Abstract
Adult T-cell leukemia (ATL) is an aggressive lymphoproliferative disease of poor clinical prognosis associated with infection by the human T-cell leukemia virus type I (HTLV-I). The use of arsenic trioxide (As2O3) has been shown to effectively treat acute promyelocytic leukemia (APL) with greater than 80% of patients achieving complete remission. The combination of arsenic and interferon has also shown promising results in the treatment of ATL. The requirement for slow dosage increases of arsenic and the time required to achieve a pharmacologic active dose in patients is a major obstacle because median survival of patients with ATL is about 6 months. In this study we report a potent synergistic effect of the combination of arsenic trioxide and interferon α (As/IFN-α) with emodin and DHA on cell-cycle arrest and cell death of HTLV-I–infected cells. Importantly, we found that clinically achievable doses of DHA and emodin allowed for reduced arsenic concentrations by 100-fold while still remaining highly toxic to tumor cells. Our data provide a rationale for combined use of As/IFN-α with emodin and DHA in patients with ATL refractory to conventional therapy.
Introduction
Adult T-cell leukemia (ATL) is associated with a poor clinical prognosis, and treatment of patients using conventional chemotherapy has limited benefit given that HTLV-I cells are resistant to conventional anticancer, apoptosis-inducing agents.1–3 Cyclophosphamide, adriamycin, vincristine, and prednisolone (CHOP) protocol has a reported 17% rate of remission but a predicted survival of less than 15% after 3 years.4–6 Some success has been reported in treating ATL with a combination of zidovudine (AZT) and IFN-α.7–10 However, this success is fleeting as many patients relapse, because response to therapy requires the presence of a transcriptionally active p53 gene.11 Other treatments include bone marrow transplantation,12–14 topoisomerase inhibitors,15,16 anti-Tac monoclonal antibody,17 inhibitors of NF-kB18,19 and proteasome inhibitors.20
Arsenic trioxide in combination with IFN-α is very effective in treating APL21–23 and showed promising results in the treatment of patients with ATL.24–29 However, differences in sensitivity to arsenic, as well as the toxicity associated with its use, require a slow dose increase in vivo, and treatment may need to be discontinued in some patients with ATL. Thus, it is worthwhile to investigate the effectiveness of docosahexaenoic acid (DHA) a nontoxic ω-3 polyunsaturated fatty acid found in fish oil. HL-60, an acute myeloid leukemia cell line that is resistant to clinically relevant doses of arsenic, shows a 90% reduction in viability along with an increase in apoptosis, an increase in intracellular reactive oxygen species (ROSs) and lipid peroxidation, as well as an up-regulation of Bax when treated with a combination of arsenic and DHA.30 Further, treatment with oleic acid, a monounsaturated fatty acid that is not susceptible to lipid peroxidation, did not enhance the effect of arsenic treatment.31 In breast cancer cells, the addition of DHA results in an increase in malondialdehyde, an end product of lipid peroxidation, which increases sensitivity to doxorubicin treatment through oxidative stress. Additionally, DHA failed to increase sensitivity to mitoxantrone, a drug that does not alter hydroperoxide levels, indicating that the addition of DHA is only effective in combination with drugs that induce oxidative stress.32 This presents a rationale for use of DHA in combination with emodin, a naturally occurring anthraquinone that has been shown to generate intracellular ROSs in cancer cells.33 Treatment with arsenic increases the concentration of intracellular ROSs,34 and it has been shown that lower levels of ROSs promote apoptosis, whereas higher levels cause necrosis. An increase in ROSs has been tied to the activation of Bax, a proapoptotic member of the Bcl-2 family, at the mitochondrial membrane.35 It has recently been shown that arsenic treatment induces activation of Bax and that the activation can be blocked by overexpression of Bcl-2 or ROS scavengers but not by pan-caspase inhibitors, indicating that ROSs, or changes in redox states, are involved in cellular death upstream of Bax activation.36
In this study we found a potent synergistic effect of the combination of As/IFN-α with emodin and DHA in HTLV-I–infected cells. The mechanism underlying these effects was in part linked to ROS production which leads to an increase in Bax and a decrease in Bcl-2 expression. In addition, combinatorial use of emodin and DHA with As/IFN-α resulted in the inhibition of the AP-1 and Akt pathways in HTLV-I cells, as demonstrated by a decrease in JunD, JAB1, and Akt expression. Our results suggest that As/IFN-α in combination with ROS-inducing/sensitizing agents may have benefits in ATL therapy.
Materials and methods
Drugs
IFN-α 2 (Cell Sciences, Canton, MA) and arsenic trioxide (Sigma, St Louis, MO) were used at 100 U/mL and 1 μM, respectively, as previously described.1 DHA (Sigma) was dissolved in ethanol and used at 10 to 50 μM. Emodin (Sigma) was dissolved in DMSO and used at 10 to 50 μM. Ascorbic acid (Sigma) was dissolved in sterile dH2O and used at 5 mM.
Cell lines
C8166 and MT-2 are HTLV-I–transformed T-cell lines. Jurkat and CEM HTLV-I–negative T-cell lines were grown in RPMI-1640 (Invitrogen Carlsbad, CA) supplemented with 10% fetal bovine serum, gentamycin, and penicillin-streptomycin. 1185 and LAF, IL-2–dependent T-cell lines immortalized by HTLV-I, and peripheral blood mononuclear cells (PBMCs) were cultured in 20% serum and 40 U/mL IL-2.
Cell-proliferation assay
Cell proliferation was measured using the Cell Proliferation Kit II (XTT; Roche, Mannheim, Germany) according to manufacturer's instructions. Cells (105/mL) were treated 48 to 72 hours in a 96-well plate and then incubated with tetrazolium salt XTT for 4 hours. Proliferation was quantified by measuring cleavage of XTT to an orange formazan dye using an enzyme-linked immunoabsorbent assay (ELISA) reader at 450 nm.
Cell-cycle analysis
Cells (2 × 106) were treated 24 to 72 hours then washed with PBS. After resuspension, cells were fixed on ice with 80% EtOH for 30 minutes. Cells were washed with PBS and treated with RNase for 15 minutes at 37°C, followed by staining with 50 μg/mL propidium iodide for 15 minutes at room temperature. Cells were then washed and resuspended in PBS and analyzed by flow cytometry.
Measurement of intracellular ROS production
Production of ROSs in cells was measured using 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Sigma), an oxygen-sensitive fluorescent probe. Cells (5 × 105) were incubated for 12 hours, as indicated, with arsenic and IFN-α with or without ascorbic acid, then DHA and emodin were added for another 12 hours. DCFH-DA was added at 5-nM final concentration to each sample and incubated at 37°C for 15 minutes. Cells were then collected and washed with cold PBS. Cells were analyzed by flow cytometry after resuspension in 500 μL PBS.
Apoptosis assay
Cells (5 × 105) were treated as described in “Measurement of intracellular ROS production” for the measurement of ROS production, then collected and washed with cold PBS. Cells were then stained with annexin V/propidium iodide using the Vybrant Apoptosis Assay Kit no. 2 (Molecular Probes, Eugene, OR) according to the manufacturer's instructions.
Mitochondrial membrane potential
Cells (1 × 106) were treated for 24 hours, then changes in the mitochondrial membrane potential (ΔΨm) were measured using the ApoAlert Mitochondrial Membrane Sensor Kit (Clontech, Mountain View, CA) according to the manufacturer's instructions.
Western blot analysis
Cells were washed with PBS containing phosphatase inhibitors and lysed in RIPA buffer with phosphatase and protease inhibitors (Complete Cocktail; Roche) after 24 to 48 hours of treatment. Protein concentration was determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred to Immobilon PVDF membranes (Millipore, Billerica, MA), visualization was done with the Supersignal West Dura enhanced chemiluminescence system (Pierce, Rockford, IL). Spot densitometry quantification of expression was performed as previously reported37 and was expressed as a percentage of the appropriate control sample referred as 100%. The study protocol was approved by the institutional review board at the University of Kansas Medical Center.
Results
Effects of As/IFN and their combination with DHA and emodin on cell proliferation of HTLV-I–immortalized and –transformed T cells
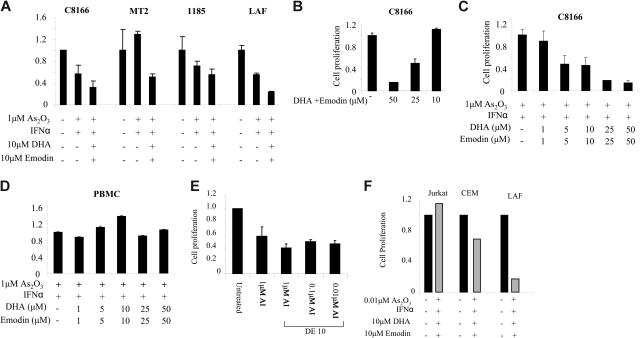
Cellular proliferation was assessed using the Cell Proliferation Kit II XTT, according to the manufacturer's instructions. Proliferation was quantified by measuring cleavage of XTT to an orange formazan dye using an ELISA reader at 450 nm, as previously reported.26 Results are expressed as the percentage of control-treated cells and represent the mean values of at least 2 independent experiments performed in triplicate. Our results indicated a very strong synergy between As/IFN-α and DHA/emodin at all time points and doses used in the HTLV-I–infected cells C8166 and MT-2, as well as the IL-2–dependent 1185 and LAF cell lines. This was in contrast to those effects not seen in the control PBMCs (Figure 1A-D) or with buffer controls (data not shown). Consistent with previous reports, MT-2 cells were more resistant to As/IFN-α–induced cell death when compared with C8166, LAF, or 1185 cells. However, all HTLV-I cell lines appeared to be equally and highly sensitive to the combination of As2O3/IFN-α with emodin and DHA (AIDE) (Figure 1A).
Figure 1.
Emodin and DHA increase sensitivity of HTLV-1–transformed cell lines to As2O3 ± IFN-α treatment. (A) HTLV-1–transformed cells (C8166, MT-2) and IL-2–dependent immortalized cells 1185 and LAF were treated with buffer control, 1 μM As2O3/IFN-α (100 U/mL), As2O3/IFN-α with 10 μM emodin and DHA (AIDE) for 60 hours, and cellular proliferation was measured using the XTT assay. Results are representative of 3 independent experiments performed in duplicate. (B) Proliferation assay of HTLV-I–transformed C8166 cells treated with increasing amounts of emodin and DHA from 10 to 50μM. (C) Proliferation assay of HTLV-I–transformed C8166 cells treated with increasing amounts of emodin and DHA from 1 to 50 μM in the presence of 1 μM As2O3/IFN-α. (D) Proliferation assay of normal PBMCs treated with increasing amounts of emodin and DHA from 1 to 50 μM in the presence of 1 μM As2O3/IFN-α. (E) Proliferation assay of HTLV-I–transformed C8166 cells treated with 10 μM emodin and DHA, IFN-α (100 U/mL) and decreasing amounts of As2O3, 1 μM, 0.1 μM, and 0.01 μM. (F) Comparison of CEM, Jurkat, and LAF proliferation following treatment with As2O3/IFN-α and emodin/DHA. Error bars represent SD.
The effect of emodin and DHA was also tested in the absence of arsenic trioxide and IFNα. As reported in Figure 1B, no effect on cell growth was detected for C8166 cells when cultured in the presence of emodin and DHA at a concentration of 10 μmol/L, which confirms the synergistic action of emodin and DHA with arsenic trioxide and IFN-α (Figure 1A). Because of arsenic trioxide toxicity, a major limitation to its use as a therapeutic agent for patients with ATL is the time required to achieve the clinically active dose. Because emodin and DHA showed a strong synergy with As/IFN-α, we tested whether we could lower the dose of arsenic while still preventing proliferation of tumor cells. The combination of emodin and DHA allowed up to a 100-fold decrease in arsenic concentrations with little loss in efficacy against tumor-cell proliferation (Figure 1E), whereas control cell lines Jurkat and CEM were not significantly affected (Figure 1F).
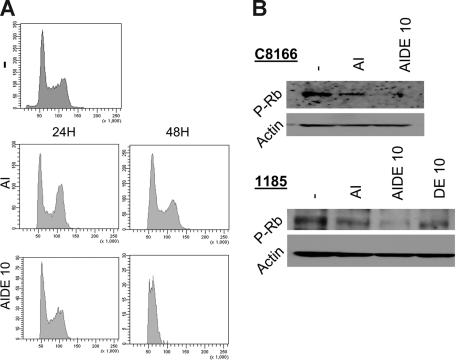
We next analyzed cell-cycle progression following treatment of HTLV-I cells with As/IFN-α (AI) or AIDE.
Results revealed a marked G0/G1 arrest of tumor cells cultured in the presence of emodin and DHA along with As/IFN-α (Figure 2A). In HTLV-I–infected cells the retinoblastoma protein (Rb) is constitutively phosphorylated, thereby allowing tumor cells to progress to S phase.38,39 In agreement with cell-cycle results presented in Figure 2A, we found that Rb accumulated in its hypophosphorylated form in 1185 and C8166 cells (Figure 2B). This effect was more pronounced in cells treated with As/IFN-α with emodin and DHA than those treated with As/IFN alone.
Figure 2.
Emodin and DHA reduce the level of phosphorylated Rb and induce G1 cell-cycle arrest in HTLV-I–transformed cells. (A) Cell-cycle analysis of propidium iodide–stained HTLV-I–transformed C8166 cells treated with As2O3/IFN-α in the absence or the presence of emodin and DHA. (B) Western blot analysis of phosphorylated Rb in C8166 and 1185 cells treated with As2O3/IFN-α in the absence or the presence of emodin and DHA or emodin and DHA alone. Actin was used as loading control.
Emodin and DHA reroute As/IFN-treated cells to caspase-independent cell death
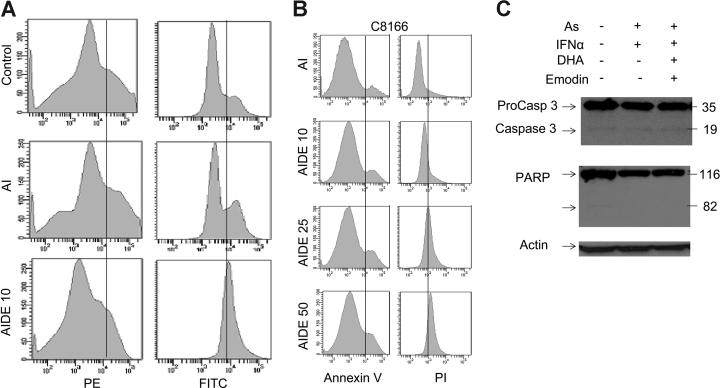
Changes in mitochondrial membrane potential, ΔΨm, are a critical step in cells undergoing apoptosis, regardless of the death signal. Therefore, ΔΨm was measured in C8166 cells (Figure 3A). As previously reported As2/IFN-α–treated cells that entered apoptosis showed an altered mitochondrial membrane permeability that led to ΔΨm collapse. Consistent with the increased ROS generation, mitochondrial membrane permeability collapse was much more pronounced when DHA and emodin were added to As/IFN-α (Figure 3A). By contrast, control C8166 cells treated with buffer did not show any change in ΔΨm.
Figure 3.
AIDE induces the collapse of mitochondrial membrane potential and increased Bax and decreased Bcl-2 expression and tumor-cell death. (A) ΔΨm collapse was measured using the Apoalert Mitochondrial Membrane Sensor kit. Results are representative of at least 2 experiments performed with different HTLV-1–transformed cells. (B) C8166 cells were treated with buffer control, As/IFN-α for 60 hours with or without increasing emodin and DHA. Cells were then harvested, washed in PBS without Ca++/Mg2+, and stained using the Vybrant Apoptosis kit. Annexin V conjugated to fluorescein allowed the identification of apoptosis (AV+/PI−) versus necrosis cell death (AV−/PI+) by fluorescence-activated cell sorting (FACS). (C) Western blot analysis of PARP and caspase 3 activation. Actin was used to confirm equal loading.
ΔΨm collapse is usually associated with the activation of caspases. Caspase-3 is a critical downstream protease in the caspase cascade, and poly-ADP-ribose polymerase (PARP) is 1 substrate of caspase-3 protease activity that is associated with apoptosis. Several studies have reported that As/IFN-α–mediated apoptosis of HTLV-I–infected cells is partially a caspase-dependent process and partially caspase independent. To gain insight into the mechanism used by emodin and DHA, cells were analyzed by flow cytometry using double staining (annexin V/PI). Apoptotic cells were scored as annexin V+/PI− versus necrotic dead cells, which were PI+. Results indicated that DHA and emodin did not significantly increase the apoptotic activity of As/IFN-α but, instead, triggered a potent necrotic-cell death signal, resulting in nearly 90% cell death after 48 hours of treatment (Figure 3B). Similar results were observed with lower doses of DHA and emodin and longer incubation times (data not shown).
These results were further confirmed by Western blot analysis of procaspase 3 and PARP (Figure 3C). Our results indicate that DHA and emodin are not associated with cleavage of the PARP, because the 85-kDa fragment representing the cleaved form was not detected after treatment of HTLV-I cells by As/IFN-α with DHA and emodin.
Together, with the flow cytometry data, this suggests that the combination of As/IFN-α with emodin and DHA is a potent inducer of necrotic-cell death in HTLV-I– infected cells (Figure 3B-C). Spot densitometry quantification analyses of the expression of several proteins implicated in survival pathways revealed a decrease in Bcl-2 expression of 37% and 68% following treatment with AI and AIDE, respectively (data not shown). Concomitantly, we found an increase in Bax expression, 200% and 150%, following treatment with AI and AIDE, respectively (data not shown). In contrast to a previous report, the proapoptotic cleaved form of Bcl-2 was not detected in our assay (data not shown). This is possibly due to differences in experimental procedures because we did not use purified mitochondria in our study. Other markers, such as Bcl-xL, were not significantly affected in our experimental conditions (data not shown).
Emodin and docosahexaenoic acid dramatically increase generation of ROSs and loss of mitochondrial membrane potential in As/IFN-treated cells
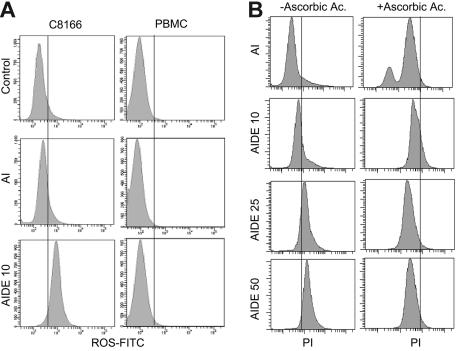
Because previous studies have shown that lower levels of ROSs promote apoptosis and higher intracellular levels of ROSs are associated with necrosis, we tested whether emodin and DHA treatment may increase the concentration of intracellular ROSs in HTLV-I cells following treatment with As/IFN-α and DHA/emodin. ROS production was not changed in As/IFN-α–treated C8166 cells, but ROS production significantly increased when DHA and emodin were added with As/IFN-α. PBMCs used as control were not affected under the same experimental conditions (Figure 4A).
Figure 4.
AIDE increases generation of ROS and Bax expression. (A) Measure of ROS production in PBMC control and HTLV-I–transformed C8166 cells treated with AI or AIDE. (B) Measure of cell death in AIDE-treated C8166 cells in the presence or absence of ascorbic acid, a ROS scavenger.
To demonstrate that ROSs were involved in HTLV-I–cell death, we used ascorbic acid, a well-known ROS scavenger. Treatment with ascorbic acid effectively reduced the generation of ROSs in C8166 cells treated with As/IFN-α and emodin and DHA (data not shown). In parallel experiments the presence of ascorbic acid efficiently prevented the death of HTLV-I cells treated with As/IFN-α and emodin and DHA as demonstrated by propidium iodide and FACS analysis (Figure 4B; compare left and right panels). Generation of ROSs has been linked to increased Bax expression, and ROS scavengers can prevent cell death by reducing Bax protein expression. In fact, when we performed Western blot analysis in HTLV-I cells treated with As/IFN-α and emodin and DHA in the absence or in the presence of ascorbic acid, we found that the presence of ascorbic acid reduced Bax expression by 30% to 35% (data not shown).
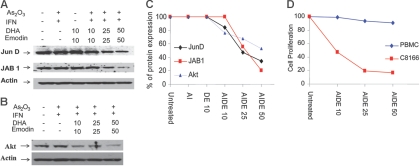
As/IFN with emodin and docosahexaenoic acid inhibit Akt pathway and reduce levels of JunD and JAB1 expression in HTLV-I cells
Recent studies suggest that DHA inhibits activator protein 1 (AP-1) activity. AP-1 plays a critical role in HTLV-I oncogenesis, is expressed at high levels in ATL patient samples, and may be required for maintenance of the malignant phenotype.40 Among AP-1's components, c-Jun is a potent transcriptional activator, and its activity is regulated through phosphorylation by Jun N-terminal kinase (JNK), a member of the MAP kinase family.58 AP-1 becomes activated through phosphorylation of Jun family transcription factors by members of the c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) family. Previously, constitutive JNK activation has been reported in HTLV-I–infected T cells,41,42 suggesting that HTLV-I–induced JNK activation may contribute to the activation of AP-1 in T cells. However, in our experiments only a small decrease in phosphorylation of JNK2 or JNK3 was detected in HTLV-I cells treated with As/IFN-α with emodin and DHA (data not shown). Previous studies found that JunD is the major component of the AP-1–DNA complex in HTLV-1–infected T-cell lines.43–45 Others have reported that the Jun-activating binding protein (JAB1) selectively potentiates transactivation by JunD.46 Interestingly, the expression of both JunD and JAB1, in cells treated with As/IFN with DHA and emodin, was reduced (Figure 5A). Because recent studies suggest that Akt, an essential survival pathway, is activated by HTLV-I,47,48 we also tested expression of Akt in HTLV-I cells treated with As/IFN-α and with emodin and DHA. Although As/IFN-α had no significant effect on Akt expression, the addition of emodin and DHA strongly down-regulated Akt expression by 25% to 50% (Figure 5B).
Figure 5.
AIDE inhibits Akt and AP-1 pathways in HTLV-I–transformed cells. (A) Western blot analysis of AP-1 members JunD and JAB1 in C8166 cells after treatment with As2O3/IFN-α in the absence or presence of increasing doses of emodin and DHA. Actin was used to confirm equal loading. (B) Western blot analysis of Akt in C8166 cells treated with As2O3/IFN-α in the absence or presence of increasing doses of emodin and DHA. (C) Spot densitometry quantification expressed as the percentage of untreated control (100%) for Akt, JunD, and JAB1. (D) Proliferation assay of C8166 cells and PBMCs treated with As2O3/IFN-α and increasing doses of emodin and DHA.
Spot densitometry quantification indicated that a longer incubation time or a higher dose was required to achieve inhibition levels comparable to those observed on Akt, and that JunD was more affected than JAB1 (Figure 5C). However, proliferation assays demonstrated that HTLV-I–transformed cells are more susceptible to As/IFN-α and with emodin and DHA than normal PBMCs (Figure 5D). Antiproliferative effects were more pronounced when emodin and DHA were used at 25 and 50 μmol/L, suggesting that the inhibition of both AP-1 and Akt may have an additive effect on preventing growth of HTLV-I–transformed cells.
Discussion
Disruption of cellular death pathways can contribute to a number of diseases, including cancer. It is now well established that anticancer agents induce apoptosis or necrosis and that disruption of cellular death pathways can reduce treatment sensitivity. In vitro and in vivo HTLV-1–transformed cells are highly resistant to most death-inducing agents. Although the basis for this resistance is not fully understood, it has been reported that HTLV-I–infected cells overexpress antiapoptotic factors such as Bcl-2, Bcl-xL, survivin, and I-309.49–52
Studies suggest a broad spectrum of antileukemic activity for As2O3 because of its ability to increase intracellular ROS production.53 In As2O3-sensitive cells, such as the APL-derived cell line NB-4, increased intracellular levels of ROSs lead to collapse of the mitochondrial membrane potential, activation of the caspases, and apoptosis. However, cells with intracellular antioxidant defense mechanisms, such as the acute myeloid leukemia (AML)–derived cell line HL-60, are resistant to As2O3. To render As2O3-resistant tumor cells more susceptible to As2O3-mediated cell death, ways to enhance the effects of As2O3 have been investigated. Importantly, agents modulating the GSH redox system as well as agents increasing intracellular ROS concentrations have been shown to increase As2O3 efficacy in cells otherwise resistant to As2O3.54 It is well known that clinically achievable concentrations of As2O3 (that is, 1-2 μM) induce insufficient amounts of ROSs to induce apoptosis of HL-60 cells in vitro. However, recent studies have shown an enhancing effect of DHA on As2O3-mediated cytotoxicity in some As2O3-resistant cell lines, indicating that the combined effect of As2O3 and DHA is not restricted to AML-derived HL-60 cells but may also be observed in other As2O3-resistant hematologic malignancies, including Burkitt lymphoma and hairy-cell leukemia. Recent clinical studies have shown some antileukemic effect of As/IFN-α, suggesting that ATL cells may respond by apoptosis or cell-cycle arrest in vivo. Differences in sensitivity to arsenic, as well as the toxicity associated with its use, make it worthwhile to investigate drug combinations that could enhance the actions of arsenic and reduce the level necessary for treatment. In this study, we found a synergistic effect of the combination of As/IFN-α with emodin and DHA in HTLV-I–infected cells. IL-2–dependent or IL-2–independent HTLV-I–infected cells, as well as freshly isolated patient samples, were all highly sensitive to this drug combination. The rationale for this drug combination was because emodin has been shown to reduce invasiveness of tumor cells55 and to enhance As-mediated ROS production.56,57 In addition, DHA was added to this combination because DHA sensitizes tumor cells to ROS-inducing agents (As and emodin in our case).
Our results indicate a potent antiproliferative effect on tumor cells, which was linked to the accumulation of hypophosphorylated Rb and G0/G1 arrest. Previous reports have shown that Tax can induce JNK activity and c-Jun phosphorylation, leading to the formation of Smad3/c-Jun complexes and inhibition of TGF-β signaling.41 In fact, in ATL and in normal T cells transduced by Tax, c-Jun is constitutively phosphorylated and acts as a repressor of TGF-β signaling. Thus, our results suggest that As/IFN-α in the presence of emodin and DHA may lead to reactivation of TGF-β, thereby preventing the growth of tumor cells. Further investigations in this area are warranted. We also found a significant reduction in Akt expression which may in part be responsible for increased cell death in treated cells.
Previous studies have shown that HL-60 cells are resistant to clinically relevant doses of arsenic but have 90% reduction in viability, increased intracellular ROSs, and up-regulation of Bax when treated with a combination of arsenic and DHA. Similarly, we readily detected increased ROS production in HTLV-I–infected cells, but not in healthy PBMCs, treated with As/IFN-α and emodin and DHA. To further demonstrate that ROS production played a role in tumor growth suppression and death of HTLV-I–expressing cells we measured cell death by FACS in the absence or presence of ascorbic acid, a ROS scavenger. Results clearly demonstrate the importance of ROSs, because significant cell death was prevented by ascorbic acid with concomitant down-regulation of Bax to normal endogenous levels. In human hematopoietic malignancies, continuous AP-1 DNA binding activity involving JunD has been found in cell lines of cutaneous T-cell lymphoma and patients with Sézary syndrome. Previously, it has been shown that HTLV-I–transformed cells and samples from patients with ATL have constitutive AP-1 and JNK activity and that these may play a role in the maintenance of the tumor growth potential. In fact, our data are in agreement with these observations, because we found an inhibition of the AP-1 pathway characterized by a dose-dependent decrease of JunD and JAB1. Inhibition of NF-kB pathway or survivin expression by As/IFN-α have also been reported by other groups and are likely to play a role in the suppression of tumor-cell proliferation by arsenic. However, they do not appear to be involved in the synergy described here between As/IFN-α, emodin, and DHA and, therefore, were not further investigated.
In conclusion, we have shown high synergy of As/IFN-α with emodin and DHA treatment with regard to the inhibition of proliferation and induction of cell death of HTLV-I–transformed T cells. In addition, the synergistic effectiveness of the combination treatment at pharmacologic doses was specific for HTLV-I–infected cells and had marginal effect on PBMCs isolated from healthy donors. The drug combination described here allows the As concentration to be decreased by 100-fold while preserving its antitumor activity, suggesting a broad application in the treatment of leukemias and lymphomas and warrants consideration for a phase 2 clinical study.
Acknowledgment
This work was supported by the National Cancer Institute (grant RO1CA106258) (C.N.). The flow cytometry core is supported in part by National Institutes of Health grant PZO RR016443 from the National Center for Research Resources.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.B. performed experiments described in Figures 1 to 5; M.B. performed some experiments described in Figure 3C; C.N. is responsible for the design of the project and interpretation of the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Nicot, University of Kansas Medical Center, Department of Microbiology, Immunology, and Molecular Genetics, 3025 Wahl Hall West, 3901 Rainbow Blvd, Kansas City, KS 66160; e-mail: cnicot@kumc.edu.
References
- 1.Bazarbachi A, Hermine O. Treatment of adult T-cell leukaemia/lymphoma: current strategy and future perspectives. Virus Res. 2001;78:79–92. doi: 10.1016/s0168-1702(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 2.Bazarbachi A, Ghez D, Lepelletier Y, et al. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 2004;5:664–672. doi: 10.1016/S1470-2045(04)01608-0. [DOI] [PubMed] [Google Scholar]
- 3.Mahieux R, Hermine O. In vivo and in vitro treatment of HTLV-1 and HTLV-2 infected cells with arsenic trioxide and interferon-alpha. Leuk Lymphoma. 2005;46:347–355. doi: 10.1080/10428190400019966. [DOI] [PubMed] [Google Scholar]
- 4.Besson C, Panelatti G, Delaunay C, et al. Treatment of adult T-cell leukemia-lymphoma by CHOP followed by therapy with antinucleosides, alpha interferon and oral etoposide. Leuk Lymphoma. 2002;43:2275–2279. doi: 10.1080/1042819021000039983. [DOI] [PubMed] [Google Scholar]
- 5.Taguchi H, Kinoshita KI, Takatsuki K, et al. An intensive chemotherapy of adult T-cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, ranimustine, and mitoxantrone with granulocyte colony-stimulating factor support. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:182–186. doi: 10.1097/00042560-199606010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- 7.White JD, Wharfe G, Stewart DM, et al. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2001;40:287–294. doi: 10.3109/10428190109057927. [DOI] [PubMed] [Google Scholar]
- 8.Matutes E, Taylor GP, Cavenagh J, et al. Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol. 2001;113:779–784. doi: 10.1046/j.1365-2141.2001.02794.x. [DOI] [PubMed] [Google Scholar]
- 9.Pagano JS. Interferon alfa and zidovudine in adult T-cell leukemia-lymphoma. N Engl J Med. 1995;333:1285–1286. [PubMed] [Google Scholar]
- 10.Hermine O, Bouscary D, Gessain A, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332:1749–1751. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- 11.Datta A, Bellon M, Sinha-Datta U, et al. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108:1021–1029. doi: 10.1182/blood-2006-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajima K, Amakawa R, Uehira K, et al. Adult T-cell leukemia successfully treated with allogeneic bone marrow transplantation. Int J Hematol. 2000;71:290–293. [PubMed] [Google Scholar]
- 13.Obama K, Tara M, Sao H, et al. Allogenic bone marrow transplantation as a treatment for adult T-cell leukemia. Int J Hematol. 1999;69:203–205. [PubMed] [Google Scholar]
- 14.Borg A, Yin JA, Johnson PR, et al. Successful treatment of HTLV-1-associated acute adult T-cell leukaemia lymphoma by allogeneic bone marrow transplantation. Br J Haematol. 1996;94:713–715. doi: 10.1046/j.1365-2141.1996.02338.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohno R, Masaoka T, Shirakawa S, et al. Treatment of adult T-cell leukemia/lymphoma with MST-16, a new oral antitumor drug and a derivative of bis(2,6-dioxopiperazine). The MST-16 Study Group. Cancer. 1993;71:2217–2221. doi: 10.1002/1097-0142(19930401)71:7<2217::aid-cncr2820710709>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsuda H, Takatsuki K, Ohno R, et al. Treatment of adult T-cell leukaemia-lymphoma with irinotecan hydrochloride (CPT-11). CPT-11 Study Group on Hematological Malignancy. Br J Cancer. 1994;70:771–774. doi: 10.1038/bjc.1994.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldmann TA, Goldman CK, Bongiovanni KF, et al. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988;72:1805–1816. [PubMed] [Google Scholar]
- 18.Tomita M, Kawakami H, Uchihara JN, et al. Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006;118:765–772. doi: 10.1002/ijc.21389. [DOI] [PubMed] [Google Scholar]
- 19.Mori N, Yamada Y, Ikeda S, et al. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood. 2002;100:1828–1834. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- 20.Satou Y, Nosaka K, Koya Y, et al. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–1363. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- 21.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL), I: As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 22.Zhu J, Koken MH, Quignon F, et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 24.Hermine O, Dombret H, Poupon J, et al. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J. 2004;5:130–134. doi: 10.1038/sj.thj.6200374. [DOI] [PubMed] [Google Scholar]
- 25.Nasr R, Rosenwald A, El Sabban ME, et al. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood. 2003;101:4576–4582. doi: 10.1182/blood-2002-09-2986. [DOI] [PubMed] [Google Scholar]
- 26.Mahieux R, Pise-Masison C, Gessain A, et al. Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1- and type 2-infected cells by a caspase-3-dependent mechanism involving Bcl-2 cleavage. Blood. 2001;98:3762–3769. doi: 10.1182/blood.v98.13.3762. [DOI] [PubMed] [Google Scholar]
- 27.El Sabban ME, Nasr R, Dbaibo G, et al. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 2000;96:2849–2855. [PubMed] [Google Scholar]
- 28.Bazarbachi A, El Sabban ME, Nasr R, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999;93:278–283. [PubMed] [Google Scholar]
- 29.Ishitsuka K, Hanada S, Suzuki S, et al. Arsenic trioxide inhibits growth of human T-cell leukaemia virus type I infected T-cell lines more effectively than retinoic acids. Br J Haematol. 1998;103:721–728. doi: 10.1046/j.1365-2141.1998.01068.x. [DOI] [PubMed] [Google Scholar]
- 30.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 31.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner M, Sturlan S, Roth E, Wessner B, Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int J Cancer. 2004;112:707–712. doi: 10.1002/ijc.20462. [DOI] [PubMed] [Google Scholar]
- 33.Yi J, Yang J, He R, et al. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 34.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 35.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem. 2004;279:6753–6760. doi: 10.1074/jbc.M310145200. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Yamaguchi H, Tian C, et al. Arsenic trioxide (As(2)O(3)) induces apoptosis through activation of Bax in hematopoietic cells. Oncogene. 2005;24:3339–3347. doi: 10.1038/sj.onc.1208484. [DOI] [PubMed] [Google Scholar]
- 37.Bellon M, Datta A, Brown M, et al. Increased expression of telomere length regulating factors TRF1, TRF2 and TIN2 in patients with adult T-cell leukemia. Int J Cancer. 2006;119:2090–2097. doi: 10.1002/ijc.22026. [DOI] [PubMed] [Google Scholar]
- 38.Kehn K, Fuente CL, Strouss K, et al. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene. 2005;24:525–540. doi: 10.1038/sj.onc.1208105. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Yamada Y, Koji T, et al. Expression and phosphorylation status of retinoblastoma protein in adult T-cell leukemia/lymphoma. Leuk Res. 2000;24:299–305. doi: 10.1016/s0145-2126(99)00186-1. [DOI] [PubMed] [Google Scholar]
- 40.Mori N, Fujii M, Iwai K, et al. Constitutive activation of transcription factor AP-1 in primary adult T-cell leukemia cells. Blood. 2000;95:3915–3921. [PubMed] [Google Scholar]
- 41.Arnulf B, Villemain A, Nicot C, et al. Human T-cell lymphotropic virus oncoprotein Tax represses TGF-beta 1 signaling in human T cells via c-Jun activation: a potential mechanism of HTLV-I leukemogenesis. Blood. 2002;100:4129–4138. doi: 10.1182/blood-2001-12-0372. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, Heidenreich O, Kitajima I, et al. Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene. 1996;13:135–142. [PubMed] [Google Scholar]
- 43.Tomita M, Kawakami H, Uchihara JN, et al. Curcumin suppresses constitutive activation of AP-1 by downregulation of JunD protein in HTLV-1-infected T-cell lines. Leuk Res. 2006;30:313–321. doi: 10.1016/j.leukres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Thebault S, Basbous J, Hivin P, Devaux C, Mesnard JM. HBZ interacts with JunD and stimulates its transcriptional activity. FEBS Lett. 2004;562:165–170. doi: 10.1016/S0014-5793(04)00225-X. [DOI] [PubMed] [Google Scholar]
- 45.Fujii M, Iwai K, Oie M, et al. Activation of oncogenic transcription factor AP-1 in T cells infected with human T cell leukemia virus type 1. AIDS Res Hum Retroviruses. 2000;16:1603–1606. doi: 10.1089/08892220050193029. [DOI] [PubMed] [Google Scholar]
- 46.Chamovitz DA, Segal D. JAB1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep. 2001;2:96–101. doi: 10.1093/embo-reports/kve028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Wang Y, Yamakuchi M, et al. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene. 2001;20:2514–2526. doi: 10.1038/sj.onc.1204364. [DOI] [PubMed] [Google Scholar]
- 49.Nicot C, Mahieux R, Takemoto S, Franchini G. Bcl-X(L) is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood. 2000;96:275–281. [PubMed] [Google Scholar]
- 50.Nicot C, Astier-Gin T, Guillemain B. Activation of Bcl-2 expression in human endothelial cells chronically expressing the human T-cell lymphotropic virus type I. Virology. 1997;236:47–53. doi: 10.1006/viro.1997.8720. [DOI] [PubMed] [Google Scholar]
- 51.Mori N, Yamada Y, Hata T, et al. Expression of survivin in HTLV-I-infected T-cell lines and primary ATL cells. Biochem Biophys Res Commun. 2001;282:1110–1113. doi: 10.1006/bbrc.2001.4708. [DOI] [PubMed] [Google Scholar]
- 52.Ruckes T, Saul D, Van Snick J, Hermine O, Grassmann R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 2001;98:1150–1159. doi: 10.1182/blood.v98.4.1150. [DOI] [PubMed] [Google Scholar]
- 53.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 54.Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T. Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood. 2003;101:4990–4997. doi: 10.1182/blood-2002-08-2391. [DOI] [PubMed] [Google Scholar]
- 55.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 2004;68:361–371. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 56.Yi J, Yang J, He R, et al. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 57.Yi J, Gao F, Shi G, et al. The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis. 2002;7:209–215. doi: 10.1023/a:1015331229263. [DOI] [PubMed] [Google Scholar]
- 58.Natoli G, Costanzo A, Moretti F, Fulco M, Balsano C, Levrero M. Nuclear factor kappaB (NFkappaB)-inducing kinase requirement for activation of activating protein 1 and NFkappaB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem. 1997;272:26079–26082. doi: 10.1074/jbc.272.42.26079. [DOI] [PubMed] [Google Scholar]