Abstract
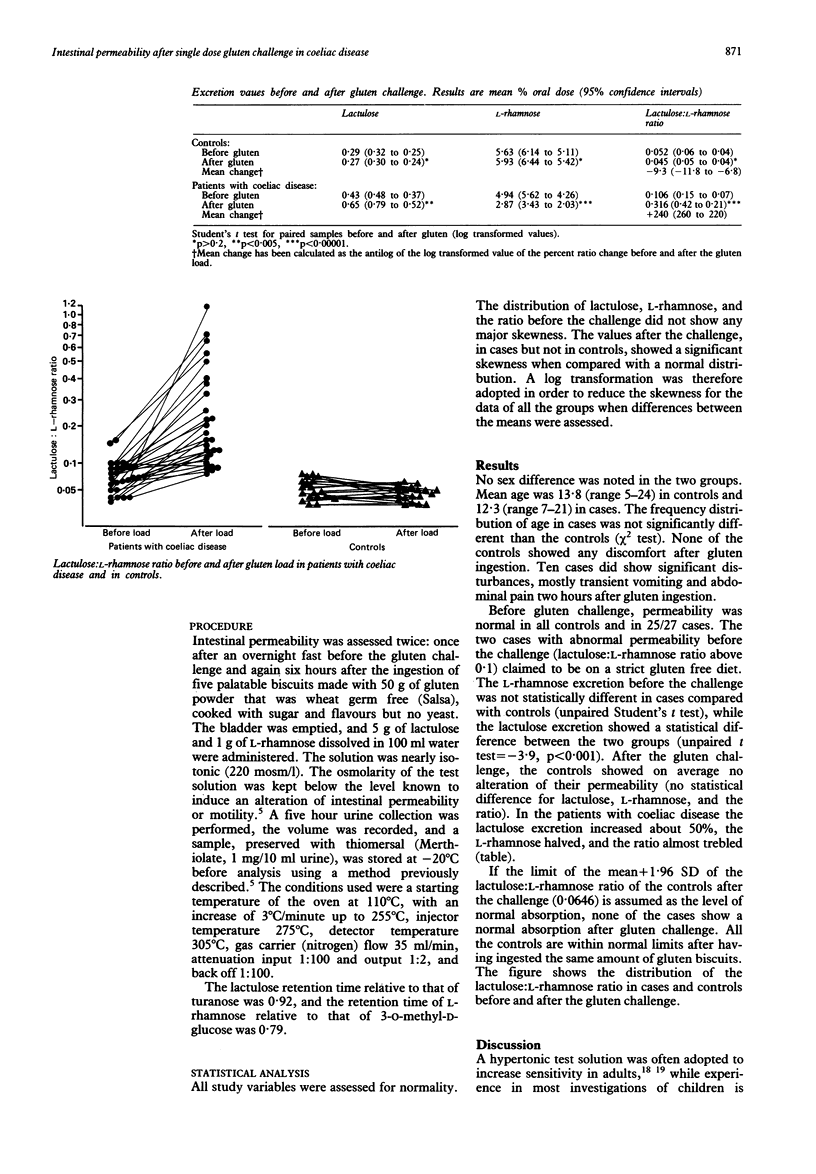
The changes of intestinal permeability before and after a gluten load were studied. The study group comprised 27 patients with coeliac disease (mean age 12.3 years) and 19 healthy controls matched by sex and age. Intestinal permeability was studied by measuring the urinary excretion of two sugars, lactulose and L-rhamnose, before and six hours after the ingestion of five palatable biscuits made with 50 g of gluten powder. The patients with coeliac disease had been on a gluten free diet during the previous two years. After the gluten load lactulose and L-rhamnose urinary excretion changed significantly in patients, and a significant increase in the lactulose: L-rhamnose ratio was also observed. No significant changes were observed in the controls. In view of the modification of the three biopsies diagnostic protocol made by the European Society for Paediatric Gastroenterology and Nutrition, permeability tests associated with single gluten challenges may be an added contribution to the accuracy of the diagnosis in childhood.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., Marsh M. N., Price A., Levi A. J., Peters T. J. Intestinal permeability in patients with coeliac disease and dermatitis herpetiformis. Gut. 1985 Nov;26(11):1214–1219. doi: 10.1136/gut.26.11.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Peters T. J. In vitro determination of small intestinal permeability: demonstration of a persistent defect in patients with coeliac disease. Gut. 1984 Feb;25(2):145–150. doi: 10.1136/gut.25.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budillon G., Parrilli G., Pacella M., Cuomo R., Menzies I. S. Investigation of intestine and liver function in cirrhosis using combined sugar oral loads. J Hepatol. 1985;1(5):513–524. doi: 10.1016/s0168-8278(85)80749-2. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology. 1977 Aug;73(2):247–251. [PubMed] [Google Scholar]
- Cobden I., Dickinson R. J., Rothwell J., Axon A. T. Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. Br Med J. 1978 Oct 14;2(6144):1060–1060. doi: 10.1136/bmj.2.6144.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Hamilton I., Rothwell J., Axon A. T. Cellobiose/mannitol test: physiological properties of probe molecules and influence of extraneous factors. Clin Chim Acta. 1985 May 15;148(1):53–62. doi: 10.1016/0009-8981(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Intestinal permeability and screening tests for coeliac disease. Gut. 1980 Jun;21(6):512–518. doi: 10.1136/gut.21.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford R. P., Menzies I. S., Phillips A. D., Walker-Smith J. A., Turner M. W. Intestinal sugar permeability: relationship to diarrhoeal disease and small bowel morphology. J Pediatr Gastroenterol Nutr. 1985 Aug;4(4):568–574. [PubMed] [Google Scholar]
- Hamilton I., Cobden I., Rothwell J., Axon A. T. Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut. 1982 Mar;23(3):202–210. doi: 10.1136/gut.23.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I., Hill A., Bose B., Bouchier I. A., Forsyth J. S. Small intestinal permeability in pediatric clinical practice. J Pediatr Gastroenterol Nutr. 1987 Sep-Oct;6(5):697–701. doi: 10.1097/00005176-198709000-00006. [DOI] [PubMed] [Google Scholar]
- Hodges S., Ashmore S. P., Patel H. R., Tanner M. S. Cellobiose: mannitol differential permeability in small bowel disease. Arch Dis Child. 1989 Jun;64(6):853–855. doi: 10.1136/adc.64.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker M. F., Menzies I. S. Increase in human intestinal permeability following ingestion of hypertonic solutions. J Physiol. 1977 Mar;265(3):881–894. doi: 10.1113/jphysiol.1977.sp011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxton D. G., Bjarnason I., Reynolds A. P., Catt S. D., Peters T. J., Menzies I. S. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986 Jul;71(1):71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- Mayer M., Greco L., Troncone R., Grimaldi M., Pansa G. Early prediction of relapse during gluten challenge in childhood celiac disease. J Pediatr Gastroenterol Nutr. 1989 May;8(4):474–479. doi: 10.1097/00005176-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Nathavitharana K. A., Lloyd D. R., Raafat F., Brown G. A., McNeish A. S. Urinary mannitol: lactulose excretion ratios and jejunal mucosal structure. Arch Dis Child. 1988 Sep;63(9):1054–1059. doi: 10.1136/adc.63.9.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilli G., Cuomo R., Nardone G., Maio G., Izzo C. M., Budillon G. Investigation of intestine function during acute viral hepatitis using combined sugar oral loads. Gut. 1987 Nov;28(11):1439–1444. doi: 10.1136/gut.28.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Brydon W. G., Ferguson A. Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut. 1984 Nov;25(11):1241–1246. doi: 10.1136/gut.25.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]



