Abstract
Maturation promoting factor (MPF), a complex of cyclin-dependent kinase 1 and cyclin B, drives oocyte maturation in all animals. Mechanisms to block MPF activation in developing oocytes must exist to prevent precocious cell cycle progression prior to oocyte maturation and fertilization. This study sought to determine the developmental consequences of precociously activating MPF in oocytes prior to fertilization. Whereas depletion of Myt1 in Xenopus oocytes causes nuclear envelope breakdown in vitro, we found that depletion of the Myt1 ortholog WEE-1.3 in C. elegans hermaphrodites causes precocious oocyte maturation in vivo. Although such oocytes are ovulated, they are fertilization incompetent. We have also observed novel phenotypes in these precociously maturing oocytes, such as chromosome coalescence, aberrant meiotic spindle organization, and the expression of a meiosis II post-fertilization marker. Furthermore, co-depletion studies of CDK-1 and WEE-1.3 demonstrate that WEE-1.3 is dispensable in the absence of CDK-1, suggesting that CDK-1 is a major target of WEE-1.3 in C. elegans oocytes.
Keywords: Oocyte maturation, Meiotic maturation, Myt1, Wee1, Cdk1
INTRODUCTION
Oocyte growth and differentiation (oogenesis) are crucial for fertilization competency and the rapid cell divisions of early embryonic development. The developing oocyte is essentially a synthetic factory and maternal storehouse for the factors required for these processes. Maternal factors that trigger cell proliferation must be strictly inhibited so that the oocyte remains in meiotic prophase and does not advance in the cell cycle precociously. How these factors are inhibited is poorly understood.
In most animals, oocytes arrest in prophase of meiosis I once oocyte growth is complete. In contrast to mitotic prophase, meiotic prophase in immature oocytes can be a long-lived stage that is marked by extensive transcriptional and translational activity, analogous to a G2-like phase. The growth phase of oogenesis is followed by oocyte maturation, whereby an external signal instructs the oocyte to mature. The source and form of this signal varies throughout the animal kingdom (Voronina and Wessel, 2003). The hallmarks of oocyte maturation in many organisms are nuclear envelope breakdown (NEBD), chromosome congression, and the resumption of meiosis. The meiotic divisions generate the fertilization-competent haploid oocyte and the polar bodies. The timing of fertilization relative to the completion of the meiotic divisions varies among species (Voronina and Wessel, 2003). In C. elegans, fertilization triggers the completion of the meiotic divisions and the initiation of zygotic development.
MPF is universally required for oocyte maturation and the transition from G2 to M during the mitotic divisions (Schmitt and Nebreda, 2002). MPF is a complex of a cyclin-dependent kinase, Cdk1, and its partner, cyclin B (Doree and Hunt, 2002). This complex phosphorylates key substrates to promote chromosome condensation, NEBD, and spindle assembly in meiotic and mitotic cells. MPF is synthesized during oogenesis but must be kept inactive until it is required for oocyte maturation and the subsequent embryonic mitoses.
The activity of Cdk1/cyclin B is modulated by phosphorylation, dephosphorylation and cyclin degradation (Coleman and Dunphy, 1994). Although the complex exists in interphase, it is inactive. Cdk1 is phosphorylated by the Cdk-activating kinase CAK, on Thr161 (for human Cdk1), and by an inhibitory kinase, Wee1/Myt1, on Thr14 (T14) and Tyr15 (Y15). This triply phosphorylated Cdk1 is activated at the G2/M transition by the dephosphorylation of T14 and Y15 by the dual-specificity phosphatase Cdc25. Likewise, the kinases and phosphatases that regulate Cdk1 are also subject to positive and negative regulation. Cyclin B degradation during mitotic exit ensures that the complex is inactivated for the next cell cycle. It is thus the fine balance of these positive and negative regulators that controls Cdk1 activity during meiosis and mitosis.
Although the biochemistry of MPF and its regulation have been extensively studied (Schmitt and Nebreda, 2002), much less is understood about the mechanism(s) used to maintain a G2-like state within the immature oocyte. This developmental program might rely upon a Wee1 family member to ensure that MPF is kept inactive. Myt1 is a membrane-associated member of the Wee1 family and antibody depletion in Xenopus oocytes leads to precocious NEBD, presumably through the activation of Cdk1 (Nakajo et al., 2000). Evidence suggests that Myt1 is inhibited and Cdc25 is stimulated to promote the activation and onset of oocyte maturation in Xenopus oocytes (Schmitt and Nebreda, 2002). Abnormalities in the coordination between the cell cycle regulators and the developmental program of oocytes are likely to have devastating consequences.
In this study, we used C. elegans as a model to investigate the influences of cell cycle regulators on oocyte maturation, cell cycle progression, and fertilization. We have used a variety of markers to help to distinguish oocytes that are G2-like (immature) from those that display characteristics of M-phase (mature). Using RNAi to deplete developing oocytes of MPF and a Myt1 ortholog, WEE-1.3, we demonstrate that the depletion of a MPF inhibitor results in precocious oocyte maturation, as well as aberrant chromosome and microtubule organization.
MATERIALS AND METHODS
Nematode strains and culture methods
Nematodes were cultured using standard techniques (Brenner, 1974). The following strains were used:
N2 (wild type);
CB4108, fog-2 (q71) V;
BS939, emo-1 (oz1)V/nT1 [unc-?(n754) let-?] (IV;V);
AZ212, unc-119 (ed3); ruIs32 [pAZ132: pie-1::gfp::his2B (F54E12.4)] (Praitis et al., 2001);
JH1576, unc-119 (ed3); axIs1140 [pJP1.02: unc-119(+) pie-1::gfp::mbk-2] (Pellettieri et al., 2003); and
WH210, unc-119 (ed3); ojIs2 [pLM6: unc-119(+) pie-1::gfp::tba-1].
Identification of genes and ORFs
All C. elegans genes and open reading frames (ORFs) studied in this report were identified through BLAST searches of the C. elegans genome sequence using mammalian, Xenopus or yeast orthologs as the query sequence. Many C. elegans orthologs were also identified using WormBase (http://www.wormbase.org/).
Although cDNAs have been identified and sequenced for wee-1.1 and wee-1.3, no confirmatory wee-1.2 ESTs exist. The Genome Consortium refers to wee-1.2 (C01G12.4) as a pseudogene (http://www.wormbase.org/db/gene/gene?name=C01G12.4).
RNAi
RNAi feeding
For cdk-1, pMW1.04 was transformed into HT115 (DE3) (Wallenfang and Seydoux, 2000). For wee-1.3, the entire coding sequence was PCR-amplified from an ORFeome clone (Reboul et al., 2003) and recombined into a Gateway vector expressing two T7 promoters. The resulting plasmid, pAG-92, was transformed into HT115 (DE3). Standard RNAi feeding conditions were used (Wang and Barr, 2005). The RNAi effects described in this report were obvious within 24-36 hours of placing L4 animals on this food source.
Double-stranded RNA (dsRNA) injections
PCR fragments of 0.5-1.0 kb were amplified from wild-type genomic DNA using primers with T7 promoter sequences on their 5′ ends. PCR products were then used for dsRNA synthesis using gene-specific primers and the Megascript T7 in vitro transcription kit (Ambion, Austin, TX). Young wild-type adult hermaphrodites were microinjected with dsRNAs using standard microinjection protocols (Fire et al., 1998). For studies in which multiple genes were targeted by RNAi, combinations of dsRNAs were co-injected into a single animal. Individual dsRNAs at the appropriate dilution were injected separately as controls. RNAi injections were performed for all of the genes discussed in this study. However, because feeding and injections were equally efficient, all of the figures (except Fig. 2H and Fig. 5) are from RNAi feeding experiments. We have observed that RNAi feeding works efficiently but usually takes 24-36 hours, whereas dsRNA injections reveal phenotypes in 12-24 hours.
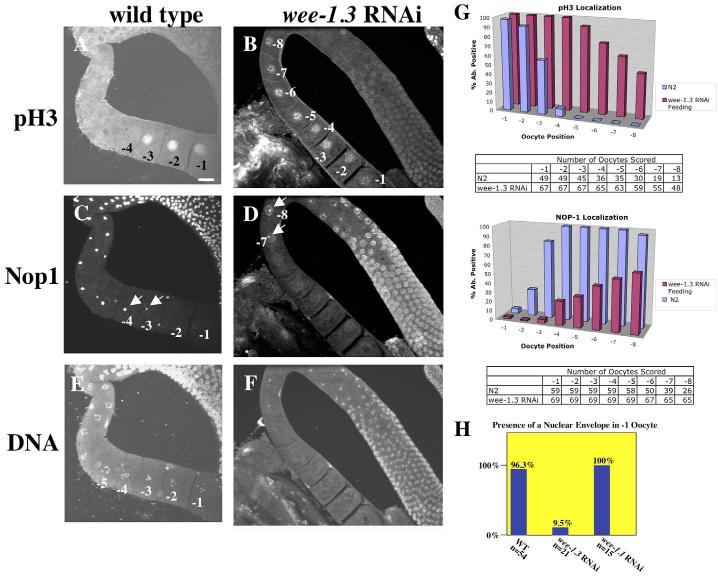
Fig. 2.
WEE-1.3 depletion causes precocious oocyte maturation. (A-F) Wild-type (A,C,E) and WEE-1.3-depleted (B,D,F) animals stained with pH3 antibodies (A,B), NOP1 antibodies (C,D), and TOTO-3 (E,F). Scale bar: 20 μm. (G) Quantitation of pH3- and NOP1-positive nuclei by oocyte position in the proximal gonad. Below each graph is a table showing the number of oocytes analyzed. (H) Quantitation of the presence of an intact NE in the −1 oocyte from adult hermaphrodites untreated (WT) or depleted of WEE-1.3 or WEE-1.1 (control). Animals were analyzed 16-20 hours post-injection. n, number of gonad arms analyzed.
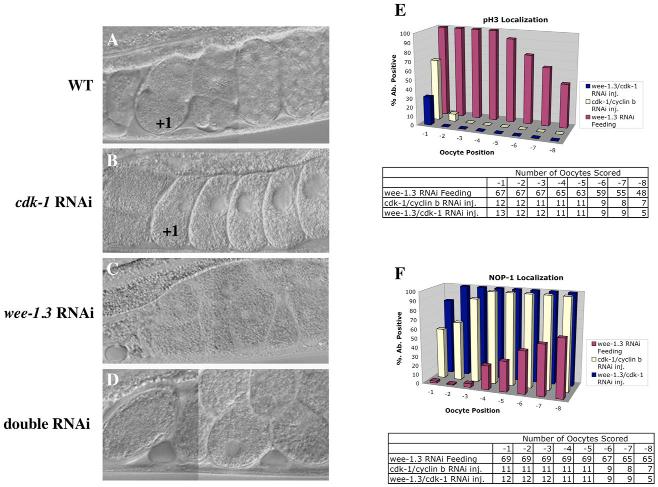
Fig. 5.
CDK-1 depletion suppresses the infertility of WEE-1.3-depleted animals. (A-D) DIC images of uteri from wild-type (A), CDK-1-depleted (B), WEE-1.3-depleted (C), and co-depleted (D) adult hermaphrodites. Wild-type animals have embryos of all stages (A), whereas CDK-1-depleted animals contain only one-cell arrested embryos (B). WEE-1.3-depleted animals have uteri containing unfertilized ‘mushy’ oocytes (C), whereas the co-depleted animals contain only one-cell arrested embryos (D). Embryos are ∼50 μm in length. (E,F) Quantitation of (E) pH3- and (F) NOP1-positive nuclei by oocyte position in the proximal gonad. Below each graph is a table showing the number of oocytes analyzed for each RNAi condition.
Although there are four cyclin B genes, only three PCR fragments were amplified for dsRNA synthesis; CYB-2.1 and CYB-2.2 are 95% identical except for an insertion of 24 amino acids in CYB-2.2 (Nieduszynski et al., 2002), and thus the dsRNA for one should interfere with the expression of the other.
Brood count assay
Young adult animals were injected with dsRNAs and picked to fresh plates every 24 hours (20°C) until they no longer produced embryos. Unhatched and hatched embryos were counted 24-48 hours after removal of the mothers. For wee-1.3 brood counts from RNAi feeding experiments, L4 animals were fed wee-1.3 bacteria and picked to fresh plates every 24 hours (20°C) until they no longer produced embryos.
Differential interference contrast (DIC) microscopy
Oocytes and embryos were observed under DIC optics in living adult animals mounted in M9 on 3% agarose pads (Sulston and Horvitz, 1977). To record oocyte maturation and ovulation in live animals, young hermaphrodites were anesthetized in a watchglass containing 0.1% tricaine and 0.01% tetramisole (in M9) for 20-30 minutes (McCarter et al., 1999). Sedated animals were mounted in M9 on 3% agarose pads and subjected to four-dimensional DIC microscopy for up to 1.5 hours.
Antibodies
The anti-phospho-histone H3 (Upstate Biotechnology, Waltham, MA), anti-NOP1 (Encor Biotech, Alachua, FL), and anti-AIR-2 antibodies have been previously described (Aris and Blobel, 1988; Hendzel et al., 1997; Schumacher et al., 1998). Alexa Fluor 488 and 568 anti-mouse and anti-rabbit secondary antibodies were obtained from Molecular Probes (Eugene, OR).
Phospho-specific and non-phospho-specific ICP-1 polyclonal rabbit antibodies were raised against the following ICP-1 peptides (amino acids 591-604): cys-VKVKKRG(pS)(pS)AVWKK and cys-VKVKKRGSSAVWKK. The antibodies were affinity purified against the appropriate peptide. Peptide and antibody production was performed by Bethyl Laboratories (Montgomery, TX).
Immunohistochemistry and microscopy
Oocyte and embryo chromosomes were analyzed by UV epifluorescence in whole-mount, DAPI-stained animals that were fixed with Carnoy II fixative (6:3:1 ethanol: acetic acid: chloroform).
For indirect immunofluorescence analysis of germline and embryos, adult animals were transferred to egg buffer (Edgar, 1995) or M9 on a Color Frost Plus slide (Fischer Scientific, Pittsburgh, PA). Embryos and gonads were extruded using a syringe needle, freeze-cracked and immediately fixed in −20°C methanol for 1-3 days. Specimens were incubated with the appropriate primary and secondary antibodies. For DNA staining, TOTO-3 iodide (Molecular Probes) was included in the next-to-last wash.
Confocal imaging of live or fixed fluorescent specimens was performed on a Nikon Eclipse E800 microscope equipped with a PerkinElmer Ultraview LCI CSU10 scanning unit (PerkinElmer, Freemont, CA), an Argon/Krypton ion laser (Melles Griot, Carlsbad, CA), and an ORCA ER cooled CCD camera (Hamamatsu, Japan). Image acquisition, analysis, and processing were done with Openlab 3 software (Improvision, Lexington, MA).
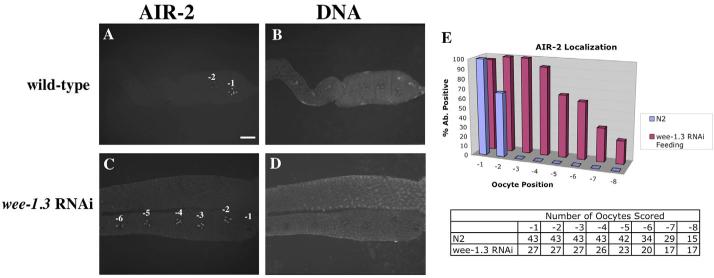
Note that the AIR-2 antibody, under our specific methanol fixation conditions, only labels the chromosomes of the −1 oocyte in wild-type animals. Although it has been reported that AIR-2 can stain the chromosomes of more immature germ cells (Chan et al., 2004; Nabeshima et al., 2005), our staining conditions reproducibly show AIR-2 staining in the −1 oocyte and faintly in the −2 oocyte.
GFP images were also acquired with the microscope described above. Live strains expressing a histone H2B::GFP fusion, a GFP::MBK-2 fusion, or an α-tubulin::GFP fusion were subjected to RNAi feeding and subsequently imaged.
RESULTS
C. elegans germline development
The C. elegans hermaphrodite gonad consists of two U-shaped arms that each connect to a common uterus via their spermathecae (see Fig. S1 in the supplementary material), where sperm are stored (Schedl, 1997). The single file arrangement of oocytes in each oviduct restricts the onset of oocyte maturation to the oldest primary oocyte in each arm. Oocytes in diakinesis of prophase I sequentially undergo oocyte maturation and ovulation such that an oocyte is fertilized in the spermatheca approximately every 23 minutes at 20°C (McCarter et al., 1999). The oocyte closest to the spermatheca is considered to be in position −1; those farther away are in position −2, −3, and so forth (see Fig. S1 in the supplementary material). Once ovulated and fertilized, the chromosomes of the oocyte begin to align on the metaphase plate (McCarter et al., 1999). The zygote passes through the spermatheca into the uterus where it completes two meiotic divisions, synthesizes an eggshell, and initiates embryonic mitoses.
Oocyte maturation is dependent on the presence of sperm in the spermatheca. The sperm-specific major sperm protein (MSP) is sufficient to induce oocyte maturation (Miller et al., 2001; Miller et al., 2003). To examine the specific roles of cell cycle proteins in oocyte maturation, we depleted conserved cell cycle regulators from developing oocytes via RNAi, and examined the expression of markers of oocyte maturation and cell cycle progression.
For oocyte maturation markers, we examined the presence or absence of the nucleolus and a specific chromosome modification, phosphorylation of histone H3 at serine 10. The nucleolus is prominent in immature oocytes and can be identified by DIC microscopy (McCarter et al., 1999) or by indirect immunofluorescence microscopy (MacQueen and Villeneuve, 2001) using an antibody to the nucleolar marker NOP1/fibrillarin (Aris and Blobel, 1988; Henriquez et al., 1990; Schimmang et al., 1989). Chromosome condensation can be monitored in maturing oocytes using an antibody specific for phosphorylated histone H3 (pH3) (Hendzel et al., 1997; Hsu et al., 2000). In wild-type adult C. elegans hermaphrodites, NOP1 staining was observed in all the nuclei of the loop and the proximal germline (Fig. 1C, Fig. 2C); only the nucleus of the −1 oocyte lacked any detectable NOP1 staining (n=59). In the majority of wild-type germlines, NOP1 staining was also significantly reduced within the −2 and −3 nuclei (Fig. 1C,G, Fig. 2C). This result is supported by DIC analysis of nucleolar breakdown in live animals (McCarter et al., 1999). The pH3 antibody stained the nuclei and chromosomes of the three most proximal oocytes within each gonad arm (n=45; Fig. 1A,G, Fig. 2A), consistent with previous studies (Hsu et al., 2000; Page et al., 2001). The −2 and −3 oocytes had similar staining intensities, but the staining was always greatest in the −1 oocyte (Fig. 1A,G, Fig. 2A).
Fig. 1.
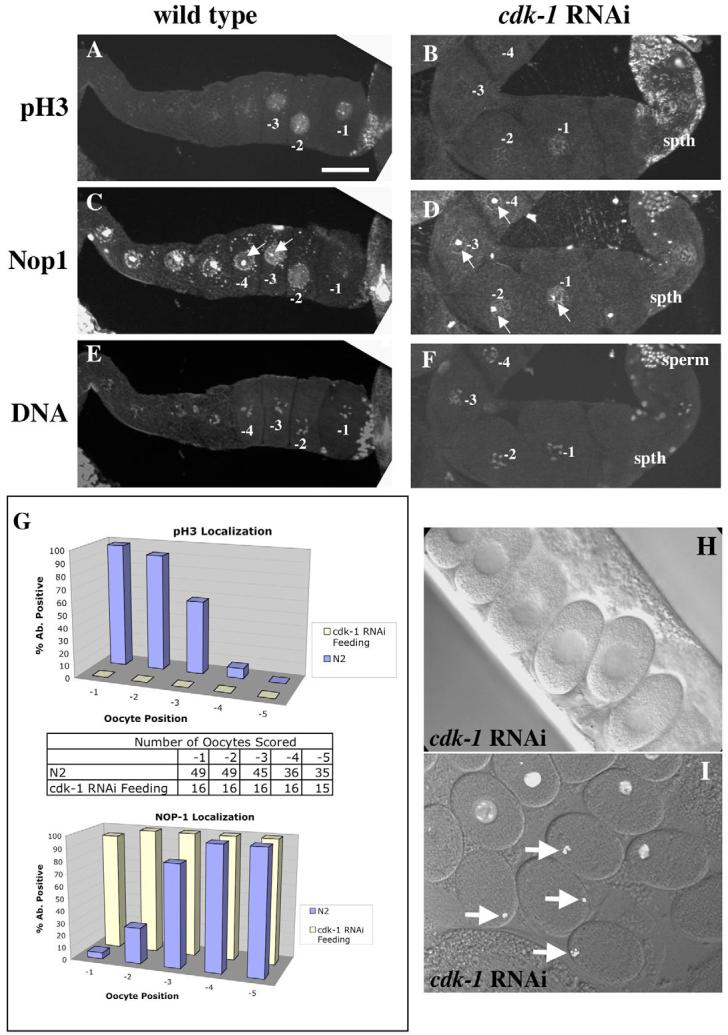
CDK-1 depletion impairs oocyte maturation. (A-F) Wild-type (WT; A,C,E) and CDK-1-depleted (B,D,F) animals stained with pH3 antibodies (A,B), NOP1 antibodies (C,D), and TOTO-3 to visualize DNA (E,F). Scale bar: 20 μm. (G) Quantitation of pH3- and NOP1-positive nuclei by oocyte position in the proximal gonad. In between the graphs is a table showing the number of oocytes analyzed. N2 is the wild-type strain. (H) DIC image of one-cell arrested embryos in the uterus of a CDK-1-depleted adult. (I) One-cell arrested embryos dissected from the uterus of a histone H2B::GFP transgenic animal depleted of CDK-1. The four embryos marked by arrows show the congression of the oocyte chromosomes, similar to metaphase of meiosis I. Older embryos remain arrested as one-cell embryos but their chromosomes have decondensed. spth, spermatheca.
Although all of the oocytes in the proximal gonad arm are in diplotene/diakinesis of prophase I, we consider those that stain with the NOP1 antibody and fail to stain with the pH3 antibody as G2-like nuclei. We define an M-phase oocyte as any oocyte that no longer stains with the NOP1 antibody but does stain with the pH3 antibody. Thus, we consider the three oocytes most proximal to the spermatheca in each arm of young hermaphrodite adults to be transitioning from G2 to M. We demonstrate below that MPF is required for this G2/M-phase transition on the basis of these markers.
The oocyte at position −1 is unique because it undergoes NEBD approximately six minutes before ovulation (McCarter et al., 1999) (see Fig. S1 in the supplementary material). Because the onset of NEBD within the oocyte occurs in the last few minutes before ovulation, random observations of −1 oocytes will reveal the presence of a nuclear envelope (NE) (96%, n=54, Fig. 2H). Approximately three minutes before ovulation, the −1 oocyte assumes a more rounded shape and becomes fully competent for fertilization (McCarter et al., 1999).
RNA interference of MPF components disrupts oocyte maturation and prevents the meiotic divisions
To determine whether MPF plays a role in oocyte maturation in C. elegans, as it does in other organisms, we altered its activity using RNAi to CDK-1, cyclin B and the negative regulator of MPF WEE-1.3. The phenotypes of the RNAi-treated hermaphrodites and their progeny were determined (see Table S1 in the supplementary material). Previous observations of CDK-1-depleted oocytes revealed a delayed NEBD (Boxem et al., 1999; Chase et al., 2000). We extended these observations by staining depleted germlines for NOP1 and pH3. In CDK-1-depleted hermaphrodites, greater than 90% of all −1 oocyte nuclei stained positive for NOP1 (n=16; Fig. 1D,G), suggesting that nucleolar breakdown is blocked. The pH3 staining was absent or greatly reduced in the −1, −2 and −3 oocytes of CDK-1-depleted animals (n=16; Fig. 1B,G). Time-lapse DIC imaging revealed the presence of a NE in the −1 oocyte in CDK-1-depleted gonads; NEBD did not occur prior to ovulation (n=10; data not shown). This delay in NEBD was evident in the one-cell fertilized embryos in the uterus because the majority still had an intact NE (Fig. 1H). The persistence of a NE and nucleolus, as well as the lack of pH3 staining in −1 oocytes, reveals that oocyte maturation is compromised in the absence of CDK-1. Similar results were obtained when all four cyb genes were subjected to combinatorial RNAi (data not shown), but not when fewer than four were depleted. Taken together, these results indicate that MPF functions in C. elegans as an oocyte maturation-promoting factor.
Despite the defects in oocyte maturation, depletion of MPF components in C. elegans did not prevent oocyte growth, ovulation or fertilization. After fertilization, MPF-depleted embryos failed to complete both meiotic divisions and arrested as one-cell meiotic embryos (Fig. 1H,I). Although these embryos resemble those depleted of the anaphase promoting complex (APC) (Davis et al., 2002; Furuta et al., 2000; Golden et al., 2000), in that they lack polar bodies and their maternal chromosomes are highly condensed in a metaphase I-like configuration, they differ in that they do not establish meiotic spindles (n=30; data not shown). Thus, a reduction in CDK-1 activity is sufficient to block the meiotic divisions in C. elegans embryos.
Depletion of WEE-1.3 causes precocious oocyte maturation
To address whether any of the wee-1 genes in C. elegans functions as a negative regulator of CDK-1, we performed RNAi with each of the predicted wee-1 genes. The depletion of a negative regulator of CDK-1 may result in the premature activation of CDK-1 in oocytes and cause precocious oocyte maturation. Of the three wee-1 genes found in C. elegans (see Table S1 in the supplementary material) (Wilson et al., 1999), wee-1.3 was the only one to reveal a phenotype by RNAi. The wee-1.3 gene product contains a hydrophobic membrane-spanning domain and is closely related to Myt1 (Lamitina and L'Hernault, 2002; Wilson et al., 1999).
Animals depleted of WEE-1.3 became infertile within 24 hours of exposure to dsRNA. In the WEE-1.3-depleted germlines, the number of NOP1-positive oocyte nuclei was dramatically reduced. On average, the seven most proximal oocytes within a gonad arm lacked NOP1 staining (n>50; Fig. 2D,G). However, pH3 staining of WEE-1.3-depleted germlines revealed far more pH3-positive oocyte nuclei (Fig. 2B,G) than were observed in untreated animals (Fig. 2A). In animals that were scored after long exposures to RNAi, both the NOP1 and pH3 staining patterns described above were even more dramatic (data not shown). In gonad arms stained with both the NOP1 and pH3 antibodies, there was frequently only a slight overlap in staining, suggesting that most germ cell nuclei that stained with pH3 had already undergone nucleolar breakdown. DIC observation of the oocytes revealed the presence of a NE in only 9.5% of the WEE-1.3-depleted −1 oocytes (Fig. 2H), suggesting that NEBD was occurring precociously in these treated animals. In addition, we observed precocious NEBD in up to four consecutive oocytes adjacent to the spermatheca when animals were subjected to longer periods of wee-1.3 RNAi treatment (data not shown).
On average, WEE-1.3-depleted animals laid fewer than 10 embryos before becoming infertile (Table 1). Despite the observation that these oocytes precociously express markers of oocyte maturation (Fig. 2), the oocytes apparently failed to mature properly. These oocytes were ovulated, but were not fertilized despite exposure to wild-type sperm. Upon longer RNAi treatments, we observed that the oocytes in the oviduct were smaller than oocytes from wild-type controls (data not shown). These observations suggest that oocyte maturation may have occurred before oocyte growth was complete. In summary, our data support the model that, upon WEE-1.3 depletion, MPF is precociously activated in the developing germline and disrupts normal oocyte development.
Table 1.
Brood counts for animals subjected to RNAi
| RNAi | n | Average brood |
% Unhatched 24 hours post-injection |
|---|---|---|---|
| wee-1.3 | 41 | 8 | * |
| cdk-1 | 15 | 145 | 100 |
| wee-1.3/cdk-1 | 21 | 109 | 85 |
| zyg-1 | 12 | 82 | 92 |
| wee-1.3/zyg-1 | 30 | 13 | * |
Young adult animals were injected with dsRNA and the brood size for each animal was determined. For the percentage unhatched embryos in the last column, only embryos laid after 24 hours post-injection were assayed. As previously reported, cdk-1 RNAi results in a reduction in brood size compared with those in wild type (Boxem et al., 1999).
No embryos were laid at this time.
WEE-1.3-depleted oocytes display additional characteristics of precocious oocyte maturation
Other than NOP1 and pH3, very few molecular markers exist to distinguish the maturing oocyte from those less mature. AIR-2, an Aurora/Ipl1 ortholog, was previously shown to associate with the chromosomes of the −1 oocyte, and only in the presence of sperm (Schumacher et al., 1998). AIR-2 is the kinase responsible for histone H3 phosphorylation (Hsu et al., 2000) and thus is a useful marker of oocyte maturation. To address whether the chromosomes of wee-1.3 RNAi oocytes showed signs of precocious oocyte maturation, we used an AIR-2 antibody and an antibody (pICP-1) that recognizes the phospho-epitope of an AIR-2 substrate, ICP-1 (Bishop and Schumacher, 2002). In wild-type animals, AIR-2 and pICP-1 antibodies stained the diakinetic chromosomes of the −1 oocyte, and faintly stained the chromosomes of the −2 and −3 oocytes (Fig. 3A, see also Fig. S2A in the supplementary material) (Hsu et al., 2000; Schumacher et al., 1998). However, in WEE-1.3-depleted oocytes, the chromosomes of −1, −2, −3, and more distal oocytes stained with both antibodies (Fig. 3C, Fig. S2C in the supplementary material). This staining pattern, like that of pH3, was observed soon after the onset of wee-1.3 RNAi.
Fig. 3.
WEE-1.3 depletion causes an increase in the number of oocytes that stain for AIR-2. (A-D) Wild-type (A,B) and WEE-1.3-depleted (C,D) animals stained with an AIR-2 antibody (A,C) and TOTO-3 (B,D). Scale bar: 20 μm. (E) Quantitation of AIR-2-positive nuclei by oocyte position in the proximal gonad. Below the graph is a table showing the number of oocytes analyzed.
WEE-1.3-depleted oocytes progress through meiosis
To determine whether the WEE-1.3-depleted oocytes advanced in the meiotic cell cycle, we examined transgenic animals expressing GFP::MBK-2. MBK-2 is a C. elegans DYRK family kinase that regulates the degradation of several maternal proteins shortly after fertilization (Pellettieri et al., 2003; Quintin et al., 2003). MBK-2 is found uniformly on the cortex of oocytes and newly fertilized embryos and in cortical aggregates in meiosis II embryos (Fig. 4A) (Pellettieri et al., 2003). Thus, the ‘speckled’ pattern of expression is a good marker of embryos in meiosis II. GFP::MBK-2 animals depleted of WEE-1.3 have this speckled pattern in one or two oocytes that are usually quite distal from the spermatheca (Fig. 4B,D). These speckled oocytes are distally flanked by oocytes with the uniform cortical pattern (Fig. 4B). Interestingly, MEI-1, a meiotic spindle-specific target of MBK-2-dependent degradation, is prematurely absent in oocytes depleted of WEE-1.3 (Stitzel et al., 2006). These observations suggest that precociously matured oocytes are progressing through meiosis even though they have not been fertilized and have not segregated their chromosomes (see below).
Fig. 4.
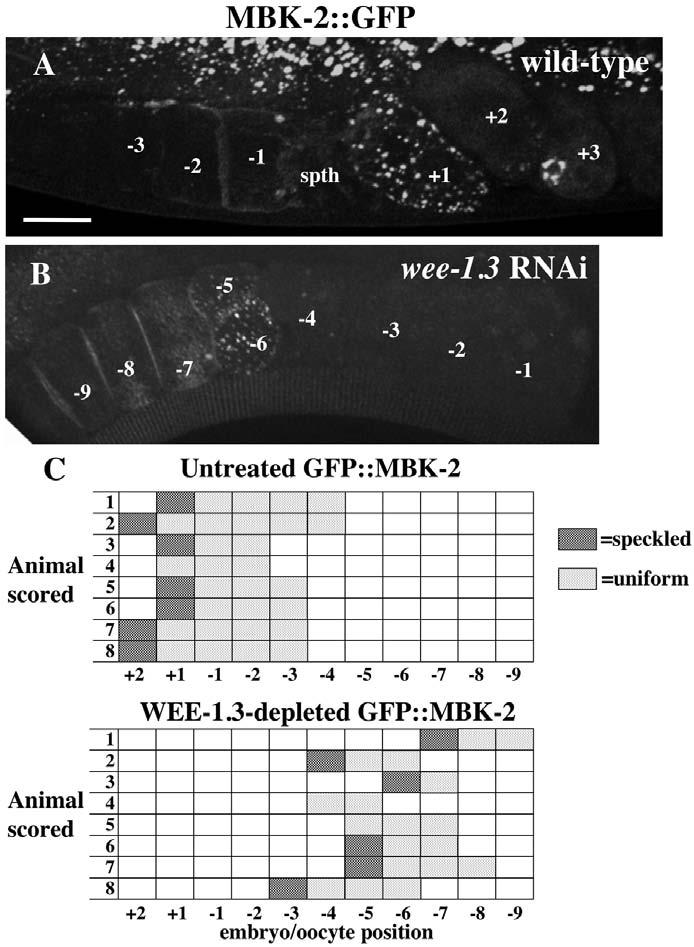
GFP::MBK-2 aggregates in WEE-1.3-depleted oocytes. (A,B) Fluorescence images of wild-type GFP::MBK-2 (A) and WEE-1.3-depleted (B) oviducts. The speckled pattern is present in the +1 embryo in A and in the −5 and −6 embryos in B. Scale bar: 20 μm. (C,D) The position of each oocyte with a speckled GFP pattern and a uniformly cortical pattern is indicated for eight representative animals of each. n=19 for the total number of untreated GFP::MBK-2 animals, and n=22 for WEE-1.3 depleted. spth, spermatheca.
WEE-1.3 is dispensable when CDK-1 is depleted
To test our hypothesis that WEE-1.3 functions to specifically prevent the precocious activation of CDK-1 in growing oocytes, hermaphrodites were subjected to CDK-1 and WEE-1.3 combinatorial RNAi. If the primary function of WEE-1.3 in oocytes is to directly inhibit CDK-1, the co-depletion of both should result in a cdk-1 RNAi phenotype. Animals depleted of WEE-1.3 alone produced broods of eight (Fig. 5; Table 1), whereas CDK-1-depleted animals produced broods of greater than 100 embryos, the majority of which arrested at the one-cell stage (average=145; Fig. 5; Table 1). Animals depleted of both WEE-1.3 and CDK-1 were fertile and produced broods of greater than 100 embryos (average=109; Fig. 5; Table 1). These embryos arrested as meiotic one-cell embryos, identical to CDK-1-depleted embryos. NOP1 antibody staining was present in the majority of −1 oocytes (Fig. 5F), whereas pH3 staining was absent (Fig. 5E). The presence of a NE in −1 oocytes was evident based on DIC observation (data not shown). When compared with the wee-1.3 or cdk-1 single RNAi phenotypes, co-depletion of these genes results in phenotypes identical to CDK-1 depletion alone, suggesting that WEE-1.3 is dispensable when CDK-1 levels are reduced. These results imply that WEE-1.3 acts primarily to inhibit the premature activation of CDK-1 in the hermaphrodite germline. Similar results were also observed when WEE-1.3 and the B-type cyclins were co-depleted (data not shown).
As a control, animals were co-injected with wee-1.3 and an unrelated dsRNA that results in embryonic lethality (zyg-1, Table 1); this treatment did not suppress the infertility of WEE-1.3-depleted animals. Other cell cycle regulatory genes known to yield one-cell arrested meiotic embryos upon RNAi did not suppress the infertility either. RNAi of plk-1 (Chase et al., 2000), air-2 (Schumacher et al., 1998) or a combination of cdc-25 genes did not suppress the wee-1.3 RNAi-induced infertility (data not shown). In addition, temperature-sensitive mutants of emb-27, a subunit of the APC (Golden et al., 2000), were depleted of WEE-1.3 at the non-permissive temperature and did not suppress the wee-1.3 RNAi phenotype. CDK-1 or cyclin B depletion were the only conditions that suppressed the wee-1.3 RNAi phenotype, suggesting that CDK-1 is likely to be the major substrate of WEE-1.3 in maturing oocytes.
Tubulin organization is aberrant in WEE-1.3-depleted oocytes
In wild-type oocytes, the cytoskeleton comprises a cytoplasmic microtubule meshwork (Yang et al., 2003). Upon NEBD in the −1 oocyte, tubulin concentrates near the compacted oocyte chromosomes (Albertson and Thomson, 1993; Yang et al., 2003). Meiotic spindle assembly is evident in fertilized embryos present in the spermatheca and is often completed by the time the zygote enters the uterus (Fig. 6B) (Yang et al., 2003). We have examined the tubulin organization of CDK-1- and WEE-1.3-depleted oocytes by performing RNAi in live α-tubulin::GFP transgenic animals. In CDK-1-depleted oocytes, the tubulin meshwork appeared normal. In fertilized embryos depleted of CDK-1, the arrangement of the oocyte bivalents was normal, yet no meiotic spindles were observed (n=30; data not shown) (Wallenfang and Seydoux, 2000). In WEE-1.3-depleted oocytes in the oviduct, perinuclear tubulin foci were often observed (n=71; Fig. 6C,D). Although these foci resembled mitotic centrosomes, they did not stain for a centrosome marker (data not shown). Once the NE broke down in the proximal oocytes and the oocyte chromosomes coalesced into one mass (see below), we frequently observed a disorganized tubulin cloud associated with this chromatin mass (Fig. 6E). This tubulin cloud was also observed in the unfertilized oocytes in the uterus. These aberrant tubulin patterns were also observed by α-tubulin antibody staining (data not shown).
Fig. 6.
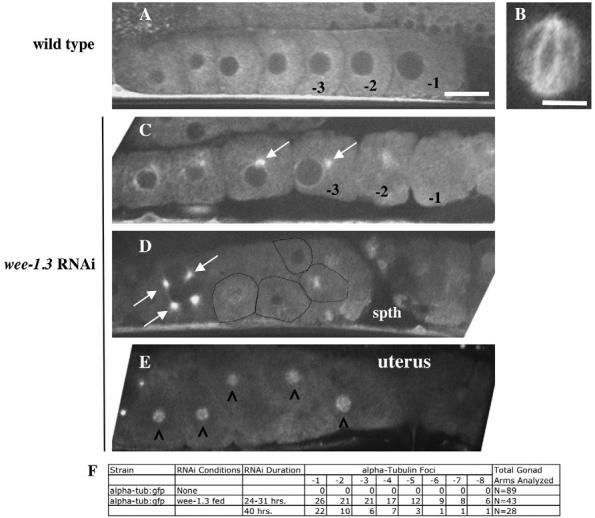
WEE-1.3 depletion results in the appearance of aberrant microtubules in developing oocytes. (A-E) Live α-tubulin::GFP animals were subjected to wee-1.3 RNAi. (A,B) Untreated animals. (A) Oocytes with typical cytoplasmic tubulin cytoskeletons. (B) A wild-type meiotic spindle; such a structure is normally observed in the oocyte upon fertilization in the spermatheca, or in the +1 embryo in the uterus, and is never observed in WEE-1.3-depleted oocytes in the spermatheca or uterus. (C-E) WEE-1.3-depleted oocytes in the oviduct (C,D) or the uterus (E). White arrows mark the perinuclear tubulin foci. Oocytes in D are outlined in black. (E) Tubulin clouds (marked by carets) form around the coalesced chromosomes of WEE-1.3-depleted oocytes that fail to be fertilized but nonetheless end up in the uterus. The spermatheca is to the right in each gonad shown. Scale bars: in A, 20 μm for A,C-E; 10 μm in B. (F) Quantitation of the number of oocytes with tubulin foci scored by oocyte position in the proximal gonad. spth, spermatheca.
Depletion of WEE-1.3 causes chromosome coalescence
To examine oocyte chromosomes in WEE-1.3-depleted animals, we stained with DAPI. Six highly condensed distinct bivalents were found in wild-type proximal oocytes (Fig. 7A). In WEE-1.3-depleted animals, the chromosomes of the −1 oocyte were no longer recognizable as six distinct bivalents. Instead, they coalesced into a single mass of chromatin (Fig. 7; n>200). This defect was observed in the −2 and −3 oocytes as well. The longer the animals were subjected to the effects of RNAi, the more widespread this effect was. We often detected ‘stringy’ chromosomes in one oocyte in each arm, in between oocytes with normal-looking diakinetic chromosomes and those with abnormal coalesced chromatin masses (Fig. 7E; see also Table S2 in the supplementary material). This ‘stringy’ phenotype may represent a transition from a normal to a defective state. This chromatin phenotype was also evident in WEE-1.3-depleted animals expressing a histone H2B::GFP transgene (Fig. 7K,L). Once the chromosomes coalesced into a single mass, the intensity of DAPI staining (or GFP) increased, suggesting that these oocyte chromosomes were undergoing limited endo-replication, similar to that reported in a previous study (Detwiler et al., 2001). The wee-1.3 RNAi chromosome coalescence phenotype is reminiscent of the metaphase congression that bivalents undergo as they pass into the spermatheca (Fig. 7I,J). However, in WEE-1.3-depleted oocytes, the chromosomes appear to have over-congressed, in that individual chromosomes can no longer be distinguished (Fig. 7L). This chromosome coalescence phenotype is likely to be a secondary consequence of oocyte maturation defects because it always follows the expansion of pH3, AIR-2 and pICP-1 staining in the oviduct (data not shown).
Fig. 7.
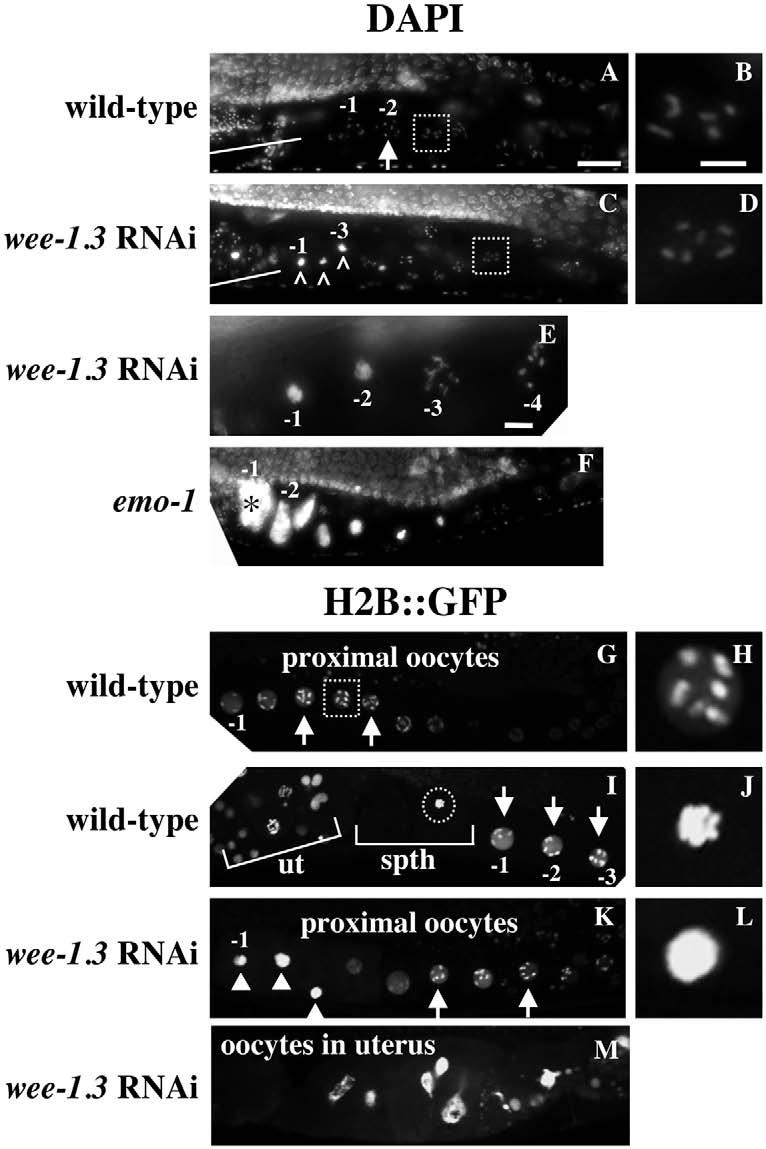
Oocyte chromosomes do not maintain a diakinetic arrangement upon WEE-1.3 depletion. (A-F) Wild-type (A,B), WEE-1.3-depleted (C-E), and emo-1 (F) animals were DAPI stained to visualize oocyte chromosomes. Arrows and white boxes mark the normal diakinetic arrangement of chromosomes in growing oocytes; those marked with a white box are enlarged in B,D. White lines mark the spermatheca and the highly condensed sperm chromosomes. Carets (^) in C mark the most proximal oocyte chromosomes that have coalesced. (E) The −3 oocyte has stringy chromosomes and is flanked by normal diakinetic oocytes to the right and oocytes with coalesced chromosomes to the left. Asterisk in F marks an endoreplicating oocyte. (G-M) Images of live, untreated (G-J) or WEE-1.3-depleted (K-M) histone H2B::GFP transgenic animals. Diakinetic chromosomes are apparent in wild-type (G-I) and WEE-1.3-depleted (K) animals, and are marked with arrows and white boxes; the one in the white box in G is enlarged in H. The chromosomes of a wild-type fertilized oocyte congress during metaphase I (marked by a circle in I and enlarged in J). The coalesced chromosomes of WEE-1.3-depleted oocytes are marked by white arrowheads (K) and one is enlarged in L. (M) The uterus of a WEE-1.3-depleted animal in which the oocyte chromosomes have begun to endoreplicate. ut, uterus; spth, spermatheca. Scale bars: in A, 20 μm for A,C,F,G,I,K,M; in B, 5 μm for B,D,H,J,L; in E, 5 μm.
WEE-1.3 is not required to maintain prophase arrest in diapaused oocytes
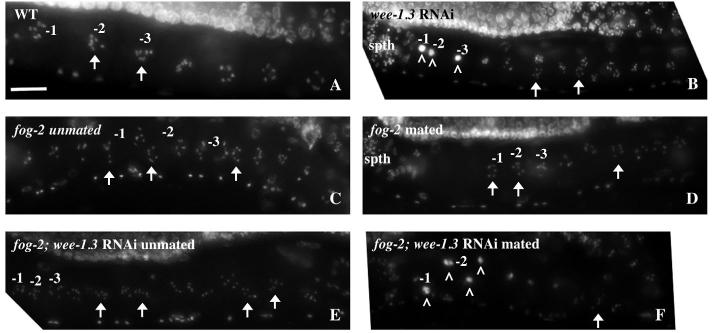
Immature oocytes in many species have the ability to ‘diapause’ or remain developmentally arrested for long periods of time prior to the initiation of oocyte maturation. How this arrest is maintained at the molecular level is poorly understood. C. elegans mutants exist with feminized germlines (Fog) and develop as females (Schedl, 1997). They lack sperm and thus the sperm-generated maturation-inducing factor, MSP. The oocytes in these animals accumulate in diakinesis of prophase I and can remain arrested in this meiotic state for days. These diapaused oocytes remain fertilization-competent; the introduction of sperm via mating results in the release from diapause and the production of progeny. To determine whether WEE-1.3 is required for diapause, fog-2 females were subjected to wee-1.3 RNAi (Fig. 8E). These oocytes remained in diakinesis (n=71) and their chromosome morphology did not resemble the morphologies described for WEE-1.3-depleted oocytes (Fig. 8B; Table S2 in the supplementary material). When fog-2 females are mated with wild-type males, the oocytes are fertilized, resulting in viable progeny. By contrast, when wild-type males were mated to the WEE-1.3-depleted fog-2 females, the fog-2 animals became infertile as early as 4 hours after the introduction of males. Upon mating, the chromosomes of the WEE-1.3-depleted fog-2 oocytes coalesced (n=35; Fig. 8F), as was observed for wild-type hermaphrodites subjected to wee-1.3 RNAi (Fig. 8B). Taken together, these data suggest that WEE-1.3 is not required to maintain diakinesis in the oocytes of animals that lack sperm. Thus, the requirement for WEE-1.3 in oocytes appears to be dependent on oocyte maturation signal(s) from sperm. This suggests that diapause requires a distinct regulator.
Fig. 8.
The chromosome coalescence phenotype of WEE-1.3-depleted oocytes is dependent on the presence of sperm. Diakinetic chromosomes (arrows) of oocytes in the proximal gonad are evident in DAPI-stained wild-type hermaphrodites (A), in unmated (C) or mated (D) fog-2 females, and in unmated WEE-1.3-depleted fog-2 females (E). Although the oocytes in fog-2 animals remain in diakinesis (C-E), the chromosomes of WEE-1.3-depleted fog-2 oocytes coalesce into one mass upon the introduction of sperm (F). This phenotype appears identical to that observed in wild-type hermaphrodites depleted of WEE-1.3 (B). The white carets (^) mark the oocyte chromosomes that have coalesced (B,F). The spermatheca (spth) is to the left in each panel. Scale bar: 20 μm.
DISCUSSION
The unique germline organization, transparency, and excellent cytology of C. elegans provide an ideal system for the observation of oogenesis, oocyte maturation and fertilization in live animals. In addition, with the advent of RNAi, the maternal functions of essential genes can readily be determined. This is particularly advantageous for the study of genes that have essential functions at several stages of the life cycle. For example, cdk-1 and wee-1.3 mutants display numerous larval defects and thus have not been useful for uncovering the roles of these genes during oogenesis, fertilization and early embryogenesis (Boxem et al., 1999; Lamitina and L'Hernault, 2002) (B.K.S. and A.G., unpublished). In the absence of conditional alleles or mosaic animals, RNAi is the only method by which these maternal functions can be discerned.
Our study is the first to examine the molecular and cellular effects of Myt1 depletion during oocyte development and maturation in vivo. We demonstrate that molecular markers of G2/M-phase progression were precociously present when WEE-1.3 was depleted, and were reduced or absent when CDK-1 was depleted. We expanded on the work of Boxem et al. (Boxem et al., 1999) and showed that the delayed nucleolar and NE breakdown upon CDK-1 depletion is accompanied by a severe reduction in pH3 staining of the compacted oocyte chromosomes. These oocyte maturation defects do not prevent ovulation and fertilization. However, MPF-depleted oocytes that are fertilized do not progress past metaphase of meiosis I, do not organize a meiotic spindle, and do not extrude polar bodies. Interestingly, the maternal chromosomes still congress into a metaphase-like plate despite the absence of a meiotic spindle. Thus, these observations suggest that MPF is required for the meiotic divisions upon fertilization.
CDK-1 antibody staining revealed that our MPF depletion was not complete in these studies (data not shown), and thus the possibility exists that a more severe depletion would reveal a strict requirement for MPF in NEBD and fertilization competency. In any case, our results reveal that later events in oocyte maturation (the meiotic divisions) are more sensitive than earlier events (NEBD, fertilization) to a decreased dose of MPF. It also remains possible that other CDKs function redundantly with CDK-1 in oocyte maturation, and that the depletion of multiple CDKs is required to fully block oocyte maturation and subsequent fertilization in C. elegans.
Precocious activation of MPF disrupts oocyte maturation and fertilization
By depleting the Myt1 kinase WEE-1.3 we were able to determine the consequences of precociously activating MPF in C. elegans oocytes. Although the resultant infertility has been previously reported (Detwiler et al., 2001), our studies are the first to demonstrate that WEE-1.3-depleted oocytes express numerous molecular markers indicative of M-phase. Our findings in C. elegans are consistent with experiments in Xenopus, in which oocytes injected with neutralizing anti-Myt1 antibodies resulted in germinal vesicle breakdown (Nakajo et al., 2000). They are also consistent with studies in which RNAi of Wee1B in murine oocytes resulted in germinal vesicle breakdown and polar body extrusion (Han et al., 2005). Markers of oocyte maturation in C. elegans, such as nucleolar breakdown, NEBD, the compaction of the oocyte chromosomes (as detected by staining with the pH3, AIR-2 and phospho-ICP-1 antibodies), and the presence of MBK-2 aggregates, revealed that oocytes were maturing prematurely in WEE-1.3-depleted animals. Thus, the precocious activation of MPF in these oocytes results in progression through the meiotic cell cycle in the absence of fertilization.
CDK-1 is a major target of WEE-1.3
The observation that CDK-1 depletion can restore fertility to WEE-1.3-depleted oocytes suggests that CDK-1 is a major target of WEE-1.3. WEE-1.3 becomes dispensable when CDK-1 is depleted. If the oocyte defects observed during WEE-1.3 depletion were caused by the activation of other CDKs, then one would not expect CDK-1 depletion to suppress the wee-1.3 RNAi-induced infertility. cdk-1 RNAi does not affect the synthesis of other related CDKs, as depletion of each has a distinct RNAi phenotype (Boxem et al., 1999). Thus, the activation of CDK-1 is the most likely cause of the phenotypes observed upon WEE-1.3 depletion.
The localization of WEE-1.3 on the nuclear envelopes of developing oocytes (S. T. Lamatina and S. L'Hernault, personal communication) may allow WEE-1.3 to phosphorylate and inhibit CDK-1 as it passes into the nucleus. It may also provide a mechanism to control the timing of NEBD by regulating CDK-1 at the nuclear envelope.
Chromosomal maturation
The chromosomes of oocytes depleted of WEE-1.3 display a unique phenotype in that they coalesce into one indistinct mass. Individual bivalents or chromosomes are no longer distinguishable. Is this due to inappropriate cell cycle progression? The DAPI staining intensity of fixed gonads suggests that WEE-1.3-depleted oocytes have undergone additional rounds of DNA synthesis. This replication is unlike the Emo phenotype (Iwasaki et al., 1996) in that WEE-1.3-depleted oocytes appear to undergo far fewer cycles of endoreplication and do not cycle through mitosis, as condensed, distinct chromosomes are not observed. However, like Emo oocytes, there does appear to be a progressive aspect to the chromosome morphology phenotype of WEE-1.3-depleted oocytes in that the oocytes closest to the spermatheca are more affected than those more distal.
Altogether, our results suggest that the oocyte chromosomes undergo a maturation phase in which they bind and release chromatin-associated factors. These associations may occur in stages as the oocyte grows. Factors required for homolog pairing and synaptonemal complex (SC) formation are known to associate with the germ cell chromosomes early in meiotic prophase, before diakinesis. As the chromosomes compact further during diakinesis, some of the SC proteins are released as other factors, such as the condensins, are added. Condensin complexes are required for chromosome compaction and the formation of discrete diakinetic bivalents (Chan et al., 2004). These progressive modifications may be required to prepare the chromosomes for their upcoming meiotic divisions. Precocious maturation would disrupt these events. Aberrant chromatin modifications are evident based on the persistence of pH3, AIR-2 and phospho-ICP-1 on WEE-1.3-depleted oocyte chromosomes throughout the proximal gonad. These modifications may lead to the aberrant loading of kinetochore, cohesin and condensin proteins, and may thus account for their inability to exist as discrete bivalents.
Alternatively, the chromosome coalescence phenotype might be due to aberrations in the histone code. In mammalian cells, H3 phosphorylation is important for heterochromatin protein 1 (HP1) dissociation (Fischle et al., 2005). C. elegans depleted of an HP1 ortholog (HPL-2) are infertile and display endoreplicated oocytes (Couteau et al., 2002). The precocious phosphorylation of histone H3 on serine 10 could trigger a cascade of events (i.e. subsequent histone modifications or protein associations) that lead to deregulated chromosome condensation, thus perturbing the chromosomal architecture. Thus, the precocious H3 phosphorylation triggered by WEE-1.3 depletion could account for alterations in HPL-2 association with chromatin and could contribute to the aberrant chromosomal architecture.
Microtubule organization in maturing oocytes
In addition to an essential role in oocyte maturation, CDK-1 appears to be required for meiotic spindle assembly. In most metazoans, the meiotic spindle lacks centrioles and centrosomal proteins, and the meiotic chromosomes themselves organize the meiotic spindle (Varmark, 2004). These acentriolar spindles are bipolar and thus an MT bundling activity must exist to give the spindle its organization and shape. In live animals expressing α-tubulin::GFP, we observed perinuclear tubulin foci in the cytoplasm of WEE-1.3-depleted oocytes. Interestingly, ectopic MT foci are also observed in Drosophila Wee1 mutant embryos (Stumpff et al., 2005). Our observations in C. elegans suggest that precociously activated MPF attempts to organize tubulin into spindles, perhaps through the activation of a MT bundling or motor protein. Inappropriate MT bundling might also contribute to the coalescence of the meiotic chromosomes. Future studies will be needed to address the contribution of meiotic motor proteins towards the wee-1.3 RNAi phenotype.
WEE-1.3-depleted oocytes are fertilization-incompetent
The inability of WEE-1.3-depleted oocytes to be fertilized is likely to be a consequence of precocious or aberrant oocyte maturation. Although there are several hypotheses as to why these WEE-1.3-depleted oocytes may not be competent for fertilization, we favor the model whereby aberrant chromatin modifications are incompatible with fertilization. This model predicts the presence of a novel checkpoint that monitors the chromosome status of the oocyte during these final stages of oocyte maturation and fertilization. The observation that emo-1 oocytes are also not fertilized supports the existence of such a checkpoint, whereby fertilization is blocked if the oocyte chromosomes are in any stage other than diakinesis.
Maintenance of diapause does not require WEE-1.3
We do not observe precocious oocyte maturation in feminized animals depleted of WEE-1.3, suggesting that other factors are functioning to maintain the chromosomes of immature oocytes in diakinesis (or that feminized animals are less sensitive to a decreased dose of WEE-1.3). Presumably MPF is present but inactive in such oocytes. Perhaps, in addition to inhibiting WEE-1.3, sperm or MSP activates CAK. CAK, in turn, activates CDK-1. In hermaphrodites, where sperm are always present, CAK activation or synthesis may be constitutive, and thus the stimulation/inhibition of WEE-1.3 activity may be the primary focus of regulation. Alternatively, there may be a second factor that negatively regulates MPF to maintain the diakinetic state of immature oocytes in the absence of a sperm maturation signal. An RNAi screen in fog-2 animals for oocyte chromosome coalescence would likely identify such a factor.
Working model
Our working model is that CDK-1 activity is normally low in immature oocytes and that, upon WEE-1.3 depletion, this activity is increased. We propose that all of the wee-1.3 RNAi phenotypes described in this study are the direct result of precocious CDK-1 activity in oocytes. Two novel oocyte phenotypes were observed upon the depletion of WEE-1.3: (1) the coalescence of the meiotic chromosomes; and (2) the presence of perinuclear tubulin foci in oocytes. Of numerous sterility screens performed in C. elegans (Colaiacovo et al., 2002; Hanazawa et al., 2001; McDowall and Rose, 1997), none have reported either of these phenotypes. Aberrant diakinesis has been observed previously in other studies (Colaiacovo et al., 2002; Dernburg et al., 1998; Pasierbek et al., 2001), yet these have always been characterized by the presence of 12 univalents or 24 dyads, not chromosome coalescence. Furthermore, chromosomes have not been observed to coalesce upon the depletion of cohesin (Chan et al., 2003; Mito et al., 2003) or condesin subunits (Chan et al., 2004; Hagstrom et al., 2002), kinetochore components (Cheeseman et al., 2004; Oegema et al., 2001), or kinesins (Bishop et al., 2005; Powers et al., 2004; Segbert et al., 2003). We propose that chromosome coalescence is the result of perturbation of chromatin modifications that are important for maintaining the chromosomes as condensed diakinetic bivalents and for preparing them for their meiotic divisions.
Supplementary Material
Acknowledgments
We are grateful to T. Schedl and members of the Baltimore Worm Club for their input and suggestions, to S. T. Lamatina and S. L'Hernault for sharing unpublished results, to the NCI Fellows Editorial Board for their editorial assistance, and to M. Lilly, K. O'Connell, J. Dean, and members of our laboratory for critical reading of this manuscript. We also thank C. Malone for the WH210 strain, J. Austin for the AZ212 strain, M. Wallenfang, J. Pellettieri and G. Seydoux for the cdk-1 RNAi feeding construct and the JH1576 strain, and the C. elegans Genetics Center (University of Minnesota), funded by the NIH National Center for Research Resources, for providing most strains. This research was supported, in part, by the Intramural Research Program of the NIH National Institute of Diabetes, Digestive and Kidney Diseases.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/133/4/697/DC1
References
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chrom. Res. 1993;1:15–26. doi: 10.1007/BF00710603. [DOI] [PubMed] [Google Scholar]
- Aris JP, Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J. Cell Biol. 1988;107:17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J. Biol. Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JD, Han Z, Schumacher JM. The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol. Biol. Cell. 2005;16:742–756. doi: 10.1091/mbc.E04-08-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxem M, Srinivasan DG, van den Heuvel S. The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development. 1999;126:2227–2239. doi: 10.1242/dev.126.10.2227. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Chan A, Jeon M, Wu TF, Pasqualone D, Rougvie AE, Meyer BJ. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature. 2003;423:1002–1009. doi: 10.1038/nature01697. [DOI] [PubMed] [Google Scholar]
- Chan RC, Severson AF, Meyer BJ. Condensin restructures chromosomes in preparation for meiotic divisions. J. Cell Biol. 2004;167:613–625. doi: 10.1083/jcb.200408061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D, Serafinas C, Ashcroft N, Kosinski M, Longo D, Ferris DK, Golden A. The polo-like kinase PLK-1 is required for nuclear envelope breakdown and the completion of meiosis in Caenorhabditis elegans. Genesis. 2000;26:26–41. doi: 10.1002/(sici)1526-968x(200001)26:1<26::aid-gene6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaiacovo MP, Stanfield GM, Reddy KC, Reinke V, Kim SK, Villeneuve AM. A targeted RNAi screen for genes involved in chromosome morphogenesis and nuclear organization in the Caenorhabditis elegans germline. Genetics. 2002;162:113–128. doi: 10.1093/genetics/162.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr. Opin. Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Couteau F, Guerry F, Muller F, Palladino F. A heterochromatin protein 1 homologue in Caenorhabditis elegans acts in germline and vulval development. EMBO Rep. 2002;3:235–241. doi: 10.1093/embo-reports/kvf051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ES, Wille L, Chestnut BA, Sadler PL, Shakes DC, Golden A. Multiple subunits of the Caenorhabditis elegans anaphase-promoting complex are required for chromosome segregation during meiosis I. Genetics. 2002;160:805–813. doi: 10.1093/genetics/160.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell. 1998;94:387–398. doi: 10.1016/s0092-8674(00)81481-6. [DOI] [PubMed] [Google Scholar]
- Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Dev. Cell. 2001;1:187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Doree M, Hunt T. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 2002;115:2461–2464. doi: 10.1242/jcs.115.12.2461. [DOI] [PubMed] [Google Scholar]
- Edgar LG. Blastomere culture and analysis. In: Shakes DC, editor. Caenorhabditis elegans: Modern biological analysis of an organism. Vol. 48. Academic Press; New York: 1995. pp. 303–321. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- Furuta T, Tuck S, Kirchner J, Koch B, Auty R, Kitagawa R, Rose AM, Greenstein D. EMB-30: an APC4 homologue required for metaphase-to-anaphase transitions during meiosis and mitosis in Caenorhabditis elegans. Mol. Biol. Cell. 2000;11:1401–1419. doi: 10.1091/mbc.11.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A, Sadler PL, Wallenfang MR, Schumacher JM, Hamill DR, Bates G, Bowerman B, Seydoux G, Shakes DC. Metaphase to anaphase (mat) transition-defective mutants in Caenorhabditis elegans. J. Cell Biol. 2000;151:1469–1482. doi: 10.1083/jcb.151.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Echeverri C, Oegema K, Coulson A, Jones SJ, Copley RR, Duperon J, Oegema J, Brehm M, Cassin E, et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002;16:729–742. doi: 10.1101/gad.968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Hanazawa M, Mochii M, Ueno N, Kohara Y, Iino Y. Use of cDNA subtraction and RNA interference screens in combination reveals genes required for germ-line development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2001;98:8686–8691. doi: 10.1073/pnas.141004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Henriquez R, Blobel G, Aris JP. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J. Biol. Chem. 1990;265:2209–2215. [PubMed] [Google Scholar]
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, McCarter J, Francis R, Schedl T. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 1996;134:699–714. doi: 10.1083/jcb.134.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le, Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Lamitina ST, L'Hernault SW. Dominant mutations in the Caenorhabditis elegans Myt1 ortholog wee-1.3 reveal a novel domain that controls M-phase entry during spermatogenesis. Development. 2002;129:5009–5018. doi: 10.1242/dev.129.21.5009. [DOI] [PubMed] [Google Scholar]
- MacQueen AJ, Villeneuve AM. Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C. elegans chk-2. Genes Dev. 2001;15:1674–1687. doi: 10.1101/gad.902601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 2001;11:171–176. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 1999;205:111–28. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- McDowall JS, Rose AM. Genetic analysis of sterile mutants in the dpy-5 unc-13 (I) genomic region of Caenorhabditis elegans. Mol. Gen. Genet. 1997;255:60–77. doi: 10.1007/s004380050475. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito Y, Sugimoto A, Yamamoto M. Distinct developmental function of two Caenorhabditis elegans homologs of the cohesin subunit Scc1/Rad21. Mol. Biol. Cell. 2003;14:2399–2409. doi: 10.1091/mbc.E02-09-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K, Villeneuve AM, Colaiacovo MP. Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC. J. Cell Biol. 2005;168:683–689. doi: 10.1083/jcb.200410144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo N, Yoshitome S, Iwashita J, Iida M, Uto K, Ueno S, Okamoto K, Sagata N. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000;14:328–338. [PMC free article] [PubMed] [Google Scholar]
- Nieduszynski CA, Murray J, Carrington M. Whole-genome analysis of animal A- and B-type cyclins. Genome Biol. 2002;3:R0070.1–R0070.8. doi: 10.1186/gb-2002-3-12-research0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 2001;153:1209–1226. doi: 10.1083/jcb.153.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page BD, Guedes S, Waring D, Priess JR. The C. elegans E2Fand DP-related proteins are required for embryonic asymmetry and negatively regulate Ras/MAPK signaling. Mol. Cell. 2001;7:451–460. doi: 10.1016/s1097-2765(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 2001;15:1349–1360. doi: 10.1101/gad.192701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, Reinke V, Kim SK, Seydoux G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev. Cell. 2003;5:451–462. doi: 10.1016/s1534-5807(03)00231-4. [DOI] [PubMed] [Google Scholar]
- Piano F, Schetter AJ, Mangone M, Stein L, Kemphues KJ. RNAi analysis of genes expressed in the ovary of Caenorhabditis elegans. Curr. Biol. 2000;10:1619–1622. doi: 10.1016/s0960-9822(00)00869-1. [DOI] [PubMed] [Google Scholar]
- Powers J, Rose DJ, Saunders A, Dunkelbarger S, Strome S, Saxton WM. Loss of KLP-19 polar ejection force causes misorientation and missegregation of holocentric chromosomes. J. Cell Biol. 2004;166:991–1001. doi: 10.1083/jcb.200403036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S, Mains PE, Zinke A, Hyman AA. The mbk-2 kinase is required for inactivation of MEI-1/katanin in the one-cell Caenorhabditis elegans embryo. EMBO Rep. 2003;4:1175–1181. doi: 10.1038/sj.embor.7400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul J, Vaglio P, Rual JF, Lamesch P, Martinez M, Armstrong CM, Li S, Jacotot L, Bertin N, Janky R, et al. C. elegans ORFeome version 1.1: experimental verification of the genome annotation and resource for proteome-scale protein expression. Nat. Genet. 2003;34:35–41. doi: 10.1038/ng1140. [DOI] [PubMed] [Google Scholar]
- Schedl T. Developmental Genetics of the Germ Line. In: Priess JR, editor. C. elegans II. Cold Spring Harbor Laboratory Press; New York: 1997. pp. 241–270. [PubMed] [Google Scholar]
- Schimmang T, Tollervey D, Kern H, Frank R, Hurt EC. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Nebreda AR. Signalling pathways in oocyte meiotic maturation. J. Cell Sci. 2002;115:2457–2459. doi: 10.1242/jcs.115.12.2457. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Golden A, Donovan PJ. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segbert C, Barkus R, Powers J, Strome S, Saxton WM, Bossinger O. KLP-18, a Klp2 kinesin, is required for assembly of acentrosomal meiotic spindles in Caenorhabditis elegans. Mol. Biol. Cell. 2003;14:4458–4469. doi: 10.1091/mbc.E03-05-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer F, Moorman C, Van Der Linden AM, Kuijk E, Van Den Berghe PV, Kamath R, Fraser AG, Ahringer J, Plasterk RH. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:77–84. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel ML, Pellettieri J, Seydoux G. The C. elegans DYRK kinase MBK-2 marks oocyte proteins for degradation in response to meiotic maturation. Curr. Biol. 2006;16:56–62. doi: 10.1016/j.cub.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Stumpff J, Kellogg DR, Krohne KA, Su TT. Drosophila Wee1 interacts with members of the gammaTURC and is required for proper mitotic-spindle morphogenesis and positioning. Curr. Biol. 2005;15:1525–1534. doi: 10.1016/j.cub.2005.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Varmark H. Functional role of centrosomes in spindle assembly and organization. J. Cell Biochem. 2004;91:904–914. doi: 10.1002/jcb.20013. [DOI] [PubMed] [Google Scholar]
- Voronina E, Wessel GM. The regulation of oocyte maturation. Curr. Top. Dev. Biol. 2003;58:53–110. doi: 10.1016/s0070-2153(03)58003-6. [DOI] [PubMed] [Google Scholar]
- Wallenfang MR, Seydoux G. Polarization of the anterior-posterior axis of C. elegans is a microtubule-directed process. Nature. 2000;408:89–92. doi: 10.1038/35040562. [DOI] [PubMed] [Google Scholar]
- Wang J, Barr MM. RNA interference in Caenorhabditis elegans. Methods Enzymol. 2005;392:36–55. doi: 10.1016/S0076-6879(04)92003-4. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Hoch RV, Ashcroft NR, Kosinski ME, Golden A. A Caenorhabditis elegans wee1 homolog is expressed in a temporally and spatially restricted pattern during embryonic development. Biochim. Biophys. Acta. 1999;1445:99–109. doi: 10.1016/s0167-4781(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Yang HY, McNally K, McNally FJ. MEI-1/katanin is required for translocation of the meiosis I spindle to the oocyte cortex in C. elegans. Dev. Biol. 2003;260:245–259. doi: 10.1016/s0012-1606(03)00216-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.