Abstract
BCG vaccines are the oldest vaccines in use today, but the protective effect of the vaccination is still controversial. The risk of contracting tuberculosis is low compared with the possible complications after this vaccination. In Austria the formerly used BCG vaccine was not available in the required amount and another vaccine was released by the drug authorities. This product, with a more virulent strain, was used between August and December 1990, and this increased the incidence of complications. Eighty four of 1950 vaccinated newborn babies developed severe suppurative lymphadenitis three to 28 weeks after the vaccination, and surgical treatment was found to be necessary. Isoniazid treatment did not prove to be successful when the lymph node exceeded a certain size. Culture was successful in 46% up to week 20; after 20 weeks no culture became positive. All cultured bacteria were isoniazid sensitive. The question of general vaccination is raised and several points were considered before we came to the conclusion that except for high risk groups a general vaccination programme for neonates is not justified in Western countries.
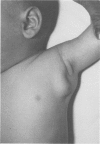
Full text
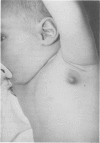
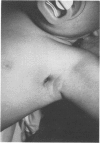
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag U., Bollag-Albrecht E. Tuberkulinreaktion und Ausmass von Impfnarben nach BCG-Impfung im frühen Neugeborenenalter. Schweiz Med Wochenschr. 1988 Jul 2;118(26):1001–1003. [PubMed] [Google Scholar]
- Brehmer W., Falkenberg N., Hussels H., Otto H. S., Preussler H., Waldschmidt J. Regionale suppurative Lymphadenitis nach BCG-Impfung. Dtsch Med Wochenschr. 1977 Sep 2;102(35):1251–1255. doi: 10.1055/s-0028-1105489. [DOI] [PubMed] [Google Scholar]
- Caglayan S., Yegin O., Kayran K., Timocin N., Kasirga E., Gun M. Is medical therapy effective for regional lymphadenitis following BCG vaccination? Am J Dis Child. 1987 Nov;141(11):1213–1214. [PubMed] [Google Scholar]
- Epstein P. R. BCG vaccination and nutrition. Lancet. 1990 Jun 23;335(8704):1536–1537. doi: 10.1016/0140-6736(90)93087-6. [DOI] [PubMed] [Google Scholar]
- Hanimann B., Morger R., Baerlocher K., Brunner C., Giger T., Schopfer K. BCG-Osteitis in der Schweiz. Ein Bericht über 6 Fälle. Schweiz Med Wochenschr. 1987 Feb 7;117(6):193–198. [PubMed] [Google Scholar]
- Hengster P., Fille M., Menardi G. Suppurative lymphadenitis in newborn babies after change of BCG vaccine. Lancet. 1991 May 11;337(8750):1168–1169. doi: 10.1016/0140-6736(91)92842-p. [DOI] [PubMed] [Google Scholar]
- Lallemant-Le Coeur S., Lallemant M., Cheynier D., Nzingoula S., Drucker J., Larouze B. Bacillus Calmette-Guérin immunization in infants born to HIV-1-seropositive mothers. AIDS. 1991 Feb;5(2):195–199. doi: 10.1097/00002030-199102000-00010. [DOI] [PubMed] [Google Scholar]
- Mahmoud M. E., Monhim Attia I. A. Evaluation of BCG vaccination in Bab El Shaaria zone--Cairo. Dev Biol Stand. 1986;58(Pt A):249–256. [PubMed] [Google Scholar]
- Milstien J. B., Gibson J. J. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ. 1990;68(1):93–108. [PMC free article] [PubMed] [Google Scholar]
- Moreno L., Gottrand F., Herbaux B., Savage C., Farriaux J. P. Vertebral osteitis following BCG vaccination in a previously healthy child. Eur J Pediatr. 1990 Jun;149(9):668–668. doi: 10.1007/BF02034763. [DOI] [PubMed] [Google Scholar]
- Myint T. T., Win H., Aye H. H., Kyaw-Mint T. O. Case-control study on evaluation of BCG vaccination of newborn in Rangoon, Burma. Ann Trop Paediatr. 1987 Sep;7(3):159–166. doi: 10.1080/02724936.1987.11748499. [DOI] [PubMed] [Google Scholar]
- Nyerges G., Drinóczy M. Significance of the number of viable units in BCG vaccines. Dev Biol Stand. 1986;58(Pt A):331–336. [PubMed] [Google Scholar]
- Packe G. E., Innes J. A. Protective effect of BCG vaccination in infant Asians: a case-control study. Arch Dis Child. 1988 Mar;63(3):277–281. doi: 10.1136/adc.63.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan U., Haupt H., Scheier R., Nada A., Grimrath U., Schmitt H. G. Untersuchungen zur BCG-Impfung des Neugeborenen mit dem Stamm 1331 Kopenhagen. Klin Padiatr. 1986 Jul-Aug;198(4):295–298. doi: 10.1055/s-2008-1033875. [DOI] [PubMed] [Google Scholar]
- Teulieres L., Diouf M. A., Chaud P., Saint-Cyr A., Saliou P. Comparative trial of administration of half (0.05 mg) and quarter (0.025 mg) dose of intradermal Pasteur BCG on 291 infants from birth to 1 year in French Guyana. Vaccine. 1991 Jul;9(7):521–524. doi: 10.1016/0264-410x(91)90040-d. [DOI] [PubMed] [Google Scholar]
- Tidjani O., Amedome A., ten Dam H. G. The protective effect of BCG vaccination of the newborn against childhood tuberculosis in an African community. Tubercle. 1986 Dec;67(4):269–281. doi: 10.1016/0041-3879(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Young T. K., Hershfield E. S. A case-control study to evaluate the effectiveness of mass neonatal BCG vaccination among Canadian Indians. Am J Public Health. 1986 Jul;76(7):783–786. doi: 10.2105/ajph.76.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]