and those with extended retraining first on the ipsilateral side [Fig. 3d; Groups × Days, F10,199 = 2.80, P < 0.01; Order, F1,40 = 0.20, not significant (n.s.); Groups × Days × Order, F10,199 = 0.38; n.s.]. In each case, graft-associated recovery on the contralateral side was associated with an increase in accuracy for the far hole in grafted animals (Groups × Days, F10,199 = 2.09, P < 0.05; Order, F1,40 = 2.79, n.s.; Groups × Days × Order, F10,199 = 0.52; n.s.). This implies an absence of any significant transfer of recovery, as measured by response bias, and indicates the specificity of the relearning required within the grafted hemisphere. One possibility is that this learning is mediated by tissue approximate to the graft, rather than in the graft itself. However, it would appear unlikely that such a mechanism alone is mediating recovery here, because the residual volume of the host striatum correlated with the initial level of impairment (r = −0.62, t(17) = 3.13; P < 0.05), but not in the change in bias, or recovery, over 30 days (r = −0.01, t(17) = 0.05; n.s.). This suggests that this relearning indeed is mediated by reconstructed striatal circuitries on the grafted side of the brain.
Rats with lesions and grafts that first were retested on the ipsilateral side (“I to C” groups) also exhibited a clear ipsilateral deficit (Fig. 3c), albeit substantially smaller than that seen on the contralateral side. Because responding in the ipsilateral half of space is under the control of the intact hemisphere, this milder impairment may arise indirectly through postural imbalance (22), although the ipsilateral side was operationally defined as the side with a stronger preoperative response bias. The grafts had no immediate effect on this asymmetry, with the graft group as impaired as the lesion group, and this modest ipsilateral deficit did not recover over 30 days of retraining (Fig. 3c; Groups F2,20 = 19.37, P < 0.001). By contrast, grafted animals that received extensive contralateral training before being tested on the ipsilateral side (“C to I” subgroup) were significantly recovered from the deficit exhibited by the rats with lesions alone on the ipsilateral side and did not differ from controls (Groups × Days, F10,100 = 2.52, P < 0.01; compare Fig. 3 b with c). This suggested that extensive contralateral training could restore the postural imbalance that degraded ipsilateral responding, whereas training on the ipsilateral side was unable to alleviate even these milder deficits. Thus, contralateral training provided benefits for grafted rats on both the ipsilateral side as well as on the contralateral side.
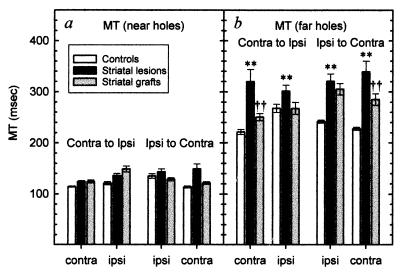
Whereas the effects of striatal grafts on response bias clearly were evident only with repeated testing, other performance indices indicated that grafted animals were less impaired than lesion animals on the resumption of retesting. This is particularly evident when considering the time taken to execute the lateralized movement to the correct response location [“movement time” (MT)]. The lesions slowed movements to both the contralateral side and, to a lesser extent, the ipsilateral side (Fig. 4). This MT deficit was apparent only when responding was to the far holes, was stable across repeated testing, and was not influenced by the order in which performance on the two sides was evaluated (Groups × Side × Distance, F2,38 = 3.83, P < 0.05; Groups × Subgroups × Side × Distance, F2,38 = 1.97, n.s.). Post hoc comparisons revealed that the grafts significantly alleviated the large lesion deficit on the contralateral side (Fig. 4a; t2,37 = 6.52, P < 0.01). On the ipsilateral side, where the lesion deficit was smaller, the graft-associated reduction in MT was also more modest and fell just short of significance (Fig. 4b; t2,37 = 2.50).
Figure 4.
(a) There were no significant differences between groups in the speed of response to near holes on either side. (b) Movement times to execute a correct response to the far holes were lengthened significantly on both sides in the lesion group (∗∗, P < 0.01), although the effect was greater on the contralateral side. The graft animals were recovered significantly from lesion levels on the contralateral (††, P < 0.01), but not the ipsilateral, side (see text).
That graft-induced alleviation of the MT deficit was immediately apparent from the outset of retesting indicates that the ability to effect efficient movements in contralateral space can recover before the exposure to the specific lateralized S-R contingencies required in the task. Thus, general aspects of visuospatial responding under the control of the grafted striatum recover without the need for specific retraining (presumably through practice in the course of the animals’ daily activities); in contrast, expression of a particular S-R task contingency in contralateral space requires specific retraining on that task.
Grafted rats also displayed an advantage over lesion rats in the speed of initiating responses to the contralateral far hole (Distance × Groups, F2,18 = 13.66; P < 0.01), although there was no impairment in the time taken to initiate contralateral responses in any group in the “Ipsi to Contra” regime (Group, F2,21 = 0.10; n.s.). Of particular interest, however, was the analysis of the reaction times of correct and incorrect responses on the contralateral side. Correct responses to the near hole were initiated faster than incorrect responses to the near hole (Outcome, F1,42 = 437.67; P < 0.01). This implies that both lesion and grafted animals were able to discriminate between the two stimuli within the contralateral visual field. More importantly, it is consistent with the concept of striatal lesions specifically impairing the expression of previously learned S-R association.
DISCUSSION
These data highlight two essential principles that may help realize the functional potential of striatal transplants. First, it was demonstrated that distinct response deficits were alleviated only if grafted animals received extensive postoperative training. Second, comparisons of different postoperative training regimes revealed that only specific training on the impaired contralateral side conferred functional benefit. Together, these results suggest that graft maturation and integration is insufficient by itself to mediate recovery on this task unless specific, extensive training subsequently is undertaken. Furthermore, this recovery would appear to be mediated by the restoration of basal ganglia circuitry, as permitted by the striatal graft.
A comparison of animals that were either (i) initially retrained on the contralateral side or (ii) tested only on the contralateral side once they had received extensive training on the ipsilateral side highlighted the degree of training specificity that is required to confer benefit. Thus, grafted animals initially demonstrated an inability to respond to the far contralateral hole regardless of whether they had received prior postoperative training on the ipsilateral side, but performance improved with repeated testing on the contralateral side. This is despite the 4-month delay between transplantation and retesting and, therefore, is consistent with the hypothesis that the restoration of appropriate anatomical connections is necessary but not sufficient for the restoration of normal striatal function (14). Furthermore, the lack of benefit derived from training on the ipsilateral side (in which responding is subject to the same abstract contingencies but is not mediated by the grafted striatum) suggests that any graft-mediated recovery in response bias must involve the specific relearning of a response. Such learning presumably is mediated by the grafted striatum and may be analogous to that seen in other forms of procedural relearning, such as delayed recovery of visual perception after alleviation of congenital blindness (23) or the relearning required after prismatic distortion of visual space (24).
Although the effects of striatal grafts on response bias in this study clearly are evident only with repeated testing, other significant performance indices (such as the latency to execute a lateralized movement) indicated that grafted animals were less impaired than lesion animals on the resumption of retesting. In keeping with previous findings (11, 25), bilateral movement time deficits were observed after unilateral striatal lesions when the response was to a distal location. Striatal transplants restored, in part, the ability to execute efficiently the series of movements that constituted such responses. That this partial recovery was seen at the onset of testing reflected functional capacities that did not depend on learning in the specific test paradigm, probably because the transplant is effectively “trained” in the course of daily activities in the home cage. In contrast, a reduction in response bias was found only after extensive retraining of the specific association. Notably, the rate of this recovery was comparable to the rate of original learning preoperatively, suggesting that grafted rats must relearn either the response or its expression. When considered together, these data highlight the importance of context in mediating responding in this task. Current models of basal ganglia function stress the importance of the striatum in determining the relevance of given sensory inputs in a specific context (26–28). In the present study, grafted rats clearly learn in the context of the home cage to move in a general fashion in contralateral space, but initially are unable to perform a S-R habit in contralateral space. Given that the data suggest that animals could both detect the stimuli and retrieve the relevant response rule, the deficit would appear to be one of expressing that rule in reconstructed response space. Grafted animals initially were unable to effect contralateral responses correctly not because they were unable to generate the required response but, presumably, because they had no postoperative experience of producing the response in that specific context. This suggests, therefore, that it is not the S-R association per se that has to be relearned, but the S-R association in the new context of the reconstructed response space (11).
The current data have important implications for our understanding of what is being relearned on the contralateral side. Although both lesion and grafted animals initially were unable to effect a correct response to the contralateral far hole at the start of retesting, only grafted animals had the capacity to learn to overcome the conflicting tendency to respond inappropriately in the contralateral near hole. It is presumably the reconstruction of comprising graft and host elements into a new striatal circuitry that allows such learning to occur at a neuronal level. The concept of behavioral intervention inducing structural changes within the brain is well documented (29, 30). We must now seek new ways to determine anatomically and physiologically how the particular S-R associations that are reestablished through retraining are mediated by synaptic plasticity at the neuronal level.
Because striatal transplantation is considered for clinical application (4, 7, 8), the present results indicate that we cannot simply rely on the grafts establishing appropriate anatomical connections at the level of overall circuits of the basal ganglia (31) for the restitution of function. Research designed to improve motor function in stroke patients centers around promoting the use of the affected arm while restricting the opportunity for the intact arm to mediate compensatory strategies (32). It may well be that graft-mediated actions will require similar encouragement, and serious attention will need to be applied to the rehabilitative training of transplanted patients (33).
Acknowledgments
We thank Drs. H. Hemmings and P. Greengard for the generous gift of antibodies. This study was supported by the Medical Research Council and Merck, Sharp and Dohme.
ABBREVIATION
- S-R
stimulus–response
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
A Commentary on this article begins on page 9976.
References
- 1.Björklund A, Campbell K, Sirinathsinghji D J S, Fricker R A, Dunnett S B. In: Functional Neural Transplantation. Dunnett S B, Björklund A, editors. New York: Raven; 1994. pp. 157–195. [Google Scholar]
- 2.Dunnett S B, Isacson O, Sirinathsinghji D J S, Clarke D J, Björklund A. Neuroscience. 1988;24:813–820. doi: 10.1016/0306-4522(88)90069-3. [DOI] [PubMed] [Google Scholar]
- 3.Isacson O, Dunnett S B, Björklund A. Proc Natl Acad Sci USA. 1986;83:2728–2732. doi: 10.1073/pnas.83.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanberg P R, Wictorin K, Isacson O. Cell Transplantation for Huntington’s Disease. Austin, TX: Landes; 1994. [Google Scholar]
- 5.Kendall A L, Rayment F D, Torres E M, Baker H F, Ridley R M, Dunnett S B. Nat Med. 1998;4:727–729. doi: 10.1038/nm0698-727. [DOI] [PubMed] [Google Scholar]
- 6.Hantraye P, Riche D, Mazière M, Isacson O. Proc Natl Acad Sci USA. 1992;89:4187–4191. doi: 10.1073/pnas.89.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peschanski M, Cesaro P, Hantraye P. Neuroscience. 1995;68:273–285. doi: 10.1016/0306-4522(95)00162-c. [DOI] [PubMed] [Google Scholar]
- 8.Kopyov O V, Jacques S, Kurth M, Philpott M, Lee A, Patterson M, Duma C, Lieberman A, Eagle K S. In: Cell Transplantation for Neurological Disorders. Freeman T B, Widner H, editors. Totawa, NJ: Humana; 1998. pp. 95–134. [Google Scholar]
- 9.Mishkin M, Malamut B, Bachevalier J. In: Memories and Habits: Two Neural Systems. Lynch G, McGaugh J L, Weinberger N M, editors. 1984. pp. 65–77. [Google Scholar]
- 10.White N M. Life Sci. 1989;45:1943–1957. doi: 10.1016/0024-3205(89)90569-9. [DOI] [PubMed] [Google Scholar]
- 11.Brasted P, Humby T, Dunnett S B, Robbins T W. J Neurosci. 1997;17:8919–8926. doi: 10.1523/JNEUROSCI.17-22-08919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown V J, Robbins T W. J Neurosci. 1989;9:3760–3765. doi: 10.1523/JNEUROSCI.09-11-03760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittleman G, Brown V J, Robbins T W. Neurosci Res Comm. 1988;2:1–8. [Google Scholar]
- 14.Mayer E, Brown V J, Dunnett S B, Robbins T W. Eur J Neurosci. 1992;4:119–126. doi: 10.1111/j.1460-9568.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 15.Coffey P J, Lund R D, Rawlins J N P. Prog Brain Res. 1990;82:269–275. doi: 10.1016/s0079-6123(08)62613-8. [DOI] [PubMed] [Google Scholar]
- 16.Coffey P J, Lund R D, Rawlins J N P. Proc Natl Acad Sci USA. 1989;86:7248–7249. doi: 10.1073/pnas.86.18.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunnett S B, Björklund A. Neural Transplantation: A Practical Approach. Oxford: IRL; 1992. [Google Scholar]
- 18.Robbins T W, Muir J L, Killcross A S, Pretsell D. In: Behavioural Neurosciences, a Practical Approach. Sahgal A, editor. Oxford: IRL Press; 1993. pp. 13–47. [Google Scholar]
- 19.Wictorin K. Prog Neurobiol. 1992;38:611–639. doi: 10.1016/0301-0082(92)90044-f. [DOI] [PubMed] [Google Scholar]
- 20.Labandeira-Garcia J L, Wictorin K, Cunningham E T, Björklund A. Neuroscience. 1991;42:407–426. doi: 10.1016/0306-4522(91)90385-2. [DOI] [PubMed] [Google Scholar]
- 21.Fricker R A, Sirinathsinghji D J S, Torres E M, Hume S, Dunnett S B. Neuroscience. 1997;79:695–710. doi: 10.1016/s0306-4522(96)00656-2. [DOI] [PubMed] [Google Scholar]
- 22.Whishaw I Q, O’Connor W T, Dunnett S B. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 23.Gregory R L. Eye and Brain: The Psychology of Seeing. Oxford: Oxford Univ. Press; 1990. [Google Scholar]
- 24.Howard I, Templeton W. Human Spatial Orientation. New York: Wiley; 1966. [Google Scholar]
- 25.Brasted P J, Döbrössy M D, Robbins T W, Dunnett S B. Brain Res Bull. 1998;46:487–493. doi: 10.1016/s0361-9230(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 26.Houk J C, Wise S P. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- 27.Houk J C, Adams J L, Barto A G. In: Models of Information Processing in the Basal Ganglia. Houk J C, Davis J L, Beiser D G, editors. London: MIT Press; 1995. pp. 249–270. [Google Scholar]
- 28.Lawrence A D, Sahakian B J, Robbins T W. Trends Cognit Sci. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- 29.Kempermann G, Kuhn H G, Gage F H. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones T A, Schallert T. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunnett S B. Behav Brain Res. 1995;66:133–142. doi: 10.1016/0166-4328(94)00134-2. [DOI] [PubMed] [Google Scholar]
- 32.Taub E, Wolf S L. Top Stroke Rehab. 1997;3:38–61. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- 33.Polgar S, Borlongan C V, Koutouzis T K, Todd S L, Cahill D W, Sanberg P R. Brain Res Bull. 1997;44:229–232. doi: 10.1016/s0361-9230(97)00109-3. [DOI] [PubMed] [Google Scholar]