Abstract
Estrogen and progesterone act on gene and protein expression in serotonin neurons in a manner that suggests serotonin neurotransmission should increase. However, measurement of extracellular serotonin in macaques was lacking. Elevated prolactin secretion can be an indicator of increased serotonergic function and prolactin is increased by combined estrogen and progesterone treatment. We examined extracellular serotonin by microdialysis in a well-characterized macaque model of steroid-induced prolactin secretion. Monkeys were fitted with 2 guide tubes directed to the arcuate nucleus of the hypothalamus. Samples (75μl/15-min interval) were obtained via a tether-swivel device through sample lines into an adjoining room. Serotonin was measured with a modified commercial enzyme linked immunoassay (ELISA) kit. Fenfluramine infused through the probe (300 μM for 2 h; n=2 trials) or administered intravenously (2.5 mg/kg; n=2 trials) caused a marked increase in extracellular serotonin and verified the efficacy of the procedure. Three monkeys were maintained with an estrogen implant for 2 weeks. Each monkey was injected with 20 mg of progesterone s.c. in oil at 1500h; microdialysis was initiated the next morning and samples were obtained for 24 h. There was a significant increase in serotonin between 40 and 43 h after the progesterone injection (P < 0.001, ANOVA). Serotonin averaged 59±1 pg/sample from 18-30 h post-progesterone injection, and averaged 76±2 pg/sample from 30-48 h post-progesterone injection (P < 0.0001; t-test). Since the increase in serotonin is delayed by ∼40 h after progesterone-injection, we speculate that the action of progesterone may involve either nuclear progestin receptors or membrane progestin receptors.
Keywords: estrogen, progesterone, hypothalamus, Macaca mulatta, fenfluramine, microdialysis
1. Introduction
Serotonin neurons in the dorsal and median raphe project to most areas of the forebrain, but the target areas vary with regard to the proportion of terminals from the two nuclei (Azmitia, 1978; Azmitia and Gannon, 1986; Azmitia and Segal, 1978). In macaques, lesion of the axons from the dorsal raphe with MDMA (ectasy; 3,4-methylenedioxymethamphetamine) causes about 50% loss of serotonin immunoreactive fibers in the hypothalamus suggesting that the hypothalamic serotonergic innervation derives from both nuclei in nearly equal proportions (Rischer et al., 1995). The 5-HT2C receptor is densely expressed in the mediobasal hypothalamus, as well (Gundlah et al., 1999).
Studies from this laboratory have shown that estrogen and progesterone act on gene and protein expression in serotonin neurons of the macaque dorsal raphe in a manner that could lead to an increase in serotonin neurotransmission (Bethea et al., 2002; Sanchez et al., 2005). Of note, estrogen and progesterone increase tryptophan hydroxylase −2 (TPH-2) expression and translation (Sanchez et al., 2005; Smith et al., 2004), while decreasing 5-HT1A autoreceptor expression, translation and coupling to the G protein (Lu and Bethea, 2002; Pecins-Thompson and Bethea, 1998). However, the effect of estrogen and progesterone treatment on extracellular serotonin in macaques needs confirmation. Therefore, we sought an appropriate hormone replacement paradigm in which to examine dynamic changes in extracellular serotonin.
In nonhuman primates, estrogen treatment alone has little effect on prolactin secretion. However, if an animal is treated with estrogen for two weeks, that is “estrogen primed”, and then additionally treated with progesterone, prolactin secretion increases significantly during the progesterone supplementation (Williams et al., 1981; Williams et al., 1985). The increase in prolactin secretion can be sustained for long periods with continuous estrogen+progesterone treatment provided with Silastic capsules containing crystalline steroid hormones. Earlier work in this laboratory showed that an injection of progesterone to an estrogen-primed monkey causes a transient increase in prolactin that occurs between 36 and 48 h after the injection (Pecins-Thompson and Bethea, 1997; Pecins-Thompson et al., 1996).
Elevated prolactin secretion can be an indicator of increased serotonergic function and some evidence suggests that progesterone acts in estrogen-primed monkeys to increase prolactin secretion through serotonin neurotransmission. In monkeys maintained with Silastic capsules containing hormones, 5 hydroxyindole acetic acid (5-HIAA) decreased in cerebral spinal fluid with estrogen+progesterone, but not estrogen alone (Bethea et al., 1999). CSF 5HIAA also decreased in male monkeys treated with a synthetic progestin, cyproterone acetate (Eaton et al., 1999). Although disputed, we believe the decrease in 5-HIAA reflects a decrease in serotonin degradation, which in turn, could promote higher serotonin levels in the extracellular space (Gundlah et al., 2001). Using the acute progesterone injection model, we found that fluoxetine augmented estrogen+progesterone-induced prolactin secretion, but had no effect on prolactin secretion during estrogen priming (Pecins-Thompson and Bethea, 1997). These data raised the possibility that progesterone treatment of an estrogen primed monkey caused an increase in extracellular serotonin.
Microdialysis in awake animals followed by serotonin measurement of the dialysate was initially successful in rodent models (Gundlah et al., 1997; Rutter et al., 1994). Extracellular serotonin in the hypothalamus was lower during estrous than diestrus in female rats (Gundlah et al., 1998). Spies and colleagues employed a variation of microdialysis, called push-pull perfusion, in awake monkeys tethered via flexible steel cable attached to a headpiece on the monkey and a swivel device at the top of the home cage (Fuchs et al., 1986; Pau et al., 1993). Later, this procedure was adapted to microdialysis enabling remote sampling of extracellular fluids in semi-freely behaving macaques (Pau et al., 2000). Recently, microdialysis was used in awake monkeys who had been adapted to primate chairs and the effect of fluoxetine on extracellular serotonin, 5-HIAA and dopamine was determined (Smith et al., 2000). However, the expertise to use the remote sampling protocol was available for this study.
Thus, direct evidence for steroid-induced serotonin release was sought with experiments utilizing microdialysis in the hypothalamus in our well-characterized macaque model of steroid-induced prolactin secretion. In this paradigm, an injection of progesterone to an estrogen-primed animal causes an increase in prolactin between 36-48 h after the injection, which provides a window during which we could measure serotonin.
2. Materials and Methods
The ONPRC Institutional Animal Care and Use Committee approved this study. All procedures were in compliance with the NIH Guidelines for the Use of Animals and with USDA regulations regarding the use of nonhuman primates. Chemicals and reagents were from Sigma unless otherwise stated.
2.1. Subjects
Adult female rhesus monkeys that had been ovariectomized for 6-12 months were selected for this study. The monkeys were of Chinese origin and weighed between 4 and 7 kg. Their ages were approximately 6-10 years as determined by dental exam. They were in overall good health and exhibited relatively calm personalities with no stereotypical behaviors.
2.2. Headpiece
The calvarium headpiece consisted of 2 major components: a nylon pedestal machined from type-1010 nylon rod with a hollow center and a catheter tunnel and guide cannulae that were fixed in position by filling the hollow pedestal with dental acrylic. The guide cannulae were fabricated from stainless steel tubing and sized to accommodate the microdialysis probes. An individual lateral roentgenogram of each animal's skull was used to predetermine the lengths of the guide cannulae. Lateral roentgenegrams were obtained continuously during placement until the cannulae reached the final destination. The headpiece was sterilized with ethylene oxide for 1 hour followed by 12 h of aeration.
2.3. Surgical Procedures
The stereotaxic procedures for implantation of a microdialysis headpiece and cannula were similar to those described previously for push-pull perfusion and microdialysis employed by Dr. Harold G. Spies for measurement of extracellular norepinephrine and GnRH in the hypothalamus (Fuchs et al., 1986; Pau et al., 1993; Pau et al., 1989; Pau et al., 2000). The heads of rhesus macaques are too variable for use of a stereotaxic atlas. Instead, placement of the guide cannulae is calculated from lateral roentgenograms that reveal critical bony structures. The landmark structure is the sella turcica, which forms the base of the medial basal hypothalamus. Anaesthetized monkeys (maintained with 2±4% isoflurane in 100% oxygen) were placed in the stereotaxic head holder and small openings in the skull and dura were made. The superior sagittal sinus was retracted for a clear view of the great longitudinal fissure and the headpiece with microdialysis guide cannula was lowered into the arcuate nucleus-median eminence of the hypothalamus. Two guide cannulae set at 2 mm apart were positioned, one behind the other, at equal distance from the anterior and posterior edges of the sella turcica on one side of the mediobasal hypothalamus. A stylette was inserted into each guide tube until time for microdialysis. The whole calvarium headpiece was precisely placed by using serial lateral roentgenograms and secured by acrylic (Co-oral-ite Dental Manufacturing Co., Santa Monica, CA) during a single intracranial surgery. The anteroposterior position and the depth of the microdialysis guide cannula were determined by the use of lateral X-rays taken at several depth levels for each animal. The lateral position was 0.6mm to the left or right of the sagittal midline. The sterilized headpiece was attached to the calvarium by bone screws and dental acrylic cement, and the microdialysis guide cannula was plugged with a sterile stylette. A subclavian catheter was also inserted at this time (silastic tubing i.d. 0.030″; o.d. 0.065″), and the catheter was brought to the headpiece via a subcutaneous tunnel. Patency was maintained with a heparin lock until microdialysis. After surgery, monkeys were given analgesics (nalbuphine, 5mg i.m., or oral acetaminophen, 160 mg) and antibiotics (chloramphenicol, 375mg i.m.) until the skin was completely healed around the headpiece. After 2 weeks, the monkeys were placed on a training swivel-tether system (Pau et al., 1989). The animals were allowed to adapt to this tether for 7-10 days, during which all animals regained their typical activity including normal appetite.
2.4. Collection of microdialysate samples
The microdialysis probe was manufactured by CMA/Microdialysis AB (Stockholm, Sweden, distributed by Carnegie Medicin, Acton, MA, USA). The probe was stock CMA/10, Catalogue no. 8309540. It had a 70mm shaft length, 0.6mm shaft outer diameter, 0.5mm membrane outer diameter, 3mm membrane length with approximately 20,000 Da molecular weight cut-off. On the day of an experiment, a new swivel-tether set including the microdialysis probe was mounted on the cage top. The microdialysis probe and its inflow/outflow lines were equilibrated using Kreb's Ringer phosphate buffer (KRP, pH=7.4) at a constant flow rate of 5 μl/min by a microdialysis pump (CMA/100, Carnegie Medicin) under sterile conditions. After air in the microdialysis system was excluded and the flow rate and membrane integrity was established (constant outflow of KRP at 75 μl/15 min for 60 min), the animal was briefly sedated with Propofol (2 mg/kg i.v.), and the microdialysis probe was inserted into the arcuate nucleus-median eminence via the guide cannula. Medical vinyl tubing (PV-6, Scientific Commodities, Inc., Lake Havasu City, AZ, USA) was also connected to the subclavian vein catheter via the headpiece connection at this time. The connection procedure usually took less than 10 min. The monkey was allowed to recover from the Propofol sedation for 1 hour, during which time the microdialysis system was evaluated for constant flow rate. Thereafter, each microdialysate sample of 75 μl per 15 min was immediately snap-frozen in a dry ice/ethanol bath. The samples were then stored at 80°C until assayed with an ELISA for serotonin. Blood samples were collected at 60 min intervals throughout the microdialysis period for serum estrogen, progesterone and prolactin measurement by radioimmunoassay (RIA).
The dead volume of a line from the pump to the tip of the microdialysis probe was approximately 300 μl. Upon completion of the dialysis, the animal was lightly sedated with Ketamine and the probes were replaced with stylettes. At the end of an entire experiment, the monkeys were deeply anesthetized with pentobarbital (3.0 mg/kg i.v.) and the brain removed for histological verification of the probe positions as described below.
2.5. Hormone Treatments
After the animals recovered from the stereotaxic surgery and accepted the tether system, they were sedated with ketamine and a 4.5 cm Silastic capsule containing crystalline estradiol (inner diameter=0.132inches; outer diameter=0.183 inches, Dow Corning, Midland, MI) was implanted subcutaneously in the periscapular region. This treatment achieves between 50 and 100 pg/ml of estrogen in the serum. After two weeks of estrogen treatment, a subcutaneous injection of progesterone (20 mg in 1 ml of oil) was administered at 1500 hr. The microdialysis was initiated the following morning at 0900 hr (17 hr post-progesterone), and sample collection began at 1000 h.
2.6. Fenfluramine treatment for determination of efficacy
At 5 sites, after the estrogen+progesterone trial was concluded, an intravenous injection of fenfluramine was administered (2.5 mg/kg) and microdialysate samples were collected for an additional 3 h. Blood samples were obtained at 15-minute intervals after fenfluramine administration.
In 2 animals, the microdialysis was initiated after estrogen priming and fenfluramine was administered via the microdialysis probe directly into the mediobasal hypothalamus. Samples were obtained for 2 h to establish a baseline. Then, fenfluramine was added to the KRP microdialysate (300μM) for 2 h. The time for the fenfluramine to reach the brain was approximately 1 hour and the time for microdialysate from the brain to reach the sample tube was approximately 1 hour. Therefore, any change in serotonin concentration was expected in samples obtained 2 hr after the syringe was changed. After 2 hr of fenfluramine treatment, the animals were dialyzed with control KRP buffer for a further 2 h.
2.7. Elisa Assay for Serotonin
ELISA assays for serotonin content were performed using a Serotonin ELISA Kit (Immuno-Biological Laboratories, Hamburg, Germany) as previously described (Bethea et al., 2003). At the time of assay, the sample was thawed on ice and 50μl was removed to which 100μl of the kit assay buffer was added. Then, 25μl of kit acylation buffer was added for a total volume of 175μl. After incubation at 37°C for 15 min, 125 μl of assay buffer was added to the tubes and the precipitated proteins were removed by centrifugation (10 min at 2000 rpm). 50 μl of the supernatant was added to duplicate wells in the ELISA plate, which was then processed according to the directions of the manufacturer and read at 406nm with a microtiter plate reader. The serotonin content was determined by comparing the optical density of the samples with a standard curve containing increasing amounts of serotonin. The lower limit of detection equaled 5 pg. The inter- and intraassay coefficients of variation were less than 5% and 10%, respectively.
2.8. Steroid Measurements
Serum estrogen, progesterone and prolactin concentrations were measured in the blood samples obtained from the subclavian catheter with radioimmunoassay by the ONPRC Endocrine Services Laboratory utilizing a Roche Diagnostics 2010 Elecsys assay instrument. Each assay had been validated for macaque plasma in comparison with traditional RIA's. Interassay coefficients of variation were less than 10% for each assay.
2.9. Histological Verification of Probe Placement
To verify the probe placement, we used the NeuroTrace BDA-10,000 Neuronal Tracer Kit (Molecular Probes, Eugene, OR). A solution of lysine-fixable biotin dextran amine (BDA) at 10% was prepared according with manufacturers instructions. Monkeys were sedated with ketamine and 0.5 μl of BDA solution was injected with a microsyringe through the microdialysis guide cannula. After the injection, monkeys were euthanized with an overdose of pentobarbital (25 mg/kg, i.v.), according to the procedures recommended by the Panel on Euthanasia of the American Veterinary Association.
The left ventricle of the heart was cannulated and the head was perfused with 1 liter of 0.9% sodium chloride at room temperature, followed by 3 liters of cold (4 °C) paraformaldehyde (4% in 0.1 M borate, pH 9.5). Blocks of tissue containing the hypothalamus were post-fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose-4% paraformaldehyde-0.1 M borate, pH 9.5, for 48-60 h at 4 °C with continuous gentle agitation before they were snap-frozen in an isopentane bath cooled to the temperature of dry ice-ethanol and stored at −80 °C. Sections at 40 μm thickness were cut on a sliding microtome and serial sections were collected in a cryoprotecting buffer (30% ethylene glycol, 20% glycerol in 0.05 M PBS).
Free floating sections were washed in 0.02 M potassium phosphate-buffered saline (KPBS) and incubated overnight at 4 °C with a solution of Avidin-horseradish peroxidase provided by the kit, at a concentration of 1 μg/ml. Visualization was obtained using 0.5% diaminobenzidine (DAB, provided by the kit) in 0.02 M KPBS and 0.003% H2O2. After KPBS washes, sections were mounted on Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA, USA) and dried overnight. The next day, the sections were counterstained with hematoxylin, dehydrated through a graded series of ethanol and xylene and coverslipped with DPX (Electron Microscopy Sciences, Hatfield, PA, USA). Sections were examined and bright field images were captured using a Marianas imaging workstation (Intelligent Imaging Innovations, Denver, CO) and compared to a monkey atlas (Paxinos et al., 2000). A montage of the MBH was constructed using the Slidebook 4.1 software.
2.10. Statistical Analysis
Serotonin concentrations are expressed as pg/50 μl sample. The results of the i.v. fenfluramine challenges were parsed into responders and non-responders, which were compared with a two way ANOVA. In the estrogen+progesterone experiments, the overall average serotonin concentration from 18-30 h post-progesterone injection was compared to the overall average serotonin concentration from 30-48 h post-progesterone injection with Student's t-test and serotonin across time was examined with a one-way ANOVA.
3. Results
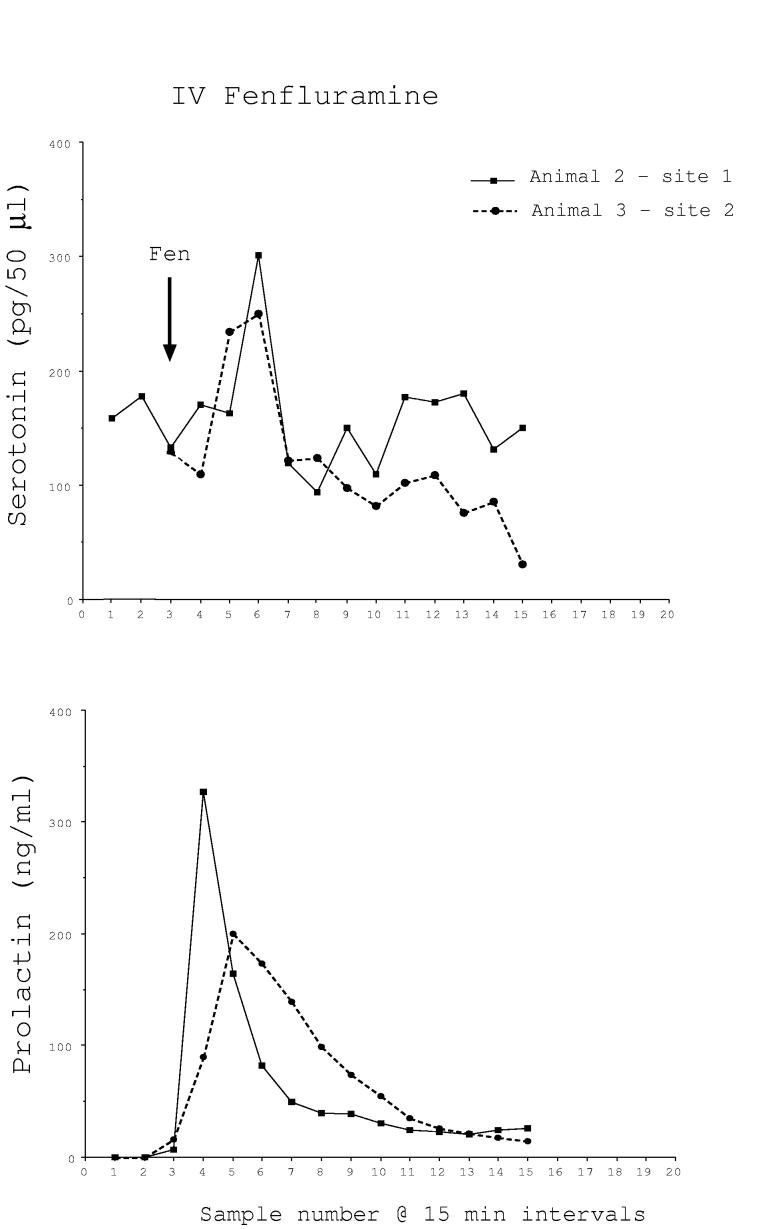
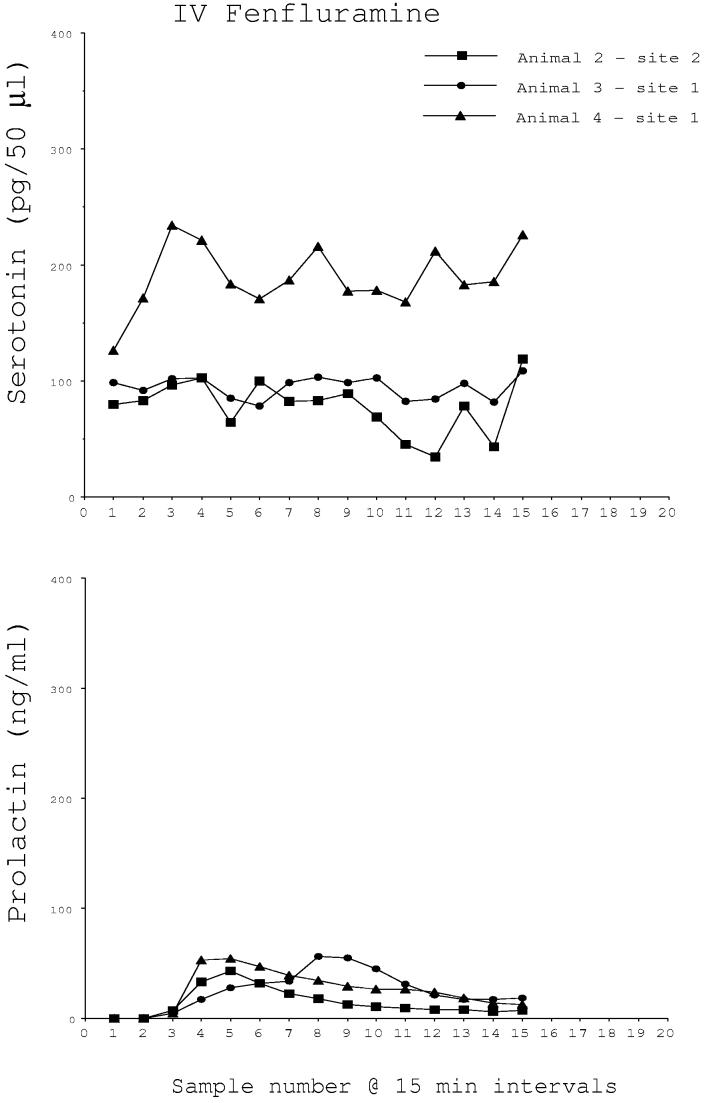
Fenfluramine (2.5 mg/kg) was injected intravenously at the end of 5 trials. Microdialysate and blood samples were collected at 15 min intervals for 3 h for serotonin and prolactin determinations. The results were variable. In 2 of 5 trials, serotonin and prolactin were markedly increased by fenfluramine. The peak of serotonin occurred in the brain at the same time as the peak of prolactin occurred in the serum, but appears delayed due to the travel time in the dialysis tubing (Fig. 1A). However, in the remaining trials, there was no apparent change in serotonin in the microdialysate and prolactin was marginally elevated (Fig. 1B). There was a significant difference in serotonin concentrations between the 5 sites by one-way ANOVA for repeated measures (P < 0.01). Fig. 1C illustrates the average serotonin and prolactin concentrations for the responders and non-responders. Two-way ANOVA found that there was a near significant difference in serotonin between the groups (P < 0.06) and there was a highly significant difference in prolactin between the groups (P<0.0001). The animals and sites included in the i.v. fenfluramine data are shown in Table 1.
Fig. 1A.
Microdialysate serotonin (pg/50μl) and serum prolactin (ng/ml) concentrations in two monkeys who responded robustly to intravenous administration of fenfluramine (2.5 mg/kg). With the time required for the serotonin in the brain to reach the sample tube equaling approximately 1 hour, the increase in serotonin and the increase in prolactin are temporally correlated.
Fig. 1B.
Microdialysate serotonin (pg/50μl) and serum prolactin (ng/ml) concentrations in 3 monkeys who did not respond to intravenous administration of fenfluramine (2.5 mg/kg). There was no apparent increase in extracellular serotonin although prolactin exhibited a modest increase.
Fig. 1C.
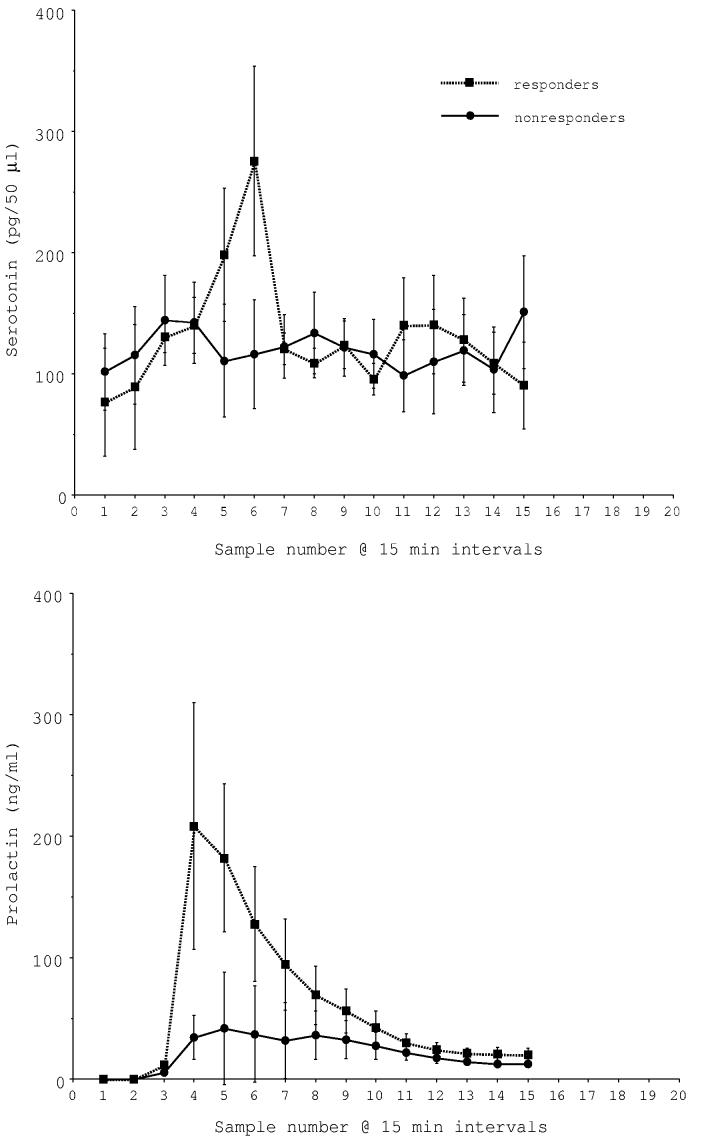
Average serotonin and prolactin concentrations in i.v. fenfluramine responders (n=2) and non-responders (n=3). There was a near significant difference in serotonin between the groups (P < 0.05; 2 way ANOVA) and a highly significant difference in prolactin between the groups (P < 0.0001; 2 way ANOVA).
Table 1.
Animals treated with i.v. fenfluramine (2.5 mg/kg) after microdialysis for analysis of steroid hormone effects. The location of the probe did not appear to impact the ability to measure serotonin or to affect prolactin secretion in response to fenfluramine.
| Site 1 | Site 2 | |||||
|---|---|---|---|---|---|---|
| Animal | Treatment | Probe Location |
Results | Treatment | Probe Location |
Results |
| 2 | E+P | Anterior | Responder | E+P | Anterior | Non-responder |
| 3 | E | MBH | Non-responder | E+P | MBH | Responder |
| 4 | E+P | MBH | Non-responder | |||
Abbreviations: E-estrogen; P-progesterone; MBH- medial basal hypothalamus
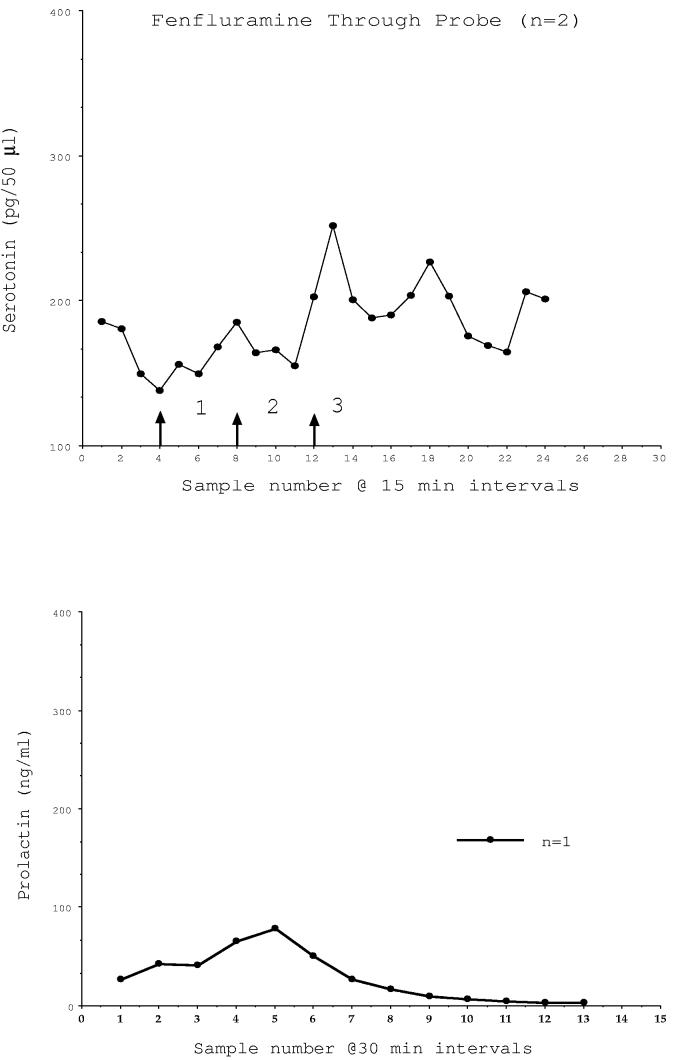
In two E-primed animals, fenfluramine administered directly to the mediobasal hypothalamus through the microdialysis probe caused a marked increase in the concentration of serotonin detected in the collected microdialysate (Fig. 2, top). Prolactin also increased in one of these animals in which the venous catheter remained patent (Fig. 2, bottom). The animals and sites included in the data for probe administration of fenfluramine are shown in Table 2.
Fig. 2.
Microdialysate serotonin (pg/50μl) and serum prolactin (ng/ml) concentrations in animals that were administered fenfluramine through the microdialysis probe. In 2 monkeys, an increase in extracellular serotonin was detected at the time expected based upon a traverse time to the brain of 1 hour and a traverse time from the brain to the sample tube of 1 hour. An increase in serum prolactin was detected at the time when fenfluramine reached the brain in one animal with a patent venous catheter. The arrows demarcate (1) the time at which the fenfluramine buffer was initiated, (2) the time at which the fenfluramine reached the brain and (3) the time that the potential increase in serotonin could first reach a sample tube.
Table 2.
Animals treated with fenfluramine (300 μM) for 2 h through the microdialysis probe.
| Animal | Site 1 | Site 2 | ||||
|---|---|---|---|---|---|---|
| Treatment | Probe Location | Results Used | Treatment | Probe Location | Results Used | |
| 5 | E | ventricle | no | E | MBH | yes |
| 6 | E | blocked | no | E | MBH | yes |
Abbreviations: E-estrogen; MBH-medial basal hypothalamus
Fig. 3 illustrates the average concentrations of serotonin from 4 microdialysis sites conducted with 3 animals (2 sites in one animal) primed with estrogen and then injected with progesterone. In one experiment, the microdialysis was started earlier than in the remaining experiments and prior to the injection of progesterone. Serotonin was low for many h in this animal, indicating that we could safely start the microdialysis at a later time point and reduce the potential for damage to the site. Serotonin began to increase between 15 and 20 h after the progesterone injection. The highest concentrations of serotonin were observed between 40 and 43 h which coincides with the expected progesterone-induced elevation in prolactin as previously described (Pecins-Thompson and Bethea, 1997; Pecins-Thompson et al., 1996). The average of the 4 serotonin determinations at each time point were further averaged across time intervals of 18-30 h and 30-48 h post progesterone injection. Serotonin averaged 59±1 pg/sample from 18-30 h post-progesterone injection and averaged 76±2 pg/sample from 30-48 h post-progesterone injection (P < 0.0001; t-test). The average serotonin concentrations of the 4 sites were further parsed into time brackets containing 3 means per bracket from 17.5 to 48 h post progesterone injection, which yielded 41 time brackets. There was a significant difference in serotonin across time (P < 0.0001, one way ANOVA). Time brackets 32, 33, and 34 corresponding to 40.75 to 42.75 h post progesterone injection were significantly different from all other time brackets (P < 0.05, Student-Newman-Keuls posthoc pairwise comparison).
Fig. 3.
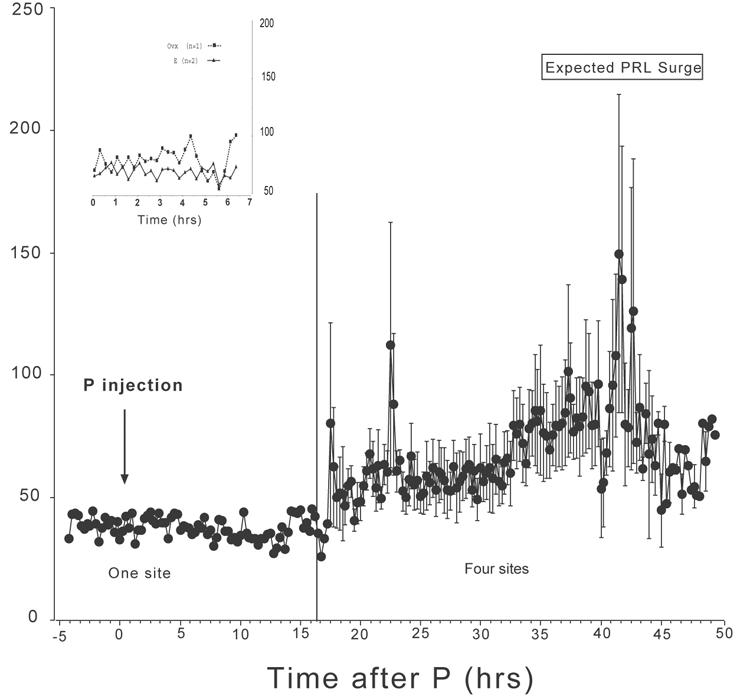
Microdialysate serotonin (pg/50μl) from 18 to 48 h at 15-minute intervals after an injection of progesterone to estrogen-primed animals (n=4). In one animal, samples were collected prior to and immediately following progesterone injection, as well. Microdialysate concentrations of serotonin were elevated over baseline from 40-43 h post progesterone injections and correlate temporally with progesterone-induced prolactin secretion from earlier studies. The overall concentration of serotonin is significantly higher between 30 and 48 h than between 15 and 30 h post progesterone injection (P < 0.001; t-test). The average serotonin concentrations of the 4 sites were further parsed into time brackets containing 3 means per bracket from 17.5 to 48 h post progesterone injection, which yielded 41 time brackets. There was a significant difference in serotonin across time (P < 0.0001, one way ANOVA). Time brackets 32, 33, and 34 corresponding to 40.75 to 42.75 h post progesterone injection were significantly different from all other time brackets (P < 0.05, Student-Newman-Keuls posthoc pairwise comparison). Insert. Microdialysate serotonin (pg/50μl) from 0-7 h at 15-minute intervals in an untreated ovariectomized monkey and in 2 monkeys primed with estrogen. Concentrations of serotonin remained low during this period.
In the animal in which the microdialysis was started early, the dialysate was collected for 7 h in the estrogen-primed state. We also collected dialysate from an untreated animal and an additional animal in the estrogen primed state. These data are shown in the inset to Fig. 3. Serotonin concentrations from the 2 sites with only estrogen priming are compared to concentrations from one site in an ovariectomized animal. There is little difference in serotonin concentrations between these sites.
Estrogen (pg/ml) concentrations in serum averaged 103.6±14.5 throughout the 4 trials. Progesterone (ng/ml) concentrations in the serum averaged 12.9±1.7 at 18 hr post injection and declined to 4.9±2.1 by 48 hr post-injection. The previously documented progesterone-induced increase in prolactin secretion was not observed in all animals. The animals and sites included in the estrogen+progesterone data are shown in Table 3.
Table 3.
Animals that were untreated, or treated with estrogen plus or minus progesterone injection, prior to microdialysis, to determine the effect of steroid treatment on serotonin concentrations. If the probe location was in the medial basal hypothalamus, then the data from the site was included in the estrogen+progesterone analysis.
| Animal | Site1 | Site2 | ||||
|---|---|---|---|---|---|---|
| TRT | Probe Location |
Results Used |
TRT | Probe Location |
Results Used |
|
| 1 | none | MBH | yes | E+P* | MBH | yes |
| 2 | E+P | Anterior | no | E+P | Anterior | no |
| 3 | E | MBH | yes | E+P | MBH | yes |
| 4 | E+P | MBH | yes | E+P | MBH | yes |
Abbreviations: E-estrogen; P-progesterone; MBH-medial basal hypothalamus
Dialysis initiated 7 h prior to P injection and 0-7 h serotonin data were used to obtain the average for E only treatment in Figure 3 insert.
Fig. 4 illustrates the probe placement, the design of the headpiece holding the guide cannulae, and a lateral roentgenogram from the same animal #4 obtained during the surgery. The guide cannulae barrels are located at the final destination. The microdialysis probe extends several millimeters beyond the end of the guide cannula. The DAB injection point is from the posterior site, which is located near the bottom of the 3rd ventricle of the mediobasal hypothalamus. The anterior site would have been located 2 mm in front of this point. All of the data reported from the estrogen+progesterone experiment are from animals with similar probe locations.
Fig. 4.
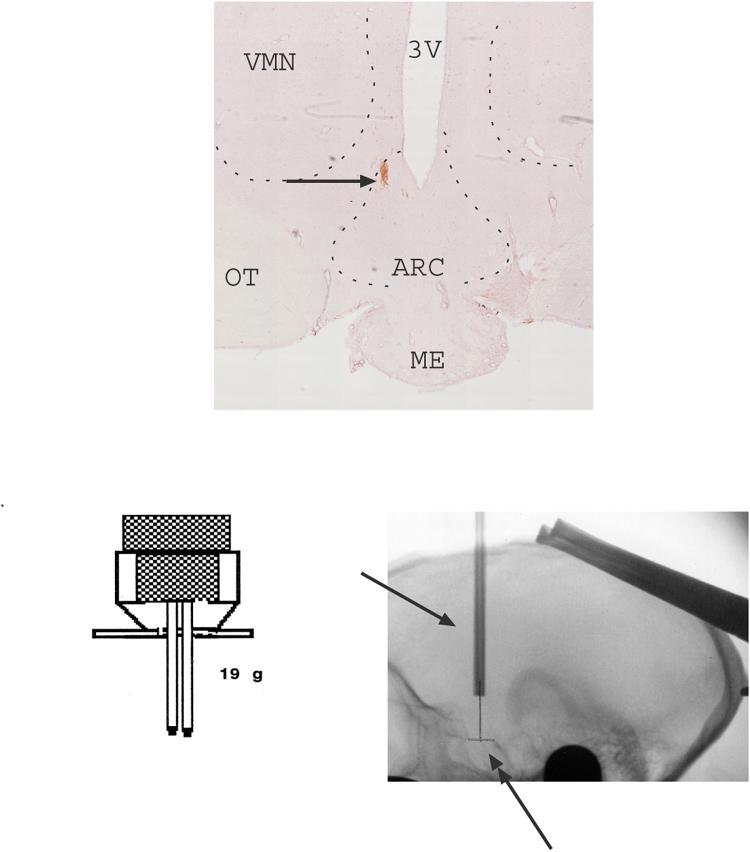
Montage of 10x images of the mediobasal hypothalamus of one representative monkey showing the posterior probe placement with DAB staining. The DAB injection site is located near the bottom of the 3rd ventricle of the mediobasal hypothalamus. The anterior site would have been located 2 mm in front of this point. In addition, the design of the headpiece holding the guide cannulae, and a lateral roentgenogram from the same animal (#4), are shown. The xray was obtained at final cannula placement and indicates the cannulae barrels (single-headed arrow). The double-headed arrow indicates the pencil mark on film, which marks the midpoint of the edges of the sella turcica. The single arrowheads indicate the anterior and posterior edges of the sella turcica, which are the critical landmarks for positioning of the guide cannulae. The guide cannulae are positioned at equal distance from the anterior and posterior points. The microdialysis probe extends several millimeters beyond the end of the guide tubes. All of the data reported for the estrogen+progesterone experiments are from animals with similar probe locations. This location contains arcuate nucleus neurons. Abbreviations: ARC-arcuate nucleus; ME- median eminence; OT- optic track; VMN- ventromedial nucleus; 3V- third ventricle.
4. Discussion
This technically challenging study found that extracellular serotonin in the mediobasal hypothalamus increases in response to fenfluramine administered directly through a microdialysis probe and that extracellular serotonin may or may not increase in response to fenfluramine administered intravenously. In addition, a significant increase in extracellular serotonin was observed in 4 animals primed with estrogen and injected with progesterone, but the increase occurred ∼40h after the progesterone injection.
The reason for the variability in the response to intravenous fenfluramine is not clear, but it is not due to animal variation. The two “responders” were also “non-responders” when the microdialysis was applied to the other site. It is also not due to the order of the microdialysis. One animal responded during her first trial and not her second, whereas the other animal responded during her second trial and not her first. One possibility is related to the order of the experiment. Fenfluramine was administered after the estrogen+progesterone trial was concluded which involved 24+h of microdialysis. This could have led to damage to the surrounding neuropile in some sites but not others. In order to assure ourselves that we could consistently detect a change in extracellular serotonin, we administered fenfluramine through the probe and observed a marked increase in extracellular serotonin in two animals. Thus, we were assured that if extracellular serotonin increased during our experimental paradigm, then it would be detectable.
Extracellular serotonin is significantly elevated between 40-43 h post-progesterone injection. We used ovariectomized and estrogen-primed monkeys to observe this regulation based upon previous data showing that this exact treatment increased prolactin secretion. Indeed, the elevation of serotonin correlates with the expected surge of prolactin. Serum prolactin did not consistently increase with progesterone injection in this study. This stimulus is milder than fenfluramine and the progesterone-induced increase in prolactin is not robust even in less challenging experimental conditions. Therefore, we cannot conclude that the elevation in serotonin causes a progesterone-induced elevation in prolactin, only that the two are temporally correlated between studies. If the probe had caused extensive damage to the tuberoinfundibular dopamine neurons, then prolactin would have been chronically elevated, but it was not. Nonetheless, with successful fenfluramine administration, we observed that robust increases in serotonin were correlated with major increases in prolactin, and modest or undetectable increases in serotonin were correlated with minor increases in prolactin. In the i.v. fenfluramine responders, the prolactin increase was detected in serum in the next blood sample, but the increase in serotonin required an hour to reach the sample tube for detection.
Serotonin remained at basal levels in an untreated ovariectomized animal and in 2 animals primed with estrogen. Unfortunately, this time period is too short to reveal a potential circadian release of serotonin, but it is worth noting that there was a spike of serotonin at 24 hr followed by a gradual increase in baseline and then a marked amplification prior to 48 h post progesterone injection. This raises the possibility that progesterone treatment amplifies endogenous circadian release of serotonin.
The 5-HT2C receptor is expressed most densely in the mediobasal hypothalamus (Gundlah et al., 1999) and may play a role in serotonin-induced prolactin secretion. We previously observed that most of the GABAergic neurons in the arcuate-infundibular nuclei in monkey express 5-HT2C receptors (Mirkes and Bethea, 2001). If serotonin, via the 5-HT2C receptor, stimulated the GABAergic inhibition of the tuberoinfundibular dopamine neurons, then prolactin would increase. Hence, we speculate that blocking the 5-HT2C receptor would also block progesterone-induced prolactin secretion, but further experiments are needed.
The mechanism by which progesterone acts in serotonin neurons to increase serotonin release in the hypothalamic terminal field is unknown, but there are at least 2 potential mechanisms that could be involved. We showed that serotonin neurons in monkeys express the nuclear form of the progestin receptor (Bethea, 1993; 1994) and this receptor is markedly induced in animals treated with estrogen for several weeks. We previously showed that the mixed progestin receptor antagonist RU486 blocked P-induced prolactin secretion (Pecins-Thompson and Bethea, 1997). Hence, we anticipate that a progestin antagonist will block P-induced serotonin release, but additional experiments are required. Nonetheless, progesterone injected into an estrogen-primed monkey could act via progestin receptors to alter gene and protein expression leading to serotonin release in the time frame observed. We recently reported that a number of genes involved in synthesis, secretion, trafficking and transmission were increased in the raphe region by progesterone treatment of estrogen primed monkeys (Reddy and Bethea, 2005).
The participation of a membrane progestin receptor in the observed action cannot be excluded. Evidence for a membrane protein(s) that binds to progesterone has accumulated in a variety of tissues (Mahesh et al., 1996; Ramirez et al., 1996). Compelling evidence has been presented that membrane progestin receptors exist in the VTA of the rat midbrain, which binds to metabolites of progesterone and plays a pivotal role in sexual behavior (Frye, 2001). Thus, progesterone injection in our paradigm may have activated membrane progestin receptors and in turn, a cascade of events leading to an increase in serotonin release.
Metabolites of progesterone are also potent neurosteroids, which can act as agonists at the GABA-A receptor (Bitran et al., 1995). Serotonin neurons express GABA-A receptors, which inhibit serotonin firing (Gao et al., 1993). Along this line of reasoning, metabolites of progesterone may play a role in termination of progesterone-induced serotonin release.
It is difficult to compare absolute concentrations of serotonin in the microdialysate between studies that employ different sampling methods. However, in the one previous paper in which serotonin was measured by repeated microdialysis of chaired macaques, the levels reported range from 5 – 35 fmol/20 μl which approaches 1 pg/μl (Smith et al., 2000). Our serotonin concentrations under basal conditions ranged from 50-100 pg/50 μl or from 1-2 pg/μl. Thus, the concentrations of serotonin in the microdialysis of chair adapted monkeys are comparable to those obtained from tethered semi-freely behaving animals.
In conclusion, progesterone added to an estrogen regimen increases hypothalamic serotonin release in primates. We know that estrogen induces progestin receptors in serotonin neurons. Estrogen also alters gene expression for pivotal serotonin regulatory genes leading to increased serotonin synthesis, transport and neuronal firing. Supplemental progesterone then acts to amplify serotonin release potentially involving action through nuclear progestin receptors or membrane progestin receptors.
Acknowledgements
We are deeply indebted to the surgical staff and the technicians of the Division of Animal Resources who very carefully monitored our animals continuously. We are doubly indebted to Dr. David Hess, who as chair of the ONPRC IACUC obtained USDA exemption to perform brain surgery on animals that were previously ovariectomized.
Footnotes
Supported by NIH grants: MH62677 to CLB, U54 contraceptive Center Grant HD 18185, Fogarty Foundation Fellowship 517-379 to MLC and RR000163 for the operation of ONPRC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Azmitia EC. The serotonin-producing neurons of the midbrain median and dorsal raphe nuclei. In: Iverson LL, Iverson SD, Synder SH, editors. Handbook of Psychopharmacology. Vol. 9. Plenum Publishing Corp.; Baltimore, MD: 1978. pp. 233–313. [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on macaca fascicularis. Adv. Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal raphe and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology. 1993;57:1–6. doi: 10.1159/000126334. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Reddy A, Shlaes T, Streicher JM, Whittemore SR. Characterization of reproductive steroid receptors and response to estrogen in a rat serotonergic cell line. J. Neurosci. Meth. 2003;127:31–41. doi: 10.1016/s0165-0270(03)00095-5. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobio. 1999;18:87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxioytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAa receptors. J. Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Worlein JM, Kelley ST, Vijayaraghavan S, Hess DL, Axthelm MK, Bethea CL. Self-injurious behavior is decreased by cyproterone acetate in adult male rhesus (macaca mulatta) Horm. Behav. 1999;35:195–203. doi: 10.1006/hbeh.1999.1513. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm. Behav. 2001;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Gliessman P, Hess DL, Pau K-YF, Spies HG, Wuttke W. Release rates of catecholamines, GABA and β-endorphin in the preoptic area and the mediobasal hypothalamus of the rhesus monkey in push-pull perfusate: correlation with blood hormone levels. Exp. Brain Res. 1986;65:224–228. doi: 10.1007/BF00243846. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Benke D, Mohler H. Neuron-specific expression of GABAa-receptor subtypes: Differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-a and -b mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2001;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Martin KR, Heal DJ, Auerbach SB. In vivo criteria to differentiate monoamine reuptake inhibitors from releasing agents: sibutramine is a reuptake inhibitor. J. Pharmacol. Exp. Therap. 1997;283:581–591. [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1a, 2a and 2c receptor mRNA in macaque hypothalamus. Mol. Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Simon LD, Auerbach SB. Differences in hypothalamic serotonin between estrous phases and gender: an in vivo microdialysis study. Brain Res. 1998;785:91–96. doi: 10.1016/s0006-8993(97)01391-7. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW, Hendry LB. Diverse modes of action of progesterone and its metabolites. J. Steroid Biochem. Mol. Biol. 1996;56:209–219. doi: 10.1016/0960-0760(95)00238-3. [DOI] [PubMed] [Google Scholar]
- Mirkes SJ, Bethea CL. Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J. Neuroendocrinol. 2001;13:182–192. doi: 10.1046/j.1365-2826.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus monkeys. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- Pau KYF, Hess DL, Kaynard AH, Ji WZ, Gliessman PM, Spies HG. Suppression of mediobasal hypothalamic GnRH and plasma LH pulsatile patterns by phentolamine in ovariectomized rhesus macaques. Endocrinology. 1989;124:891–898. doi: 10.1210/endo-124-2-891. [DOI] [PubMed] [Google Scholar]
- Pau KYF, Hess DL, Kohama S, Bao JZ, Pau KY, Spies HG. Oestrogen up-regulates norepinephrine release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J. Neuroendocrinol. 2000;12:899–909. doi: 10.1046/j.1365-2826.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; New York, NY: 2000. [Google Scholar]
- Pecins-Thompson M, Bethea CL. RU 486 blocks and fluoxetine augments progesterone-induced prolactin secretion in monkeys. Neuroendocrinology. 1997;65:335–343. doi: 10.1159/000127192. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of 5-HT1A autoreceptor messenger ribonucleic acid expression in the dorsal raphe of rhesus macaques. Neuroscience. 1998;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Widmann AA, Bethea CL. β-endorphin, but not oxytocin, substance P or vasoactive-intestinal polypeptide, contributes to progesterone-induced prolactin secretion in monkeys. Neuroendocrinology. 1996;63:569–578. doi: 10.1159/000127086. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell. Mol. Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AP, Bethea CL. Preliminary array analysis reveals novel genes regulated by ovarian steroids in the monkey raphe region. Psychopharmacology. 2005;180:125–140. doi: 10.1007/s00213-005-2154-1. [DOI] [PubMed] [Google Scholar]
- Rischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G. Reorganization of ascending 5HT axon projections in animals previously exposed to the recreational drug (±) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) J. Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter JJ, Gundlah C, Auerbach SB. Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neurosci. Lett. 1994;171:183–186. doi: 10.1016/0304-3940(94)90635-1. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res. Mol. Brain. Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29:2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Griedman K, Malley JD, Foote SL. In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse. 2000;38:460–470. doi: 10.1002/1098-2396(20001215)38:4<460::AID-SYN11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Williams RF, Barber DL, Cowan BD, Lynch A, Marut EL, Hodgen GD. Hyperprolactinemia in monkeys: induction by an estrogen-progesterone synergy. Steroids. 1981;38:321–331. doi: 10.1016/0039-128x(81)90067-2. [DOI] [PubMed] [Google Scholar]
- Williams RF, Gianfortoni JG, Hodgen GD. Hyperprolactinemia induced by an estrogen-progesterone synergy: quantitative and temporal effects of estrogen priming in monkeys. J. Clin. Endocrinol. Metab. 1985;60:126–132. doi: 10.1210/jcem-60-1-126. [DOI] [PubMed] [Google Scholar]