Abstract
The clinical data of 18 patients with X linked hypophosphataemia were analysed retrospectively. The height data were expressed as SD scores. There was no difference in the final height of patients treated with vitamin D (or 1,25-dihydroxyvitamin D) and phosphate for at least two years (n = 12) and that of 16 hypophosphataemic family members who had never been treated. The mean final SD score (-2.07) of treated patients, however, was significantly higher than the value before treatment (-2.79), which indicated an average absolute height gain of 4-4.5 cm compared with the expected height values. Six of the treated patients developed ultrasonographically detectable nephrocalcinosis with normal renal function. The daily phosphate intake and excretion of patients with nephrocalcinosis was significantly higher than that of patients with normal renal morphology. There was no difference in the doses of vitamin D between the two groups. The average urinary calcium:creatinine ratio of the two groups was similar to and below the hypercalciuric 0.6 mmol:mmol limit. The group with nephrocalcinosis, however, had a higher incidence of hypercalciuric episodes than the group without nephrocalcinosis (12 in 130 observations compared with six in 334 observations, respectively). The benefits and risks of treatment of patients with X linked hypophosphataemia must be further evaluated. The high dose of phosphate seems to be an important factor in the development of nephrocalcinosis in this group of patients.
Full text
PDF
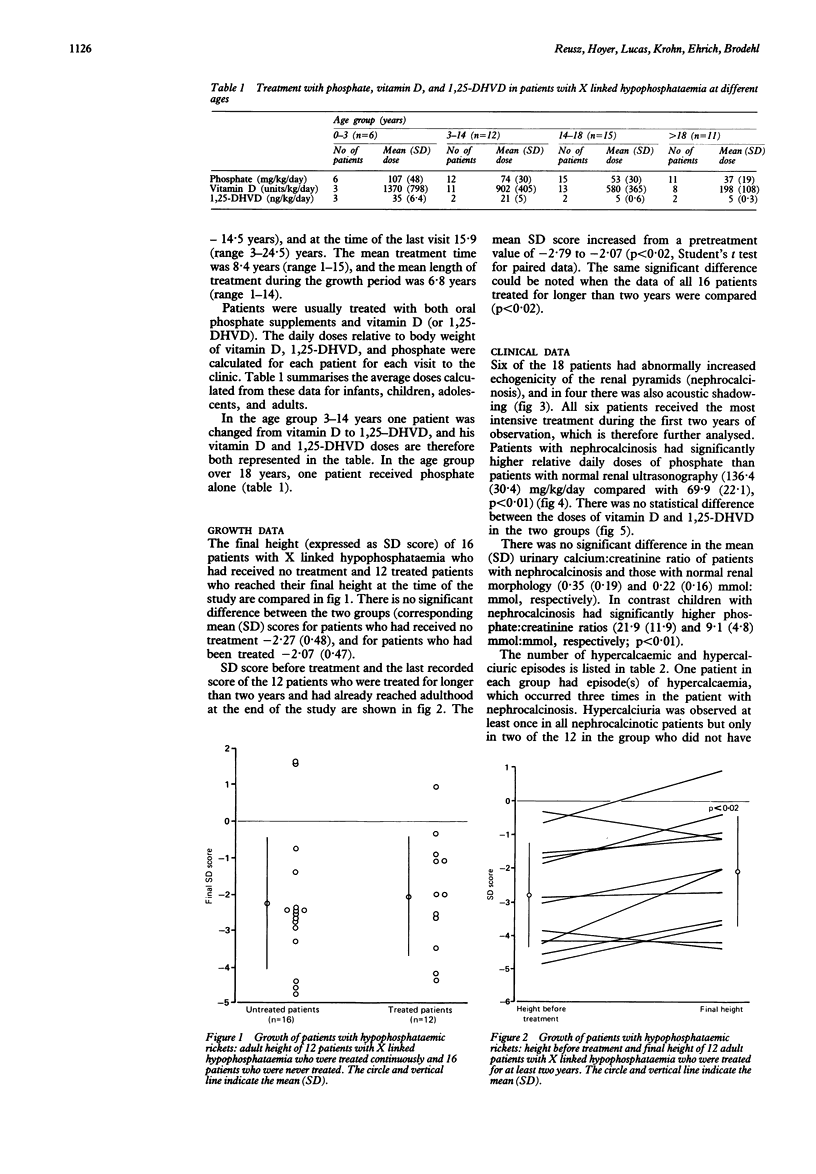
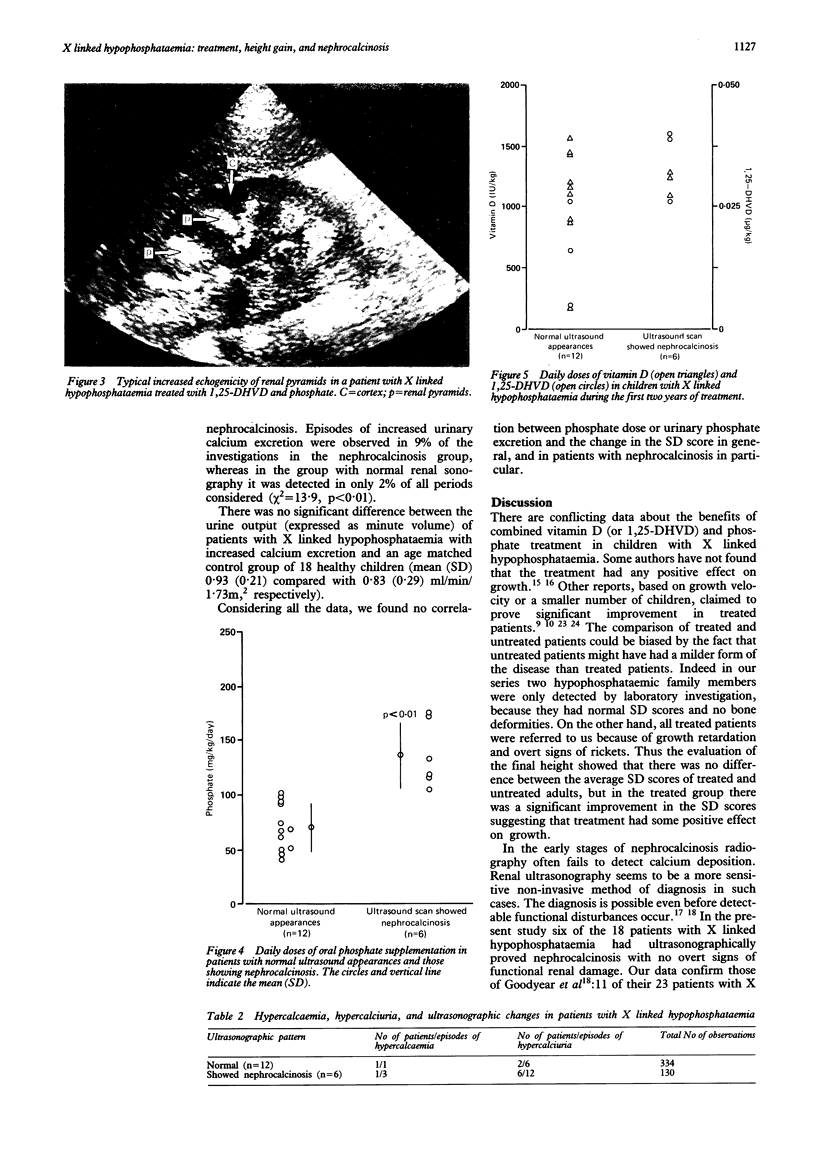

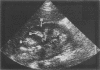
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon U., Brewer W. H., Chan J. C. Nephrocalcinosis: detection by ultrasonography. Pediatrics. 1983 Jun;71(6):970–973. [PubMed] [Google Scholar]
- Alon U., Chan J. C. Effects of hydrochlorothiazide and amiloride in renal hypophosphatemic rickets. Pediatrics. 1985 Apr;75(4):754–763. [PubMed] [Google Scholar]
- Chan J. C., Lovinger R. D., Mamunes P. Renal hypophosphatemic rickets: growth acceleration after long-term treatment with 1,25-dihydroxyvitamin-D3. Pediatrics. 1980 Sep;66(3):445–454. [PubMed] [Google Scholar]
- Chesney R. W., Mazess R. B., Rose P., Hamstra A. J., DeLuca H. F., Breed A. L. Long-term influence of calcitriol (1,25-dihydroxyvitamin D) and supplemental phosphate in X-linked hypophosphatemic rickets. Pediatrics. 1983 Apr;71(4):559–567. [PubMed] [Google Scholar]
- Chesney R. W., Mazess R. B., Rose P., Hamstra A. J., DeLuca H. F. Supranormal 25-hydroxyvitamin D and subnormal 1,25-dihydroxyvitamin D: their role in X-linked hypophosphatemic rickets. Am J Dis Child. 1980 Feb;134(2):140–143. doi: 10.1001/archpedi.1980.02130140014005. [DOI] [PubMed] [Google Scholar]
- Delvin E. E., Glorieux F. H. Serum 1,25-dihydroxyvitamin D concentration in hypophosphatemic vitamin D-resistant rickets. Calcif Tissue Int. 1981;33(2):173–175. doi: 10.1007/BF02409431. [DOI] [PubMed] [Google Scholar]
- Fraser D., Scriver C. R. Familial forms of vitamin D-resistant rickets revisited. X-linked hypophosphatemia and autosomal recessive vitamin D dependency. Am J Clin Nutr. 1976 Nov;29(11):1315–1329. doi: 10.1093/ajcn/29.11.1315. [DOI] [PubMed] [Google Scholar]
- Goodyer P. R., Kronick J. B., Jequier S., Reade T. M., Scriver C. R. Nephrocalcinosis and its relationship to treatment of hereditary rickets. J Pediatr. 1987 Nov;111(5):700–704. doi: 10.1016/s0022-3476(87)80245-7. [DOI] [PubMed] [Google Scholar]
- Harrison H. E., Harrison H. C., Lifshitz F., Johnson A. D. Growth disturbance in hereditary hypophosphatemia. Am J Dis Child. 1966 Oct;112(4):290–297. doi: 10.1001/archpedi.1966.02090130064005. [DOI] [PubMed] [Google Scholar]
- Insogna K. L., Broadus A. E., Gertner J. M. Impaired phosphorus conservation and 1,25 dihydroxyvitamin D generation during phosphorus deprivation in familial hypophosphatemic rickets. J Clin Invest. 1983 Jun;71(6):1562–1569. doi: 10.1172/JCI110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn H. P., Brandis M., Brodehl J., Offner G. Tubulärer Phosphattransport bei der Vitamin-D-resistenten Rachitis. Monatsschr Kinderheilkd. 1974 Jul;122(7):583–585. [PubMed] [Google Scholar]
- Kruse K., Kracht U., Kruse U. Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur J Pediatr. 1984 Nov;143(1):25–31. doi: 10.1007/BF00442743. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Clark A. G., Drezner M. K. Serum 1,25-dihydroxyvitamin D levels in subjects with X-linked hypophosphatemic rickets and osteomalacia. Calcif Tissue Int. 1982 Mar;34(2):125–130. doi: 10.1007/BF02411222. [DOI] [PubMed] [Google Scholar]
- McEnery P. T., Silverman F. N., West C. D. Acceleration of growth with combined vitamin D-phosphate therapy of hypophosphatemic resistant rickets. J Pediatr. 1972 May;80(5):763–774. doi: 10.1016/s0022-3476(72)80128-8. [DOI] [PubMed] [Google Scholar]
- Pak C. Y. Physicochemical basis for formation of renal stones of calcium phosphate origin: calculation of the degree of saturation of urine with respect to brushite. J Clin Invest. 1969 Oct;48(10):1914–1922. doi: 10.1172/JCI106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusz G. S., Latta K., Hoyer P. F., Byrd D. J., Ehrich J. H., Brodehl J. Evidence suggesting hyperoxaluria as a cause of nephrocalcinosis in phosphate-treated hypophosphataemic rickets. Lancet. 1990 May 26;335(8700):1240–1243. doi: 10.1016/0140-6736(90)91304-s. [DOI] [PubMed] [Google Scholar]
- Robertson W. G. Measurement of ionized calcium in biological fluids. Clin Chim Acta. 1969 Apr;24(1):149–157. doi: 10.1016/0009-8981(69)90152-1. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Reade T. M., DeLuca H. F., Hamstra A. J. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med. 1978 Nov 2;299(18):976–979. doi: 10.1056/NEJM197811022991803. [DOI] [PubMed] [Google Scholar]
- Stickler G. B., Beabout J. W., Riggs B. L. Vitamin D-resistant rickets: clinical experience with 41 typical familial hypophosphatemic patients and 2 atypical nonfamilial cases. Mayo Clin Proc. 1970 Mar;45(3):197–218. [PubMed] [Google Scholar]
- Stickler G. B., Jowsey J., Bianco A. J., Jr Possible detrimental effect of large doses of vitamin D in familial hypophysphatemic vitamin D-resistant rickets. J Pediatr. 1971 Jul;79(1):68–71. doi: 10.1016/s0022-3476(71)80060-4. [DOI] [PubMed] [Google Scholar]
- Stickler G. B., Morgenstern B. Z. Hypophosphataemic rickets: final height and clinical symptoms in adults. Lancet. 1989 Oct 14;2(8668):902–905. doi: 10.1016/s0140-6736(89)91559-6. [DOI] [PubMed] [Google Scholar]
- Tieder M., Modai D., Samuel R., Arie R., Halabe A., Bab I., Gabizon D., Liberman U. A. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med. 1985 Mar 7;312(10):611–617. doi: 10.1056/NEJM198503073121003. [DOI] [PubMed] [Google Scholar]
- Tsuru N., Chan J. C., Chinchilli V. M. Renal hypophosphatemic rickets. Growth and mineral metabolism after treatment with calcitriol (1,25-dihydroxyvitamin D3) and phosphate supplementation. Am J Dis Child. 1987 Jan;141(1):108–110. doi: 10.1001/archpedi.1987.04460010108039. [DOI] [PubMed] [Google Scholar]